Abstract
A case report of cutaneous mucormycosis and obstacles to early diagnosis is presented. A 38-year-old male was involved in a car accident that led to amputation of both lower limbs. Subsequently, he developed fungal wound infection of the left lower limb stump. The infection was detected very early, although the diagnosis was difficult because only a small area was affected and histopathological examination was initially negative. The infection was proven by microscopy, culture and histopathology. The isolate was identified by sequencing of the rDNA ITS region gene (internal transcribed spacer region of ribosomal DNA) as Lichtheimia corymbifera. Liposomal amphotericin B and surgery were successful in management of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive fungal infections are an important cause of morbidity and mortality in predisposed patients [1]. Mucormycosis is an angioinvasive infection caused by ubiquitous filamentous fungi of the order Mucorales [2]. It is the second most frequent opportunistic fungal infection causing systemic involvement [1]. The majority of cases are caused by the genera Rhizopus, Mucor, Lichtheimia, Cunninghamella, Apophysomyces and Saksenaea [3]. Underlying conditions play an important role in the development of the disease. Granulocytopenia, immunosuppression, diabetes and penetrating trauma are the most prevalent predisposing factors [4]. Based on anatomic location, mucormycosis can be classified into six forms: rhinocerebral, pulmonary, cutaneous, gastrointestinal, disseminated and uncommon presentations [3, 5]. The most common clinical manifestation is pulmonary disease, followed by rhinocerebral affection, infection of the soft tissues and disseminated disease [6]. The mortality rate remains high [3, 7]. In this respect, early diagnosis of the disease plays a crucial role [8].
A case of cutaneous mucormycosis developed after a car accident with discrepancies in the early diagnosis is presented.
Case Report
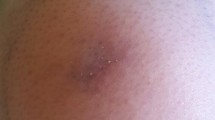
A 38-year-old healthy male was involved in a car accident in June 2014. The patient remained trapped in a car for 45 min before mechanical extrication. He was transported by helicopter to the University Military Hospital in Prague with developing hemorrhagic traumatic shock due to severe multiple trauma (an injury severity score of 66). He was treated according to the Advanced Trauma Life Support protocol. He underwent urgent abdominal exploration and amputation of the left leg below the knee joint. Laceration and open fracture of the right tibia were treated with external bone fixation. Other injuries included dissection of the right internal carotid artery, multiple rib fractures and traumatic laceration of the liver. Given the extent of the injury, a severe shock state occurred first due to the patient’s traumatic hemorrhagic injury. Multiorgan dysfunction syndrome developed that required vasopressor support, artificial ventilation and continuous renal replacement therapy for non-oliguric renal failure. Prophylactic antibiotic treatment was started with a combination of clindamycin (4.8 g i.v./day for 6 days) and gentamicin (480 mg i.v./day for 2 days). Treatment of the right leg was complicated by gas gangrene with typical local findings and a positive culture for Clostridium perfringens. Despite aggressive treatment including antibiotic therapy (metronidazole 1.5 g i.v./day for 8 days and tigecycline 100 mg i.v./day for 7 days), right leg amputation above the knee had to be performed. In addition, no signs of healing were observed in the wound on his left amputated leg. One week after amputation, on day 9 after admission, a fungus suggestive of a mucormycete was cultured from a stump swab. Thereafter, antifungal treatment with amphotericin B lipid complex (L-AmB, 400 mg i.v./day in continuous infusion for 18 days) was started. On day 16, biopsy samples were sent for histopathological analysis and for mycological microscopy and culture. In contrast to negative histopathological findings in tissue slices stained with hematoxylin–eosin, microscopic examination in the mycology laboratory using fluorescence microscopy revealed the presence of non-septate hyphae variable in width and suggestive of the presence of a mucormycete (Fig. 1). The antifungal treatment was continued. However, surgery was not indicated at that time because of the absence of clinical signs of infection and negative histological finding. Despite intensive systemic therapy and local wound care (daily bandage, debridement as necessary; povidone/iodine and hydrogen peroxide solution), the wound remained unchanged in appearance, with the defect being surrounded by gray-brownish skin without specific signs of inflammation (Fig. 2).
On day 22, tissue biopsy was sent for histopathological and mycological investigation again. This time, both microscopy examinations confirmed the presence of sparsely septate fungal hyphae in the tissue (Fig. 3). Further, histopathology showed necrotic tissue penetrated by leukocytes, fibrin and non-specific granular tissue. On day 26, based on these results, reamputation of the patient’s lower left extremity at the knee joint was performed. No complications were observed after the surgery, and a mucormycete was never found by further investigations. Based on the negative findings, the treatment with L-AmB was terminated on day 31 and the wound healed completely in the next couple of days.
