Abstract
Autism spectrum disorder (ASD) is a general term for a group of complex neurodevelopmental disorders of brain development that limits a person’s ability to function normally. Etiology has not been clearly defined up to date. However, gut microbiota and the bidirectional communication between the gastrointestinal tract and brain, the so-called microbiota–gut–brain axis, are hypothesized, which may be involved in the etiology of several mental disorders. Recent reports suggest that Candida, particularly Candida albicans, growth in intestines may cause lower absorption of carbohydrates and minerals and higher toxin levels which are thought to contribute autistic behaviors. The aim of this study was to identify the 3-year deposited yeasts isolated from stool samples of children with diagnosed or suspected ASD and to determine in vitro activity of nystatin and fluconazole against these isolates using Clinical Laboratory Standards Institute M27-A3 guidelines. A 17-year retrospective assessment was also done using our laboratory records. Among the species identified, intrinsically fluconazole-resistent Candida krusei (19.8 %) and Candida glabrata (14.8 %) with elevated MICs were remarkable. Overall, C. albicans (57.4 %) was the most commonly isolated species in 17 years. The species identification and/or antifungal susceptibility tests have to be performed using the strain isolated from stool sample, to select the appropriate antifungal agent, if antimycotic therapy is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder is a range of complex neurodevelopmental disorders characterized by language and learning deficits, difficulty with social interactions and repetitive behaviors, and a commonly diagnosed condition in childhood and adolescence. The etiology of ASD is complex and likely multifactorial [1, 2]. Recent data propose the etiopathogenetic role of gut commensal microflora in ASD by affecting the absorption of carbohydrates and minerals during digestion [3–5]. Bidirectional signaling between the gastrointestinal tract and the brain, the so-called microbiota–gut–brain axis, has been shown to play an important role in healthy brain function [6–9]. Although Candida species normally colonize mucosal surfaces of the gastrointestinal system in an asymptomatic manner and is unable to grow excessively due to the competition and suppression of bacteria, scientists have noted that some autistic people have excessive amounts of Candida albicans in their intestinal tract [10]. As Candida grows, it releases ammonia and toxins which are thought to contribute to autistic behaviors [11, 12] and high levels of the yeast in gut mean lower absorption of carbohydrates and minerals and higher toxin levels. It was considered that this hypothesis could be beneficial in explaining a certain aspect of the biochemical etiology of ASD [13].
There are safe methods to treat yeast overgrowth, rebalancing the intestinal bacteria and yeast, such as taking probiotic supplements containing benign microbes and/or taking antifungal medications, for example nystatin and/or fluconazole. Kefir, a fermented milk drink which contains a symbiotic combination of lactic acid bacteria and yeasts including Candida kefyr, Kluyveromyces lactis, and Saccharomyces cerevisiae, may be used to maintain balance of intestinal bacteria and yeasts. Candida kefyr is the yeast that helps kefir ferment.
The aim of this study was to identify the species and to determine in vitro susceptibility against nystatin and fluconazole of the deposited yeasts isolated between December 2011 and December 2014 from stool samples of pediatric patients with diagnosed or suspected ASD. Additional retrospective assessment was done using our laboratory data about the yeast C. albicans cultured per year from stool specimens of this patient population between December 1998 and December 2014.
Materials and Methods
Strains and Identification
Strains included in this study were cultured from stool samples from children with diagnosed or suspected ASD sent by the Pediatric Metabolism Department. Samples were collected by parents in sterile containers in the hospital or if needed to collect at home, kept at +4 °C overnight, and delivered to our laboratory within 8 h. Samples of children who had been treated with antibiotics and/or drunk kefir were not accepted unless these had been stopped for at least 2 weeks before the acquisition of the sample. A non-autistic healthy volunteers control group (234 males and 169 females, from 2 to 18 years of age) was included. All samples were examined freshly by direct microscopy and culture.
