The methods of scanning electron microscopy, differential scanning calorimetry and x-ray diffraction are used to study the morphological and thermal characteristics of cooling-induced martensite in alloy Fe – 30% Ni – 3% Pd. It is shown that the kinetics of the martensitic transformation is athermal and the morphology is lamellar. The mean values of the parameters of the austenite and martensite lattices are 0.35704 and 0.28614 nm respectively. The thermocycling leaves the points A s and A f invariable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Martensitic transformation is a typical phase transformation of the first kind, which develops in the absence of diffusion of atoms and has been studied in detail from the standpoints of physics, metallography and crystallography [1]. The effects of the temperature, hydrostatic pressure and uniaxial stresses are investigated for determining the special features of martensitic transformation from the standpoints of thermodynamics, kinetics and conditions of appearance of the transformation as such [2,3,4,5].
The morphology of martensite in alloys of the Fe – Ni system depends on the nickel content [4]. Introduction of a third component like Mn, Co, or C into binary Fe – Ni alloys hanges the kinetics of the transformation and the morphology of the martensite [5,6,7,8], which may be lath, butterfly, lenticular, plate, or thin-plate in different iron-base alloys [9,10,11,12].
The kinetics of the transformation may be athermal (when the fraction of the transformed volume depends only on the temperature) or isothermal (when the fraction of the transformed volume depends both on the temperature and on the time of the transformation) [13]. The athermal kinetics corresponds to a well manifested point of the start of the transformation M s. The time of formation of an individual martensite plate in Fe – Ni alloys is 1 × 10–7 sec [14].
Though the martensitic transformation in ternary Fe – Ni – X alloys has been studied well enough, the special features of the transformation in the Fe – Ni – Pd system remain little investigated. The aim of the present work was to study the morphology, the kinetics, the structure, and the temperature of cooling-induced martensitic transformation in a Fe – 30% Ni – 3% Pd alloy.
Methods of Study
We fabricated cylindrical bars with diameter 1 cm and length 10 cm from alloy Fe – 30% Ni – 3% Pd by fusing pure (99.9%) elements in a vacuum induction furnace in an argon atmosphere. The bars were placed into a quartz ampule, homogenized for 6 h at 1200°C, and quenched in water at room temperature. Then they were thinned mechanically to obtain specimens for scanning electron microscopy. The surface of the specimens was polished mechanically and cleaned in a solution containing 90 ml H2O2, 15ml H2O and 5 ml HF. The microstructure was investigated under a JEOL JSM 5600 scanning electron microscope (SEM) at an accelerating voltage of 20 kV. To determine the points M s, A s and A f of the transformation we cut discs with diameter 1 mm from the homogenized specimens and placed them into aluminum containers. The differential scanning calorimetry (DSC) curves were plotted using a Perkin-Elmer Sapphire thermal analyzer at a cooling rate of 5 K/min in the range from +20 to –150°C and a heating rate of 5 K/min in the range of 20 – 500°C. The x-ray diffraction patterns in the angle rage 2θ = 30 – 100° were obtained at room temperature using a Bruker D8 Advance diffractometer in filtered copper K 𝛼 radiation.
Results and Discussion
Figure 1 a presents the microstructure (SEM) of alloy Fe – 30% Ni – 3% Pd in secondary electrons. The size of the austenite grains is about 250 μm. After immersion of this specimen into liquid nitrogen for 5 sec we can observe formation of martensite plates (Fig. 1 b).
In accordance with the data of [15, 16], martensitic transformation is determined by its kinetics and crystallography. It is impossible to distinguish the formation of martensite under thermal and athermal kinetics on the basis of pure theory [17].
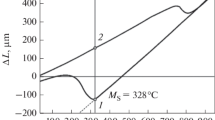
According to the results of the differential scanning calorimetry, the temperatures of the start (A s) and finish (A f) of the reverse transformation are 380 and 450°C, respectively (Fig. 2 a), and the temperature of the start of the martensitic transformation (M s) is –23°C (Fig. 2 b).
The isothermal transformation yielding new nuclei or stimulating growth of the available martensite crystals is described in detail in [13]. In our case, the specimens of alloy Fe – 30% Ni – 3% Pd were held in liquid nitrogen for 10 min, 10 h and 10 days, but new martensite crystals did not form during these holds, i.e., the kinetics of the transformation in the alloy was athermal. In [18], the points M s and A s were determined for alloys of the Fe – Ni system containing from 9.5 to 33.2 at.% Ni. By the data of this work, the γ → α′ transformation in the alloys with over 28.0 at. % Ni developed in a bust manner (burst kinetics). The point M s in the alloy with 30.43 at. % Ni was –30°C. The temperatures of the start of martensitic transformation in the alloys containing about 30% Ni are presented in Table 1 in accordance with the data of different studies and of our work.
