Abstract
Background
Gram-negative bacteria with quinolone resistance and extended-spectrum beta-lactamases (ESBLs) present significant treatment challenges. This study evaluated the prevalence and characteristics of quinolone resistance in Gram-negative strains, investigating the relationship between plasmid-mediated quinolone resistance (PMQR), ESBLs, and integrons.
Methods and results
We collected 146 Gram-negative isolates from patients in three Palestinian hospitals. For quinolone resistance isolates, the presence and characterization of PMQR, β-lactamase genes and integrons were studied by PCR and sequencing. Out of 146 clinical isolates, 64 (43.8%) were resistant to quinolones, with 62 (97%) being multidrug-resistant (MDR) and 33 (51.5%) ESBL-producers. PMQR-encoding genes were present in 45 (70.3%) isolates, including aac(6′)-Ib-cr (26.6%), qnrA (18.8%), qnrS1 (20.8%), and qnrB (6.4%). BlaCTX−M genes were detected in 50% (32/64) of isolates, with blaCTX−M−15 being the most common. BlaTEM−1, blaSHV−1 and blaVIM genes were found in 13, 6, and 4 isolates, respectively. Class I integrons were found in 31/64 (48%) of isolates, with 14 containing gene cassettes conferring resistance to trimethoprim (dhfr17, dfrA12, dfrA1) and aminoglycosides resistance genes (aadA1, aadA2, aadA5, and aadA6).
Conclusions
This study found a high rate of quinolone resistance, ESBL and integrons in clinical Gram-negative isolates from our hospitals. Urgent measures are crucial, including implementing an antimicrobial resistance surveillance system, to control and continuously monitor the development of antimicrobial resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gram-negative bacteria pose a pervasive threat as the leading causes of a spectrum of severe infections, including pneumonia, bloodstream infections, wound infections, and meningitis. These infections are primarily caused by Klebsiella, Pseudomonas aeruginosa, and E. coli [1]. Gram-negative bacteria are a major public health concern worldwide due to their high resistance to most available antibiotics, leading them to become multidrug-resistant (MDR). The prevalence of infections caused by MDR Gram-negative bacteria has witnessed a concerning increase [2]. MDR Gram-negative strains hold pivotal clinical importance, instigating a spectrum of high-risk infections that contribute to elevated morbidity and mortality rates. The global impact of these bacteria is exacerbated by the limited treatment options available for such infections [3].
Quinolone and fluoroquinolone are synthetic antimicrobials that disrupt bacterial DNA synthesis by inhibiting bacterial topoisomerase type II and topoisomerase IV. They are broad-spectrum agents used to treat infections caused by both Gram-positive and Gram-negative bacteria [4]. However, the prolonged and improper use of quinolone drugs has led to a significant increase in quinolone-resistant isolates [2]. Several mechanisms of quinolone resistance have been reported. These include mutations in chromosomal genes encoding the target enzymes DNA gyrase and topoisomerase IV, as well as a decrease in the intracellular concentration of fluoroquinolones due to efflux pump activity mediated by genes such as qepA, qepA2, and oqxAB [5]. Additionally, plasmid-mediated quinolone resistance (PMQR) determinants play a role in resistance mechanisms. These include qnr genes and aac(6’)-Ib-cr, which protect the targets of quinolones through qnr proteins and hydrolyze quinolones through the aac(6′)-Ib-cr protein [4]. There are five main family groups of qnr genes discovered on plasmids or chromosomes in bacteria, namely qnrA, qnrB, qnrC, qnrD and qnrS [2]. Plasmids carrying quinolone resistance genes can spread horizontally, leading to the accumulation of chromosomal mutations that increase the resistance rate and contribute to therapeutic failure [5].
The global incidence of extended-spectrum-beta-lactamase (ESBL) producing Gram-negative bacteria, coupled with quinolone resistance, is on the rise. Infections caused by these strains have been associated with reduced treatment efficacy, increased mortality and morbidity rates, prolonged hospitalization and higher healthcare costs [5].
Several studies have confirmed a significant association between ESBL-producing isolates and the presence of plasmid-mediated quinolone resistance (PMQR) determinants. These studies have reported the presence of qnr genes and aac (6’)-Ib-cr in ESBL-producing isolates [4,5,6,7,8].