Giemsa stained stool smears were examined under microscope in ×100 oil immersion. Samples were plated on Sabouraud dextrose agar (SDA, Himedia, Mumbai, India) supplemented with gentamicin and cooked sheep’s blood agar and incubated at 37 and 30 °C for 3 days. Isolates were checked for purity by streaking them over SDA and CHROMagar Candida (Becton–Dickinson, Sparks, MD) twice for 48 h at 35 °C. Isolates were stored on SDA slants at 4 °C by periodic subculturing until used in the study. Before testing, each isolate was passaged on SDA to ensure viability. The strains were identified by classical morphological and biochemical tests including germ tube formation in human serum, blastoconidia, pseudohyphae, true hyphae, chlamydoconidia formation on corn meal agar–Tween 80 (Difco, Detroid) [14], and urease production [14], integrating with the results of the carbohydrate assimilation reactions on the API 20 C AUX system (Biomeriéux, Marcy, l’Etoile, France).
A 17-year retrospective analysis of data accumulated between December 1998 and December 2014 about C. albicans isolation from this patient group was also conducted by year.
Antifungal Susceptibility Tests
Susceptibility patterns of the isolates and two quality control strains (Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258) to nystatin (Sigma) and fluconazole (Pfizer) were determined by broth microdilution assay according to the CLSI M27-A3 document [15, 16]. Stock solution of nystatin was prepared in dimethyl sulphoxide (DMSO, Sigma) and absolute ethanol (3:2 ratio) [17, 18], and of fluconazole in DMSO, and both were kept at −20 °C until use. Final dilutions of nystatin were prepared in antibiotic medium 3 [19], and of fluconazole in RPMI 1640 medium with glutamine, both supplemented with 2 % glucose and buffered with 0.165 M morpholinepropanesulfonic acid. Final concentrations of nystatin ranged from 16 to 0.003 and of FLZ from 64 to 0.125. Negative and positive controls were included in each microplate. The plates were incubated at 35 °C for 24 h. Endpoints were determined visually with the aid of a mirror. The MIC values for nystatin were the lowest concentration that caused complete inhibition of growth (as recommended for other polyenes, for example amphotericin B); for fluconazole it was the concentration causing approximately 50 % reduction in growth relative to the growth in the positive control wells. Interpretive criteria for FLZ were those published by CLSI [19]. Since nystatin was dissolved in DMSO and absolute ethanol, equivalent amounts of the later chemicals were tested initially as done in previous studies using the same isolates to ascertain whether they had an effect on the isolates tested. The minute volumes of the chemicals used did not have any effect on yeast survival/growth when compared with the controls [16, 17, 20, 21].
Results
Isolates
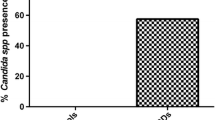
A total of 338 yeast strains were isolated from 415 stool samples of patients sent in between December 2011 and December 2014. Candida species were the most common yeasts in number (97.9 %), followed by Trichosporon mucoides (1.5 %) and S. cerevisiae (0.6 %). Among Candida spp. isolated, C. albicans was significantly higher in number (43.2 %). No yeast strain was cultured from the majority of the feces of healthy volunteers. Yeast isolates were recovered from 79 volunteers (19.6 %), and species detected were C. albicans (58.2 %) and non-albicans Candida species (41.2 %). When controls were compared with patients with ASD, the rate of yeast isolates was found notably lower in healthy subjects (p < 0.5). Candida krusei and Candida glabrata were not detected in the control group (Table 1).
Totally, 1555 stool specimens obtained from this patient group were mycologically examined over 17 years. The age range of the patients was from 9 months to 18 years. Of them, 879 were male (56.5 %) and 676 (43.5 %) were female. Totally, 1130 yeast strains were isolated. C. albicans (57.4 %) was the most commonly isolated species every year (Table 2).
Antifungal Susceptibility
The results of the MIC determination are shown in Table 3. Results for QC strains were within the acceptable range of MICs of fluconazole as stated in document M27-A3. All C. albicans, Candida tropicalis, and C. parapsilosis strains tested were found susceptible to fluconazole. Resistance to fluconazole was found only in C. krusei strains and high MIC values in C. glabrata strains. All C. albicans, T. mucoides, and S. cerevisiae strains showed low MIC values against nystatin. Low MIC50 values were observed for C. parapsilosis and C. tropicalis against nystatin.