On day 38, the patient in stable condition was transferred to a surgical intensive care unit and subsequently to a general surgical ward; after that, rehabilitation procedures were started. Eight months after the accident, the patient is able to move on a wheelchair by himself.
Mycological Examination
The schedule of sampling and results of mycological examination are summarized in Table 1.
The biopsy material was examined by fluorescence microscopy using the optical brightener Blankophor (Bayer, Leverkusen, Germany). Wide, sparsely septate hyphae were revealed (Fig. 1). All samples (swabs and biopsies) were cultured on Sabouraud dextrose agar (Trios, Prague, Czech Republic) at 25 and 35 °C. Rapidly growing colonies of the suspected mucormycete always of the same appearance were observed after 24 h. The isolates were identified as Lichtheimia sp. based on the micromorphological features in the slide culture. The strain is available from the Culture Collection of Fungi (CCF), Department of Botany, Charles University in Prague under the accession number CCF 5092.
Molecular Analysis
The identification of the isolate was verified based on rDNA ITS sequencing (internal transcribed spacer region of ribosomal DNA) and by comparison of similarities with sequences stated in a recent taxonomic monograph on the order Mucorales [9]. The rDNA ITS region (ITS1–5.8S-ITS2 cluster) was amplified using the primer set ITS1 (5′-TCCGTAGGTGAACCTGCGG) and ITS4 (5′-TCCTCCGCTTATTGATATGC). The reaction mixture and PCR protocol were described by Hubka et al. [10]. PCR product purification and sequencing were performed at Macrogen Europe (Amsterdam, the Netherlands) using the terminal primers. The sequence was deposited into the European Molecular Biology Laboratory under the accession number LN812956. The sequence showed 98 % similarity (696/710 identical base pairs) with that of the ex-type strain of Lichtheimia corymbifera CBS 429.75 (GQ342878). Some of the strains from the aforementioned study (determined as L. corymbifera) possessed 100 % conformity, e.g., CBS 100.31 (GQ342879).
Discussion
Based on the data in the literature, skin infection is the third most common clinical manifestation of mucormycosis [6, 11]. The main risk factor is disruption of the normal protective barrier [3–6, 12]. Mucormycetes are incapable of penetrating through intact skin. However, burns, traumatic disruption and persistent maceration of skin enable fungi to penetrate into deeper tissues [3, 5]. This happens particularly as a result of traumatic implantation of soil in relation to a traffic accident as shown in our report [4–6, 13].
Mucormycosis is mostly a very aggressive fungal disease [14, 15]. A hallmark of mucormycete infection is extensive angioinvasion with resultant vessel thrombosis and tissue necrosis [5]. Skin infection can be also very aggressive, and fungi can rapidly penetrate through damaged skin and subcutaneous tissues into adjacent fat, muscle, fascia and even bone [5, 16]. A typical sign of cutaneous mucormycosis is necrotic eschar surrounded by erythema and induration [3]. The infection may disseminate hematogenously, and it may occur also in immunocompetent individuals [12]. However, the skin infection itself has a favorable prognosis and a low mortality rate if radical surgical intervention is done promptly [5, 6]. In our case, the infection was only local. The wound did not show any signs of purulent inflammation, even though the skin defect in the amputation stump did not heal and was bordered by brown-gray colored skin.
Four factors are crucial for the treatment of mucormycosis: early diagnosis, elimination of predisposing factors, extensive surgical intervention and appropriate antifungal therapy [14, 15, 17]. Early diagnosis plays the most important role in the initial phase, as the infection is usually restricted only to a small body area [8, 11, 18]. Unfortunately, varied and uncommon clinical manifestations often delay the diagnosis, which may result in poor outcome [3]. Lanternier et al. [19] reported that the time elapsed between the first symptoms and diagnosis was significantly shorter in patients with cutaneous mucormycosis as compared with rhinocerebral, pulmonary or disseminated disease. In our case, the infection was revealed very early and only a small area of the stump was affected.