Integrons are genetic elements responsible for carrying antibiotic resistance genes and facilitating their dissemination among different bacterial species. These elements are transferred via mobile genetic elements, such as transposons and plasmids [9]. Integrons consist of an integrase gene (intI), an integration site (attI) and an attachment site (attC) for gene cassettes. The attI serves as the target site for cassette integration and contains a promoter [10]. Antimicrobial resistance genes are carried within integrated cassettes in integrons, and these genes can spread through the transfer of plasmids or transposons that contain the integrons. Integrons are classified into different classes based on the intI genes, with class I and II being the most predominant in resistant clinical isolates [9].
Quinolone resistance has been reported to be increasing in Palestine [11, 12], though few studies have investigated the molecular mechanisms of quinolone resistance, and the association between PMQR with ESBL and integrons [13, 14]. This study aims to evaluate the prevalence and characteristics of quinolone resistance in Gram-negative strains, as well as explore the association between PMQR with ESBL and integrons.
Materials and methods
Bacterial collection and identification
Over a 3 month period from March to June, 2013, a total of 146 non-repetitive Gram-negative bacterial isolates were collected from two Palestinian hospitals (Military Balsam Hospital and Al- Shifa Hospital) and the AL-Remal Martyrs’ Health Center in Gaza strip, Palestine. These isolates were isolated from various sources such as urine, wound infections, enteric infections, blood, ear infections, and sputum. The collected samples were transported to the Laboratory of Microorganisms and Active Biomolecules at the Faculty of Sciences of Tunis, University of Tunis El Manar (Tunisia) following standard procedures for bacterial isolation and identification. All isolates were plated on Brain Heart Infusion Agar and MacConkey Agar. Bacterial identification of the Gram-negative isolates was carried out using conventional biochemical tests and the API 20E system (BioMérieux, France). The interpretation of the results interpretation was performed using the API web database. To confirm the identity of the Gram-negative isolates, PCR amplification and sequencing of the 16 S rRNA gene were performed. After bacterial identification, the isolates were stored in skim milk broth at -20 °C and − 80 °C for further analysis.
Antimicrobial susceptibility test
The susceptibility of the Gram-negative isolates to quinolones (nalidixic acid, NAL; 30 µg) and fluoroquinolones (ciprofloxacin, CIP; 5 µg)) was determined using the Kirby-Bauer disk diffusion test. This test was performed following the guidelines of the Clinical and Laboratory Standards Institute (CLSI). In addition to quinolones, the sensitivity of the isolates to other antibiotic agents was assessed. This included β-lactam antibiotics such as ampicillin (AMP; 10 µg), cefoxitin (FOX; 30 µg), ceftazidime (CAZ; 30 µg), cefotaxime (CTX; 30 µg), and amoxicillin–clavulanic acid (AMC; 20/10 µg) (20/10). The sensitivity to imipenem (IMP; 10 µg), a carbapenem antibiotic, was also tested. Aminoglycosides including gentamicin (GM; 10 µg), tobramycin (TOB; 10 µg), and kanamycin (GM; 30 µg), were included in the susceptibility testing. Other antibiotics assessed were trimethoprim–sulfamethoxazole (SXT; 1.25/23.75 µg) and tetracycline (TET; 30 µg). Isolates that exhibited low sensitivity to third-generation cephalosporins were further screened for ESBL production using the double-disk synergy method. This method involved testing the isolates with ceftazidime and cefotaxime disks in the presence of a disk containing amoxicillin/ clavulanic acid [15].
DNA extraction and polymerase chain reaction (PCR)
Genomic extraction was performed using the boiling method. Bacterial colonies were suspended in 500 µl of sterile distilled water and heated for 10 min at 100 °C. After centrifugation for 10 min at 12,000 rpm, the supernatant containing the genomic DNA was collected and stored at -20 °C for later use in PCR [16]. The concentration and purity of the extracted genomic DNA were evaluated using a NanoDrop™ spectophotometer and UV light at 260/280 nm. The acceptable ratio value for all samples was within the range of 1.7–1.9.