Discussion
Recently, accumulating evidence suggests that gut microbiota can influence behavior by regulating brain chemistry [22–25] and the microbiota–gut–brain axis” became one of the major topics of research interest [6, 8]. Although recent few studies revealed that human gut microbiome differs among individuals in correlation with diet, age, antibiotic usage, and underlying conditions [26–29], up to 60 % of healthy people are thought as asymptomatic carriers of Candida spp. as a commensal in the gastrointestinal tract. There are some reports of associations between the occurrence of some symptoms caused by Candida in patients with ASD [11, 13]. Recently, scientists suggest that Candida, particularly C. albicans growth in intestines may cause lower absorption of carbohydrates and minerals and higher toxin levels which are thought to contribute to autistic behaviors [7, 11, 13]. However, little is known about the presence of Candida spp. in gut of this pediatric patient population.
A few reports about fecal fungal flora focused on hospitalized children [30] or those with immunosuppression, diarrhea [31, 32], or with diabetes [26], as well as healthy individuals of various age groups [33]. Recently, there are only a few reports investigating the fecal microbiota of children with ASD mainly focusing on bacteria [3, 34]. Our findings demonstrated that while the majority of healthy children did not harbor yeasts in their gut, given the fact that only a small number of Candida were isolated from stool samples of healthy volunteers studied (19.6 %), the higher prevalence was found in patients with suspected or diagnosed ASD (81.4 %). This comparative data might support the hypothesis of the importance of yeasts present in the intestines in patients with ASD.
Although Candida species were uncommon in stool samples of healthy children assessed in this study, C. albicans was the prevalent species isolated from the samples of both patients (43.2 %) and control (58.2 %) groups. In the present study, C. krusei and C. glabrata were not found in healthy children as was reported in another study [34]. Therefore, the correct identification of the species might be a crucial element for efficient therapeutic decisions in patients with ASD, when needed.
In our Deep Mycoses Laboratory, stool samples of pediatric patients with metabolic disorders and/or suspected or diagnosed ASD had been routinely examined over 17 years. Samples had been accepted for mycological examination only if the patient did not drink kefir during the last 2 weeks to avoid misinterpretation of the transient presence of Candida isolates. Isolated yeasts had been reported as C. albicans or non-albicans Candida species by performing standard tube germination and chlamydospore production tests. According to our accumulated laboratory data, a high number of yeast strains (72.7 %) and prevalently C. albicans (57.4 %) were isolated from stool samples of 1555 patients over a wide range of age (9 month–18 years).
In the present study, the non-albicans Candida species, C. krusei, C. parapsilosis, C. tropicalis, and non-Candida strains including T. mucoides and S. cerevisiae were determined among yeasts deposited in the last 3 years. Trichosporon species may be a part of human intestinal flora [35, 36]. Saccharomyces cerevisiae is known to be a transient component of the normal flora of the intestinal tract [37]. Although both of these two non-Candida yeasts were isolated in low numbers in our study and in another [37], probably, non-albicans Candida and non-Candida yeast species may also cause lower absorption of carbohydrates and minerals in the intestines and may probably take a role in the microbiota–gut–brain axis.
Many molds and yeasts are susceptible to nystatin, which is active mostly in the intestinal tract and is poorly absorbed systemically. Nystatin tablets or capsules do not dissolve until they reach the stomach or lower; therefore, this is an advantage over other antifungal agents to be limited to the intestinal tract. Fluconazole is another effective candidastatic antifungal agent. Nystatin and/or fluconazole is used to restore the proper balance of microbiota or to treat Candida overgrowth in the intestines of children with ASD.
In the present study, the vast majority of C. albicans, C. parapsilosis, and C. tropicalis isolates were inhibited at low MIC values of nystatin (MIC90, 0.5, 1, and 1 µg/ml, respectively) and also were found susceptible to fluconazole using new species-specific breakpoints [19]. These results indicate good in vitro activity of both antifungals tested against C. albicans, C. parapsilosis, and C. tropicalis [18, 37–41]. However, other non-albicans Candida species isolated were the intrinsically fluconazole-resistant C. krusei (34.9 %) and C. glabrata (26.0 %), which have intrinsically low susceptible to azole or acquire resistance during prolonged azole therapy, by undergoing mutation [42].