For the diagnosis of fungal infections including mucormycosis, microscopy, culture, serological and molecular methods can be used [20–22]. Histopathological examination, fluorescent microscopy and culture were used in the reported case. The sensitivity of microscopy techniques including histopathology and also culture is often reported to be low; nevertheless, culture and histopathology are considered as the gold standard in the laboratory diagnosis of fungal infections [20, 23]. To detect the infection in skin or subcutaneous tissue, it is necessary to take a biopsy sample [12, 13, 18, 22]. For direct examination in the mycology laboratory, fluorescence microscopy is frequently used [14]. The method is rapid, has higher sensitivity than culture and allows finding hyphae easily and rapidly [20]. Cuenca-Estrella et al. [23] state that hyphae can be detected in a sample by microscopy only in advanced stages of infection. We do not share this opinion as, in accordance with some other authors, we have good experience with fluorescence microscopy of tissue mounts stained with Blankophor [24, 25]. Hyphae of mucormycetes are different from other fungi: they are usually non-septate, 6–16 µm in width and have square branching [8, 22]. However, isolates from clinical samples, particularly from swabs, are frequently not associated with infection; they are contaminants from the air only [18].
The main problem with early diagnosis of mucormycosis in the reported case was discrepancy between histopathological and mycological results. Microscopy and culture of the tissue biopsy were repeatedly positive for the presence of the fungus in the mycology laboratory, in contrast to negative histopathology in the initial sample. This result led to the diagnostic doubts of clinicians considering surgery. However, examination of subsequent biopsy samples showed concordant findings in both laboratories and fungal infection was confirmed. Chen et al. [26] reported a similar problem with negative histopathology in early stages of L. corymbifera infection.
A consensus on treatment of soft tissue mucormycosis is available in the literature, with combined surgical and pharmacological (antifungal) treatment being recommended [14, 17, 27]. The suggested antifungal agent is amphotericin B, preferably its lipid formulations having less adverse organ toxicity [14, 15, 17]. However, antifungal therapy alone is usually insufficient for elimination of the infection. Furthermore, fungal angioinvasion which leads to thromboses and tissue necrosis is a reason for poor penetration of the drug to the site of infection [5]. Cutaneous mucormycosis treated with aggressive surgical debridement and adjunctive antifungal therapy has a mortality rate of less than 10 % [11]. In the present case, antifungal therapy was started immediately after initial detection of the mucormycete in a swab sample (Table 1). We hypothesize that administration of L-AmB in the early stage of infection when a very small area of the skin was affected prevented the mucormycete from rapidly spreading to adjacent tissues. Initially, surgical treatment was refused by a traumatologist because there were no signs of systemic infection and no histopathological evidence of the fungus. In the course of antifungal therapy, local findings remained unchanged for 2 weeks. Then, surgical reamputation was indicated as no signs of healing were observed and both histological and mycological findings were positive for fungal infection. The surgery supported by antifungal therapy finally led to eradication of the fungus.
An interesting question is whether continued refusal of surgical intervention would have led to the harm of the patient. Although the reported patient was not immunologically investigated, we can suppose that a patient with multiple traumas undergoing several surgical interventions and suffering from multiorgan dysfunction syndrome cannot be fully immunocompetent [28]. We are convinced that antifungal therapy alone, even if prolonged, would not have led to wound healing in the reported patient. A fatal course cannot be excluded because of the aggressive nature of diseases caused by mucormycetes.
References
Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13.
de Hoog GS, Guarro J, Gené J, Figueras MJ. Atlas of clinical fungi. 2nd ed. Utrecht: Centraalbureau voor Schimmelcultures; 2000.
Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54:S23–34.
Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–53.
Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–69.
Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, Lass-Florl C, Bouza E, Klimko N, Gaustad P, Richardson M, Hamal P, Akova M, Meis JF, Rodriguez-Tudela J-L, Roilides E, Mitrousia-Ziouva A, Petrikkos G. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17:1859–67.
Pagano L, Offidani M, Fianchi L, Nosari A, Candoni A, Piccardi M, Corvatta L, D’Antonio D, Girmenia C, Martino P, Del Favero A. Mucormycosis in hematologic patients. Haematologica. 2004;89:207–14.
Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis. 2012;54:S55–60.
Walther G, Pawłowska J, Alastruey-Izquierdo A, Wrzosek M, Rodriguez-Tudela JL, Dolatabadi S, Chakrabarti A, de Hoog GS. DNA barcoding in Mucorales: an inventory of biodiversity. Persoonia. 2013;30:11–47.
Hubka V, Lyskova P, Frisvad JC, Peterson SW, Skorepova M, Kolarik M. Aspergillus pragensis sp. nov. discovered during molecular reidentification of clinical isolates belonging to Aspergillus section Candidi. Med Mycol. 2014;52:565–76.
Skiada A, Rigopoulos D, Larios G, Petrikkos G, Katsambas A. Global epidemiology of cutaneous zygomycosis. Clin Dermatol. 2012;30:628–32.