For PCR amplification, a final reaction volume of 25uL was used in a DNA thermal cycle (Applied Biosystems Thermal Cycler). The PCR conditions consisted of three steps: denaturation at 94 °C for 5 min, followed by 30–40 cycles of denaturation at 94 °C for 30s, annealing step at a specific temperature listed in Supplementary file S1 (delete table 1 here and add Supplementary file S1) for 30s and extension at 72 °C for 1 min. A final extension step at 72 °C for 5 min was performed. To visualize the amplified PCR fragments, agarose gel electrophoresis was performed. A 1.5% agarose gel in 1× TBE (Tris-borate-EDTA) buffer was used, and the gel was run for 45–60 min at room temperature at 100 volts. The individual bands representing the amplified gene fragments were visualized under UV light after staining with ethidium bromide, which acts as a fluorescent DNA stain. Positive and negative controls were included in all PCR amplifications conducted in the laboratory.
Quinolone resistance elements
To investigate the presence of plasmid-mediated quinolone resistance (PMQR) genes, namely qnrA, qnrB, qnrS, qepA and aac(6’)-Ib, PCR amplification and sequencing were performed. The quinolone resistance isolates were screened for these genes using the methodology described in a previous study by [17] (Supplementary file S1).
Identification of β-lactamase genes and other resistance genes
PCR assays were performed on β-lactam resistance isolates for the detection of blaCTX−M,blaTEM,blaOXA−1,blaSHV, blaCMY−2, blaIMP, blaVIM, blaSPM,blaSIM and blaGIM (Supplementary file 1) [18].
Positive PCR reactions were sequenced to confirm the specific variant of β-lactamase genes. The sequences obtained were compared to the blast database of GenBank (www.ncbi.nlm.nih.gov) for identification and characterization purposes. In addition to β-lactamase genes, the presence of antibiotic resistance genes for sulfamethoxazole [sul1, sul3 and sul2], tetracycline [tet(A), tet(B), and tet(C)] and aminoglycosides [aac(3)-IV, aac(3)-I, and aac(3)-II] was detected using PCR [18].
Identification and characterization of integrons
In order to identify and characterize integrons, several PCR assays were performed. The presence of intI1 and intI2 genes, which are associated with integrons, was investigated in quinolone resistance isolates. PCR was used to screen for the presence of qacEΔ1 + sul1 genes in 3’-conserved regions of class 1 integrons. To detect the gene contents within the variable region of integrons, PCR amplification was performed, and the resulting amplicons were sequenced. Detailed information regarding the primer sequences and PCR conditions used for these experiments are listed in Supplementary file S1 [19].
Results
Bacterial isolates
A total of 146 samples were collected. Of these, 76 (52.1%) were obtained from patients admitted to Al-Shifa Hospital, 47 (32.2%) from Balsam Hospital and 23 (15.7%) from Al-Remal Clinic. The frequency of microbial agents in the infections in the present study was as follows: E. coli accounted for 69 (47.3%) isolates, Pseudomonas spp. for 35 (24%) isolates, Klebsiella pneumoniae for 27 (18.5%) isolates, Enterobacter cloacae for 4 (2.8%) isolates, Proteus mirabilis for 3 (2.1%) isolates, Salmonella spp. for 3 (2.1%) isolates, Serratia liquefacients for 2 (1.4%) isolates, Morganella morganii for 1 (0.7%) isolate, providencia rettegeri for 1 (0.7%) isolate, and Pasteurella pneumotropica for 1 (0.7%) isolate. The frequency of microorganisms in terms of the infection site is shown in Table 1. The most common specimen sources were urine, accounting for 86 (58.9%) samples, and wound infections, accounting for 44 (30.1%) samples. Among the urine samples, E. coli was the most prevalent strain, accounting for 60 (70%) isolates, followed by K. pneumoniae with 13(15.1%) isolates, and Pseudomonas spp. with 8 (9.3%) isolates. In the case of wound infections, Pseudomonas spp. was the most frequently isolated pathogen, accounting for 19 (43.2%) isolates, followed by K. pneumoniae with 19 (22.7%) isolates.
Resistance to quinolones
Among the 146 clinical isolates analyzed in the study, 64 (43.8%) showed resistance to quinolones and/or fluoroquinolones, specifically nalidixic acid and/or ciprofloxacin. The prevalence of quinolones resistance varied among different bacterial species. In E. coli, the resistance rate was 40.6% (28 out of 69 isolates). Pseudomonas spp. exhibited a higher resistance rate of 68.6% (24 out of 35 isolates), while K. pneumoniae had a resistance rate of 33.3% (9 out of 27 isolates). Among the smaller sample sizes, E. cloacae showed a resistance rate of 25% (1 out of 4 isolates), P. pneumotropica had a 100% resistance rate (1 out of 1 isolate), and S. liquefaciens displayed a resistance rate of 50% (1 out of 2 isolates) (Table 2).