This retrospective study provides significant baseline data for the future researches about the yeasts–gut–brain axis associated with the patients diagnosed or suspected ASD. Our results suggest that, if antifungal treatment would be necessary for otherwise healthy pediatric patients with metabolic disorders or diagnosed or suspected ASD, the species identification and/or antifungal susceptibility tests have to be performed using the strain isolated from stool sample, to select the appropriate antifungal agent.
References
Autism fact sheet. National Institute of neurological disorders and stroke. 2014. http://www.ninds.nih.gov/disorders/autism/detail_autism.htm.
Watts TJ. The pathogenesis of autism. Clin Med Pathol. 2008;1(99):103.
Tomova A, Husarova V, Lakatosova S, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–87.
Caronna EB, Milunsky JM, Tager-Flusberg H. Autism spectrum disorders: clinical and research frontiers. Arch Dis Child. 2008;93(6):518–23.
Allely CS, Gilberg C, Wilson P. Neurobiological abnormalities in the first few years of life in individuals later diagnosed with autism spectrum disorder: a review of recent data. Behav Neurol. 2014;2014:210780.
Montiel-Castro AJ, Gonzales-Cervantes RM, Bravo-Ruiseco G, Pacheco-Lopez G. The microbiota–gut–brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013;7:1–16.
Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms and therapeutic potential. Adv Exp Med Biol. 2014;817:373–403.
Foster JA, McVey Neufeld KA. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–12.
Cryan JE, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behavior. Nat Rev Neurosci. 2012;13(10):701–12.
Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism-comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22–34.
Norris S. Potential causes of autism spectrum disorders. Rep Can Libr Parlim. 2006:4–5.
Reichelt KL, Knivsberg AM. The possibility and probability of a gut-to-brain connection in autism. Ann Clin Psychiatry. 2009;21(4):205–2011.
Burrus CJ. A biochemical rationale for the interaction between gastrointestinal yeast and autism. Med Hypothesis. 2012;79(6):784–5.
Hazen KC, Howell SA. Candida, Cryptococcus and other yeasts of medical importance. In: Murray R, Baron EJ, Jorgensen JH, Phaller MA, Yolken RH, editors. Manual of clinical microbiology, vol. 9., ASM PressDC: Washington; 2007. p. 1762–88.
Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, in 3rd Informal Supplement M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth International Supplement, M27-A3, Wayne, PA: Clinical and Laboratory Standards Institute, 2012: 32.
Ellepola ANB, Samaranayake LP. Adhesion of oral C. albicans to human buccal epithelial cells following limited exposure to antifungal agents. J Oral Pathol Med. 1998;27(7):325–32.
Ellepola ANB, Samaranayake LP. The in vitro post-antifungal effect of nystatin on Candida species of oral origin. J Oral Pathol Med. 1999;28(3):112–6.
Arikan S, Ostrosky-Zeichner L, Lozano-Chiu M, et al. In vitro activity of nystatin compared with those of liposomal nystatin, amphotericin B, and fluconazole against clinical Candida isolates. J Clin Microbiol. 2002;40(4):1406–12.
Ellepola ANB, JosepH BK, Chandy R, Khan ZU. The postantifungal effect of nystatin and its impact on adhesion attributes of oral Candida dubliniensis isolates. Mycoses. 2014;57(1):56–63.
Ellepola ANB, Samaranayake LP. Impact of brief and sequential exposure to nystatin on the germ tube formation and cell surface hydrophobicity of oral Candida albicans isolates from human immunodeficiency virus-infected patients. Med Princ Pract. 2014;23(4):307–12.
Farmer AD, Randall HA, Aziz Q. It’s a gut feeling: how the gut microbiota affects the state of mind. J Physiol. 2014;592(Pt 14):2981–8.
Carabotti M, Scirocco A, Maselli MA, Severi C. The gut brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9.
Van de Sande MMH, van Buul VJ, Bruns FJPH. Autism and nutrition: the role of the gut–brain axis. Nutr Res Rev. 2014;27(2):199–214.
