The possibility of deposition of multilayer coatings with regions of titanium nitride on the surface of steel 12Kh18N10T by physical precipitation from a gas phase followed by diffusion chrome aluminizing is shown. The phase and chemical compositions, the thickness, and the microhardness of the coatings are determined. The TiN nitride is shown to exhibit barrier properties and to lower the diffusion penetration of chromium and aluminum into the matrix and of iron and titanium into the coating under subsequent chrome aluminizing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Austenitic steel 12Kh18N10T with moderate strength, high ductility and good corrosion resistance has found wide application in various branches of mechanical engineering [1, 2]. By raising the wear resistance and refractoriness of its surface layers we will obtain a new material with high properties mentioned and strength, ductility and corrosion resistance typical for steel 12Kh18N10T. We should say that it is virtually impossible to obtain a monolithic material with such properties by the known methods.
Today, the most widely used coatings consist of carbides, nitrides, and intermetallics. Titanium-, chromium- and aluminum-base coatings deserve special attention [3–6].
Coatings deposited by various methods possess specific leading properties, i.e., wear resistance, corrosion resistance or high-temperature strength. The high-temperature strength may be advanced by creating oxidation-resistant layers on the external surface of the coating [3, 7, 8]. Examples of such protective layers are silicon oxides on molybdenum disilicide or alumina-base layers on refractory alloys [7, 8].
Oxidation at high temperatures is accompanied by interaction between the coating and the substrate due to diffusion of substrate atoms into the coating and of elements of the coating into the substrate. The lower the rate of the diffusion processes the steadier the coating – substrate composition [6].
It should be noted that in many cases the formed barrier layer possesses properties matching the main functions of a coating and promotes growth in the wear resistance, corrosion resistance and refractoriness of the articles [9–11]. It has been shown in [10] that coatings based on carbides of transition metals of groups IV-VI of the periodic system may be deposited on structural and tool steels and hard alloys. The articles with carbide coatings have exhibited stability under the action of aggressive environments, sliding friction without lubrication, cavitation and cutting of metals [10]. The high operating properties of carbide and nitride coatings are a result of the combination of the characteristics of these compounds, in particular, of the considerable hardness, corrosion and heat resistances, and the presence of barriers in their structure, which are formed directly by carbide or nitride layers. Titanizing of carbon steels U8A, U10A, alloy steels 9KhS, KhVG, ShKh15, and hard alloys VK8 and T15K6 produces barrier layers based on TiC titanium carbide; vanadizing produces coatings based on VC and V2C vanadium carbides, chromizing produces coatings based on Cr7C3 and Cr23C6 chromium carbides [10]. Multilayer coatings of TiC, TiN, Al2O3 formed on steels and hard alloys by the method of chemical deposition from a vapor phase exhibit high wear resistance. The barrier functions are played by the composition of TiC and TiN, and the wear resistance is provided by the Al2O3 layer [9, 11]. The coatings on hard alloys with such arrangement of layers from the substrate to the surface possess high heat resistance, chemical stability, and resistance to formation craters on the cutting surface of tools [9, 11].
Diffusion titanoaluminizing of nitrided carbon steels and hard alloys yields on their surface multilayer coatings with a TiC, TiN barrier composition and external layer based on Al2O3 [12–14]. Thus, we may state that the barrier layers of TiN, TiC and the alumina layer lower the interaction between the coating and the substrate and between the coating and the ambient and play an important role in elevation of the protective properties of coatings.
We have mentioned already that the high-temperature strength of metals can be raised by alloying their surface with aluminum, silicon and chromium [2, 3]. It has been shown in [3, 15] that the properties of the coatings obtained by two-component saturation are superior to those of the one-component coatings. The idea of deposition of a twocomponent refractory coating containing chromium in addition to aluminum is quite obvious. Diffusion saturation with chromium and aluminum is conducted with the help of different processes of which the powder methods are the most popular [3, 15]. Complex saturation with chromium and aluminum raises the diffusion mobility of chromium, especially in austenitic steel 12Kh18N10T. Aluminum dissolves in the austenite during thermochemical treatment, which promotes a γ → α transformation and thus creates favorable conditions for the diffusion of chromium into the substrate.