Antibiotic susceptibility pattern
Sixty-two (97%) of the quinolones resistance isolates were identified as multidrug-resistant (MDR) which exhibited resistant to three or more classes of antibiotics. The majority of the quinolone-resistant isolates exhibited resistance to ampicillin, cefotaxime, trimethoprim-sulfamethoxazole, and Kanamycin. Half of the isolates showed resistance to sulfamethoxazole/trimethoprim, ceftazidime, gentamycin, and cefoxitin. The susceptibility of the isolates to imipenem was 70% (Table 3).
Detection of ESBLs
Among the 64 quinolone-resistant isolates, 51.5% (33 out of 64 isolates) were confirmed phenotypically as ESBL-producers. These 33 ESBL-producers are composed of 22 strains of E. coli with a rate of 35.5% (22 out of 60), 9 strains of K. pneumoniae (9/27; 33.3%), one strain E. cloacae (1/4; 25%), and one strains of S. liquefaciens (1/2; 50%).
Quinolone resistance elements
The presence of plasmid-mediated quinolone resistance (PMQR) genes, including qnrA, qnrB, qnrS, qepA and aac(6’)-Ib, was investigated in the quinolone-resistant isolates using PCR amplification and sequencing, following the methodology described by Rocha-Gracia et al. (2010). Among the quinolone-resistant isolates, 39% (25 out of 64) were found to harbor PMQR genes. The most prevalent gene was qnrA, detected in 18.8% (12 out of 64) of the isolates. qnrS1 was identified in 14.1% (9 out of 64) of the isolates, while qnrB was detected in two variants; qnrB1 and qnrB4, found in 6.4% (6 out of 64) and 1.6% (1 out of 64) of the isolates, respectively. Three quinolone-resistant isolates harbored two qnr encoding gene; qnrA with qnrS1 in an E. coli strain, qnrS1 with qnrB1 in Pseudomonas, and qnrB1 with qnrA in K. pneumoniae. However, qepA was not detected in any of the isolates. The distribution of quinolone resistance genes among the studied isolates, categorized by specimens and species, of the isolates is presented in Table 4. The qnrA gene was found in nine ESBL-producing isolates, while qnrS1 and qnrB1 were detected in five and three ESBL-producing isolates, respectively. Among the quinolone resistant Gram-Negative isolates, the gene aac(6′)-Ib-cr was present in 26.6% (17 out of 64). A combination of qnr genes with aac(6′)-Ib-cr was identified in 15.6% (10 out of 64) of the isolates. One ESBL-producing K. pneumoniae isolates harbored aac(6′)-Ib-cr, qnrB1, and qnrA, while one Pseudomonas isolate carried qnrS1, aac-6′-ib-cr, and qnrB1. Among the tested PMQR genes, the majority of qnr genes were found in Enterobacteriaceae isolates except for one non-fermenter isolate that carried qnrS and qnrA genes. The aac(6′)-Ib-cr gene was predominantly detected in Pseudomonas isolates (Table 4).
Identification of β-lactamase genes
In the quinolone-resistant isolates, various β-lactamase genes were identified. The blaCTX−M genes were detected in 50% (32/64) of quinolone resistant isolates. The blaCTX−M−15 was the most frequently gene detected in 26 isolates, followed by blaCTX−M−14 and blaCTX−M−1 were found in three and two isolates, respectively. While blaCTX−M−55 and blaCTX−M−27 were found in one isolate. The blaTEM−1 and blaSHV−1 genes were identified in 13 and 6 isolates, respectively. Class B beta-lactamase genes blaVIM that were detected in carbapenem-resistant isolates, three variants of blaVIM were identified (blaVIM−28 in 2 K. pneumoniae, blaVIM−1 and blaVIM−2 in Pseudomonas spp.). Three β-lactamase genes (blaCTX−M−15, blaTEM−1, blaSHV−1) and (blaCTX−M−15, blaSHV−1, blaVIM−28) were present in association with PMQR genes in 6 K. pneumoniae isolates (Table 4).