Mayer EA, Padua D, Tillisch K. Altered brain–gut axis in autism: comorbidity or causative mechanisms ? BioEssays. 2014;36(10):933–9.
Soyucen E, Gulcan A, Aktuglu-Zeybek AÇ, Onal H, Kiykim E, Aydin A. Differences in gut microbiota of healthy children and those with type 1 diabetes. Pediatr Int. 2014;56(3):336–43.
Gosiewski T, Salamon D, Spoza M, Sroka A, Malecki MT, Bulanda M. Quantitative evaluation of fungi of the genus Candida in the feces of adult patients, with type 1 and 2 diabetes—a pilot study. Gut Pathog. 2014;6(1):43–7.
Hoffmann C, Dollive S, Grunberg S, et al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS ONE. 2013;8(6):e66019.
Ekiel A, Aptekorz M, Kazek B, Wiechula B, Wilk I, Martirosian G. Intestinal microflora of autistic children. Med Dosw Mikrobiol. 2010;62(3):237–43.
Rozkiewich D, Daniluk T, Sciepuk M, Kurzqkowska B, Oldak E, Zaremba ML. Prevalence of Candida albicans in stool of hospitalized children in 2003 with or without diarrhea from the Bialystok region. Prz Epidemiol. 2005;59(1):43–51.
Agirbasli H, Keceli SAO, Gedikoglu G. Fecal fungal flora of pediatric healthy volunteers and immunosuppressed patients. Mycopathologia. 2005;159:515–20.
Klingspor L, Stitzing G, Johansen K, Murtaza A, Holmberg K. Infantile diarrhoea and malnutrition associated with Candida in a developing community. Mycoses. 1993;36(1–2):19–24.
Khatip R, Riederer KM, Ramanathan J, Baran J Jr. Faecal fungal flora in healthy volunteers and inpatients. Mycoses. 2001;44(1):151–6.
De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE. 2013;8(10):e76993. doi:10.1371/journal.pone.0076993.
Colombo AL, Padovan ACB, Chaves GM. Current knowledge of Trichosporon spp. and Trichosporonosis. Clin Microbiol Rev. 2011;24(4):682–700.
Kreger-Van Rij NJW, editor. The yeasts: a taxonomic study. 3rd ed. Amsterdam: Elsevier Science Publishers B.V; 1984.
Sanata B, Salam OA, Ibrahim S, et al. Digestive fungal flora in asymptomatic subjects in Bobo-Dioulasso, Burkina Faso. Asian Pac J Trop Biomed. 2014;4(8):658–62.
Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Echinocandin and triazole antifungal susceptibility profiles for Candida spp., Cryptococcus neoformans, and Aspergillus fumigatus: application of new CLSI clinical breakpoints and epidemiological cutoff values to characterize resistance in the SENTRY antimicrobial surveillance programme (2009). Diagn Microbiol Infect Dis. 2011;69(1):45–50.
Carrillo-Munoz AJ, Quindos G, Tur C, et al. In vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B desoxycholate, fluconazole and itraconazole. J Antimicrob Chemother. 1999;44(3):397–401.
Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol. 2005;43(5):2155–62.
Choukri F, Benderdouche M, Sednaoui P. In vitro susceptibility profile of 200 recent isolates of Candida spp. to topical antifungal treatments of vulvovaginal candidiasis, the imidazoles and nystatin agents. J Mycol Med. 2014;24(4):303–7.
Bennett JF, Izumikawa K, Marr KA. Mechanisms of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob Agents Chemother. 2004;48(5):1773–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical considerations
The study protocol was approved by Istanbul University Cerrahpasa Medical Faculty Ethics Committee (date: 02.10.2014; No. 41302).
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Kantarcioglu, A.S., Kiraz, N. & Aydin, A. Microbiota–Gut–Brain Axis: Yeast Species Isolated from Stool Samples of Children with Suspected or Diagnosed Autism Spectrum Disorders and In Vitro Susceptibility Against Nystatin and Fluconazole. Mycopathologia 181, 1–7 (2016). https://doi.org/10.1007/s11046-015-9949-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-015-9949-3