Recent reports on deposition of coatings on steel 12Kh18N10T deal primarily with the structure and properties of layers obtained by known techniques [3, 6, 15]. Data on formation of barrier structures in the coatings are scarce [13, 14]. We may expect that the further search of new wearand corrosion-resistant refractory materials with advanced properties should be directed at creation of multicomponent coatings combining high refractoriness and hardness of some layers with barrier properties of other layers.
The aim of the present work was to study the phase and chemical compositions, structure and properties of chromoaluminized steel 12Kh18N10T with a preliminarily deposited layer of titanium nitride.
Methods of Study
We studied specimens of steel 12Kh18N10T of the following chemical composition (in wt.%): 0.12 C, 18.0 Cr, 10.0 Ni, 0.6 Ti.
Chromoaluminizing of steel 12Kh18N10T was performed by the powder method in containers with fusible closure under reduced pressure. The initial mixture contained powdered chromium (46 wt.%), aluminum (10 wt.%), ammonium chloride (4 wt.%) and alumina (40 wt.%). The process of chromoaluminizing was conducted at 1050°C for 2 h. A part of the specimens of steel 12Kh18N10T was coated with TiN titanium nitride prior to the chromoaluminizing physical deposition from a vapor phase in a VU1B unit. The thickness of the layer of titanium nitride on the steel was 5.0 – 6.0 μm. The coated specimens were studied by x-ray diffraction, microscopic x-ray spectrum and metallographic analyses and measurement of the hardness.
Results and Discussion
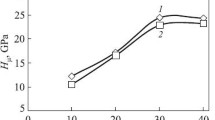
The phase and chemical compositions, the structure and some properties of the studied chromoaluminized coatings and of the coatings chromoaluminized with a layer of TiN nitride are presented in Table 1 and in Figs. 1 and 2. By the results of the x-ray diffraction analysis the lattice constant of the titanium nitride deposited onto the surface of steel 12Kh18N10T is equal to 0.4249 nm. It is somewhat less than the lattice constant of monolithic TiN with stoichiometric composition [16, 17]. It is known [16], that the lattice constants of interstitial phases in coatings are commonly greater than in massive specimens. As a rule, this is associated with the presence of residual stresses and grains of specific sizes in the coating and impurities in the nitride [16]. Closeness of the TiN phase to the stoichiometric composition is confirmed by the results of the metallographic analysis. The layer of the TiN nitride deposited on the surface of steel 12Kh18N19T is colored golden yellow on the surface and in the transverse lap under a light microscope, which matches the color of stoichiometric TiN.
The lattice constant of the TiN phase after chromoaluminizing degreases insignificantly as compared to the initial value. This is explainable by the low additives of iron and chromium, the atomic sizes of which are less than the atomic size of titanium. The color of the TiN layer in the transverse laps under the light microscope remains golden yellow. It should be noted that the chromoaluminizing has virtually not affected the thickness and the microhardness of the TiN layer (see Table 1).
A layer-after-layer x-ray diffraction analysis of the chromoaluminized steel and of the steel chromoaluminized with a layer of TiN has shown that both kinds of coating contain an Al(Fe, Cr) intermetallic on the external side and a neighboring layer of AlFeNi. In accordance with the results of the x-ray diffraction analysis and with the Al – Fe – Cr and Al – Fe – Ni phase diagrams, these compounds have an ordered structure of type CsCl [18–23]. The diffraction patterns of the layers of the AlFeCr and AlFeNi compounds have a superstructural maximum (100), which indicates ordering of the structure (see Table 1).
The layer directly adjoining the substrate is represented by an aluminum solid solution in α-iron. This solution bears nickel and chromium from the substrate. The crystals of Feα(Al, Cr, Ni) in the diffusion zone have a characteristic columnar shape. The crystals of Feα(Al, Cr, Ni) appear in the austenite as a result of a γ → α transformation after the attainment of limiting solubility of aluminum in the austenite. The motion of the Feα → Feγ boundaries from the surface into the depth of the specimen is accompanied by growth of the columnar crystals of the Feα-phase in the same direction. The thickness of the Feα(Al, Cr, Ni) layer in the chromoaluminized coatings on the steel with a layer of TiN is much lower than in the chromoaluminized coatings (see Table 1). The concentration of aluminum in the layer of Feα(Al, Cr, Ni) near the external boundary in the coatings with TiN is almost twice lower than in the chromoaluminized ones and is virtually the same at the Feα → Feγ boundary (Fig. 1).