The point M s determined in the present work was relatively insensitive to the rates of heating and cooling and to the heat treatments. Introduction of 3% Pd into the Fe – 30% Ni binary alloy changed the value of M s inconsiderably. However, the point M s in the earlier published works changed considerably upon the introduction of a third component; for the alloy Fe – 29.4% Ni – 2% Cr [12] M s = –138°C, for the alloy Fe – 30% Ni – 0.8% Mo [21] M s = –83°C, and for the alloy Fe – 28% Ni – 7.5% Si [22] M s = –180°C. A s compared to the mentioned alloying elements Pd causes growth of M s. By the data of [23], the alloy Fe – 30%Ni – 1% Pd has M s = –33°C.
Table 2 presents the values of M s after thermocycling of alloy Fe – 30% Ni – 3% Pd at from 20 to 500°C. The temperatures A s and A f remain virtually unchanged, while M s lowers with growth in the number of cycles. According to [9], the mean size of the austenite grains decreases with increase in the number of cycles, whereas growth in the fraction of retained austenite in the Fe – 31% Ni alloy is caused by stabilization of the austenite due to growth in the dislocation density. Our data agree with the results of [9], where the content of martensite formed from the reverted austenite in the Fe – Ni alloy decreased with growth in the number of thermal cycles.
Figure 3 presents the diffraction patterns of specimens before and after immersion into liquid nitrogen. We observe reflections of retained fcc austenite (γ) and bcc martensite (α′). The mean values of the lattice constants of these phases are equal to 0.35704 and 0.28614 nm respectively.
Conclusions
-
1.
The kinetics of martensitic transformation in alloy Fe – 30% Ni – 3% Pd is an athermal (burst) one; the temperature M s (–23°C) differs little from the M s of the binary Fe – 30% Ni alloy.
-
2.
Thermocycling of alloy Fe – 30% Ni – 3% Pd virtually does not change the temperatures A s and A f , while the temperature M s decreases with growth in the number cycles and attains –60°C after three cycles of heating to 500°C.
-
3.
The morphology of the martensite crystals is lamellar; the constant of the bcc lattice of the martensite is 0.28614 nm; the constant of the fcc lattice of the austenite is 0.35704 nm.
References
Z. Nishiyama, Martensitic Transformation, Academic Press, New York (1978).
T. Kakeshita and T. Fukuda, “Effects of magnetic field on martensitic transformations,” J. Phys. Conf. Ser., 165, 012051 (2009).
T. Kakeshita, K. Shimizu, S. Funada, and M. Dale, Acta Metall., 33(8), 1381 (1985).
Y. Inokuti and B. Cantor, Acta Metall., 30(2), 1381 (1982).
K. Sridharan, F. J. Worzala, and R. A. Dodd, Mater. Chem. Phys., 30, 115 (1991).
C. M. Wayman and K. Wakasa, Metallography, 14, 37 (1981).
T. N. Durlu and J. W. Christian, Acta Metall., 27, 663 (1979).
G. Krauss and A. R. Marder, Metall. Mater. Trans. B, 2, 2343 (1971).
S. Seo, D. S. Leem, J. H. Jun, and C. S. Chai, ISIJ Int., 41, 350 (2001).
M. Umemoto, E. Yoshitake, and I. Tamura, J. Mater. Sci., 18, 2893 (1983).
T. Kirindi and M. Dikici, J. Alloys Compd., 407, 157 (2006).
P. Visvesvaran, Metall. Mater. Trans. A, 27, 973 (1996).
S. Kajiwara, Philos. Mag. A, 43, 1483 (1981).
R. F. Bunshah and R. F. Mehl, J. Metals, 5, 1251 (1953).
A. R. Troiano and A. B. Greninger, Metal Progr., 50, 303 (1943).
J. W. Christian, ICOMAT, Cambridge, Massachusetts, USA (1979).
I. Ya. Georgieva and I. I. Nikitina, Metal Sci. Heat Treat., 14(5), 452 (1972).
L. Kaufman and M. Cohen, Trans. AIME J. Metals, 206, 1393 (1956).
H. Kitihara, R. Ueji, M. Ueda, et al., Mater. Charact., 54, 378 (2005).
A. Shibata, S. Morito, T. Furuhara, and T. Maki, Acta Mater., 57, 483 (2009).
E. Yasar, H. Gungunes, A. Kilic, and T. N. Durlu, J. Alloys Compd., 424, 51 (2006).
Y. Himuro, R. Kainuma, and K. Ishida, ISIJ Int., 42, 184 (2002).
G. Yildiz, Y. G. Yildiz, and S. Nezir, Bull. Mater. Sci., 36(1), 93 (2013).
The work has been supported financially by the Research Fund of the Kirikkale University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 7, pp. 11 – 13, July, 2017.
Rights and permissions
About this article
Cite this article
Yildiz, Y.G., Yildiz, G.D. Effect of Thermocycling on the Structure of Martensite and Kinetics of Martensitic Transformation in Alloy Fe – 30% Ni – 3% Pd. Met Sci Heat Treat 59, 407–409 (2017). https://doi.org/10.1007/s11041-017-0163-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-017-0163-4