Trotter DJ, Gonis G, Cottrill E, Coombs C. Disseminated Saksenaea vasiformis in an immunocompetent host. Med J Aust. 2008;189:519–20.
Ingram PR, Suthananthan AE, Rajan R, Pryce TM, Sieunarine K, Gardam DJ, Heath CH. Cutaneous mucormycosis and motor vehicle accidents: findings from an Australian case series. Med Mycol. 2014;52:819–25.
Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Freiberger T, Guinea J, Guarro J, de Hoog S, Hope W, Johnson E, Kathuria S, Lackner M, Lass-Flörl C, Lortholary O, Meis JF, Meletiadis J, Muñoz P, Richardson M, Roilides E, Tortorano AM, Ullmann AJ, van Diepeningen A, Verweij P, Petrikkos G. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20:5–26.
Skiada A, Lanternier F, Groll AH, Pagano L, Zimmerli S, Herbrecht R, Lortholary O, Petrikkos GL. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013;98:492–504.
Andresen D, Donaldson A, Choo L, Knox A, Klaassen M, Ursic C, Vonthethoff L, Krilis S, Konecny P. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet. 2005;365:876–8.
Skiada A, Petrikkos G. Cutaneous mucormycosis. Skinmed. 2013;11:155–9.
Petrikkos G, Skiada A, Drogari-Apiranthitou M. Epidemiology of mucormycosis in Europe. Clin Microbiol Infect. 2014;20:67–73.
Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M, Bitar D, Dromer F, Lortholary O. A global analysis of mucormycosis in France: the RetroZygo Study (2005–2007). Clin Infect Dis. 2012;54:S35–43.
Arendrup MC, Bille J, Dannaoui E, Ruhnke M, Heussel CP, Kibbler C. ECIL-3 classical diagnostic procedures for the diagnosis of invasive fungal diseases in patients with leukaemia. Bone Marrow Transplant. 2012;47:1030–45.
Arendrup MC, Posteraro B, Sanguinetti M, Guinea J. The state-of-the-art mycology laboratory: visions of the future. Curr Fungal Infect Rep. 2015;9:37–51.
Lass-Flörl C. Zygomycosis: conventional laboratory diagnosis. Clin Microbiol Infect. 2009;15:60–5.
Cuenca-Estrella M, Bassetti M, Lass-Flörl C, Ráčil Z, Richardson M, Rogers TR. Detection and investigation of invasive mould disease. J Antimicrob Chemother. 2011;66:S15–24.
Andreas S, Heindl S, Wattky C, Möller K, Rüchel R. Diagnosis of pulmonary aspergillosis using optical brighteners. Euro Respir J. 2000;15:407–11.
Lyskova P, Zackova P, Petecukova V, Hubka V, Vasakova M, Matej R, Cermak J, Kubatova A, Kolarik M, Hricikova I, Kozak T. Pulmonary mucormycosis caused by Rhizopus microsporus. Klin Mikrobiol Infekc Lek. 2013;19:132–7.
Chen CK, Wan SH, Kou SK. A rare cutaneous fungal infection complicating bacterial necrotising fasciitis. Hong Kong Med J. 2008;14:314–6.
Stasiak M, Samet A, Lasek J, Wujtewicz M, Witkowski Z, Komarnicka J, Golabek-Dropiewska K, Rybak B, Gross M, Marks W. Mucormycosis complicating lower limb crash injury in a multiple traumatised patient: an unusual case. BMJ Case Rep. 2009. doi:10.1136/bcr.10.2008.1170.
Hua R, Zhang Y, Chen F, Zhou Z, Li X, Shao B, Wang S, Zhang Y, Lv X. Decreased levels of perforin-positive lymphocytes are associated with posttraumatic complications in patients with major trauma. Injury. 2014;45:2089–95.
Acknowledgments
This work was supported by the Palacky University Olomouc Internal Grant Agency (project no. IGA_LF_2015_035), the Ministry of Education, Youth and Sports of the Czech Republic (SVV project), and the project “BIOCEV—Biotechnology and Biomedicine Centre of the Academy of Sciences and Charles University” (CZ.1.05/1.1.00/02.0109) from the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tyll, T., Lyskova, P., Hubka, V. et al. Early Diagnosis of Cutaneous Mucormycosis Due to Lichtheimia corymbifera After a Traffic Accident. Mycopathologia 181, 119–124 (2016). https://doi.org/10.1007/s11046-015-9943-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-015-9943-9