Analysis of the results of the microscopic x-ray spectrum analysis allowed us to determine the effect of the TiN layer on steel 12Kh18N10T and on the distribution of elements in the coating after diffusion chromoaluminizing (Fig. 2).
The distribution of the saturating elements and of the elements of the substrate in coatings of the two types has much in common. At the same time, the influence of the TiN layer manifests itself in growth in the concentration of aluminum and chromium on the external side of the chromoaluminized coating, decease in the concentration of iron, and virtual absence of titanium.
The metallographic analysis gave us the structures of the two types of coating on steel 12Kh18N10T (diffusion chromoaluminized and chromoaluminized with a layer of titanium nitride). The phase composition of the external zone of compounds is the same in both types of coating. This zone contains layers of AlFeCr and AlFeNi compounds of a light gray color with a well manifested interface. The layer of Feα(Al, Cr, Ni) on the interface with the substrate has poorly developed internal and external boundaries (the external interface with the TiN layer and the internal interface with the substrate).
The mechanism of formation of chromoaluminized coatings on the steel with a layer of TiN presents some interest with respect to the mechanism of formation of chromoaluminized coatings. In the process of chromoaluminizing of the steel with a layer of titanium nitride only one of the saturating elements (aluminum) diffuses through the layer of TiN. At the same time, the iron and nickel elements from the substrate do penetrate to the surface through the layer of TiN. The TiN layer blocks virtually fully the motion of only one element of the substrate, i.e., titanium, to the surface. The external zone of the coating forms through extraction of the elements of the substrate through the TiN layer by the aluminum and chromium atoms adsorbed by the surface and subsequent formation of a layer of Al(Fe, Ni) and Al(Fe, Cr) compounds.
Today we know of two mechanisms of formation of new phases under reactive diffusion in the process of thermochemical treatment. According to one of them, the chemical reactions yielding compounds occur directly on the interface of the metal and the saturating environment. Such a reaction may be treated as a primary process of reactive diffusion (V. Z. Bugakov). A new phase forms as a result of the action of the forces of chemical interaction of the reacting elements. This may produce a thin (monatomic) layer of a compound. Then the thickness of the layer grows due to the diffusion supply of iron and nickel from the substrate through the TiN layer and through the layer of the new phase and of chromium and aluminum from the saturating environment [21].
The other mechanism of reactive diffusion involves a stage of necessary attainment of limiting solubility upon the arrival of the diffusing elements from the external environment. This creates conditions for formation of a phase which is in equilibrium with the solid solution in accordance with the phase diagram (D. A. Prokoshkin). However, the essence of the process is determined not by the chemical reactions on the surface but by the oversaturation of the initial solution (chromium- and nickel-alloyed austenite) [21].
Thus, we may assume that the coating forms under chromoaluminizing of steel 12Kh18N10T with a TiN layer by the mechanism of V. Z. Bugakov, while in conventional chromoaluminizing it forms by the mechanism of D. A. Prokoshkin [21].
The multilayer coatings deposited in the present work on steel 12Kh18N10T with participation of TiN titanium nitride deposited by physical precipitation from vapor phase prior to the diffusion chromoaluminizing possess phase and chemical compositions, structure and microhardness, which make them appropriate for operation in aggressive environments under the action of high temperatures and friction.
Conclusions
Diffusion chromoaluminizing of steel 12Kh18N10T with a layer of TiN nitride deposited onto its surface produces coatings where the TiN layer performs barrier functions. The TiN layer hinders the diffusion penetration of chromium and aluminum into the substrate and of iron and titanium from the substrate into the layers of compounds of ordered AlFeCr and AlFeNi phases.
References
M. N. Gol’dshtein, S. V. Grachev, and G. M. Veksler, Special Steels [in Russian], Metallurgiya, Moscow (1985), 408 p.
F. F. Khimushin, Stainless Steels [in Russian], Metallurgiya, Moscow (1976), 798 p.
P. T. Kolomytsev, Refractory Diffusion Coatings [in Russian], Metallurgiya, Moscow (1979), 272 p.
M. Zheng and R. A. Rapp, “Simultaneous aluminizing and chromizing of steels to form (Fe, Cr)3Al coatings,” Oxid. Metals, 49(112), 19 – 31 (1998).
L. Hong, et al., “Corrosion resistance of stainless steel TR347N with aluminized layer,” Metalloved. Term. Obrab. Met., No. 1, 44 – 47 (2010).
P. T. Kolomytsev and V. M. Samoilenko, “Combined coating for turbine blades of high-temperature gas turbine engines,” Metalloved. Term. Obrab. Met., No. 12, 28 – 31 (2006).
L. V. Ramanathon, M. F. Pillis, and S. M. C. Fernandes, “Role of rare earth oxide coatings on oxidation resistance of chromiaforming alloys,” J. Mater. Sci., 43, 530 – 585 (2008).
M. T. Besmann, “Interface science of thermal barrier coatings,” J. Mater. Sci., 44, 1616 – 1663 (2009).
A. S. Vereshchaka and I. P. Tert’yakov, Cutting Tools with Wear Resistant Coatings [in Russian], Mashinostroenie, Moscow (1986), 192 p.
V. F. Loskutov, V. G. Khizhnyak, Yu. A. Kunitskii, and M. V. Kindrachuk, Diffusion Carbide Coatings [in Russian], Tekhnika, Kiev (1991), 168 p.
D. G. Bhat and P. F. Woener “Coatings for cutting tools,” J. Metals, 38, 68 – 69 (1986).
Khizhnyak, N. L. Kurilo, I. V. Levits’ka, and O. T. Serditov, “Nitrotitanizing of steels and hard alloys,” Naukovi Visti NTUU “KPI,” No. 6, 83 – 88 (2008).
G. V. Borisenok, L. A. Vasil’ev, L. G. Voloshin, et al., Thermochemical Treatment of Metals and Alloys [in Russian], Metallurgiya, Moscow (1981), 424 p.
R. A. Andrievskii, “Synthesis and properties of films of interstitial alloys,” Usp. Khim., No. 1, 57 – 77 (1997).
G. V. Samsonov and I. M. Vinnitskii, Refractory Compounds [in Russia], Metallurgiya, Moscow (1997), 560 p.
I. A. Al-Omari, “Structural and Moessbauer spectrographic studies of Fe0.7 – x Cr x Al0.3 alloys,” J. Magn. Mater., 225, 346 – 350 (2001).
P. Aufler, I. Faber, and G. Zahn, “X-ray single crystal diffraction investigation on Ni1 + x Al1 – x ,” Acta Crystallogr., A52, 319, 234 – 329 (1996).
M. V. Arshuk, A. V. Mikitchik, V. G. Khizhnyak, and M. V. Karpets, “Titanium-aluminide coatings on steel 12Kh18N10T with barrier layer of titanium nitride,” Sovr. Elektrometallurg., No. 2(103), 50 – 55 (2011).
G. V. Samsonov and G. L. Zhunkovskii, “Some regular features of the initial stage of reaction diffusion,” in: Protective Coatings on Metals [in Russian], Naukova Dumka, Kiev (1973), Issue 7, pp. 21 – 33.
20. J. H. De Boer, Catalysis. Some Topics of the Theory and Technology of Organic Reactions [Russian translation], Inostr. Lit., Moscow (1969), 391 p.
Yu. M. Lakhtin and B. N. Arzamasov, Thermochemical Treatment of Metals [in Russian], Metallurgiya, Moscow (1985), 256 p.
V. G. Khizhnyak and N. A. Kurilo, “Structure and mechanical properties of titanium, vanadium and chromium coatings on steel U8A,” Metalloznav. Obrob. Met., No. 3, 17 – 21 (2007).
V. Schreter, et al., Chemistry [Russian translation], Khimiya, Moscow (1989), 648 p.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 4, pp. 45 – 49, April, 2016.
Rights and permissions
About this article
Cite this article
Khizhnyak, V.G., Arshuk, M.V. & Loskutova, T.V. Chrome Aluminized Layers with Participation of Titanium Nitride on Steel 12Kh18N10T. Met Sci Heat Treat 58, 231–235 (2016). https://doi.org/10.1007/s11041-016-9995-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-016-9995-6