Resistance mechanisms to Non- β-lactam antimicrobial agents
Resistance mechanisms to non-β-lactam antimicrobial agents were investigated in the quinolone-resistant Gram-negative isolates. Table 4 shows the antibiotic resistance genotypes of the quinolone-resistant Gram-Negative isolates. Thirty-two of the quinolone resistant isolates harbored sul genes (sul1: 30 isolates; sul2: one isolate; sul1 + sul2: four isolates; and sul1 + sul3: one isolate). The aac(3)-II gene was found in thirty aminoglycoside-resistant isolates and the aac(3)-IV gene in twelve isolates. Twenty-nine of the isolates harbored tet genes [tetA: nineteen isolates; tetB: four isolates; and tetA + tetB: six isolates].
Characterization of integrons
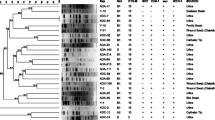
Class 1 integron has been identified in 31 of quinolone resistant isolates. They were in 13 E. coli isolates; 11 Pseudomonas spp., 5 K. pneumoniae, 1 E. cloacae and 1 S. liquefaciens. These integrons were detected in 17 ESBL producer isolates and in 14 non-ESBL producer isolates. The QacEΔ1 and sul1 genes were documented in integrons in 24 isolates while seven of those integrons lacked the qacEΔ1 and sul1 genes. The gene cassette in the variable region of the integron was demonstrated that encoded for resistance to trimethoprim (dhfr) and /or spectinomycin (aadA) with the following gene cassette arrangements: dhfr17 + aadA5 (6 isolates), dfrA12 + aadA2 (2 isolates), dfrA1 + aadA1 (1 isolate), aadA6 (4 isolates), and aadA1 (1 isolate) (Table 4). The genetic cassette content differs between bacterial species, in E. coli only dhfr17 + aadA5, in Pseudomonas spp. only aadA6, and in K. pneumoniae dfrA12 + aadA2, dfrA1 + aadA1, and aadA1.
Discussion
Limited research has been conducted in Palestine on the prevalence and molecular mechanisms of quinolone-resistant Gram-negative strains. This study aimed to fill this knowledge gap by investigating the prevalence of quinolone-resistant isolates, plasmid-mediated mechanisms of quinolone resistance and ESBL-, non-β-lactam antibiotic resistance-associated genes in these strains.
In our study, we observed that 64 out of 146 (43.8%) were resistant to quinolones and/or fluoroquinolones. This indicates a significant rate of quinolone resistance among clinical gram-negative isolates. However, our findings showed a lower quinolone resistance rate compared to previous reports from Egypt (57.2%), China (59.4%), and Iran (68%) [6, 20, 21]. Interestingly, our results contrast with a study conducted in the United States, which reported a quinolone resistance rate of 21% among uropathogenic Enterobacteriaceae [22]. This discrepancy suggests regional variations in resistance patterns and highlights the importance of local surveillance studies. The high rate of quinolone resistance observed in our country may be attributed to several factors. Overuse or misuse of these antibiotics in Palestine, both in human medicine and veterinary practices, could contribute to the development and spread of resistance. Additionally, the easy availability of quinolones and the lack of an antimicrobial treatment policy in Palestine may further exacerbate the problem. Addressing the issue of quinolone resistance in Palestine requires an accurate approach, including promoting judicious use of antibiotics, implementing effective infection control measures, and developing and enforcing antimicrobial stewardship programs. These interventions are essential to preserve the effectiveness of quinolones and combat the growing threat of antimicrobial resistance in our region. In this study, we found that quinolone-resistance isolates exhibited high levels of resistance to β-lactams, aminoglycosides and trimethoprim-sulfamethoxazole. Remarkably, 97% of the isolates were classified as multidrug-resistant (MDR), displaying resistance to at least one agent in three or more classes of antibiotics. The presence of transferable plasmid-mediated quinolone resistance determinants in quinolone-resistant Gram-negative isolates is a significant contributing factor to their resistance profiles. These resistance genes are often co-located on plasmids alongside resistance genes for other antibiotic classes, such as β-lactams and aminoglycosides [5]. This genomic organization helps elucidate the high rates of resistance to these other antibiotic classes, as well as the high prevalence of MDR strains observed in this study.
Interestingly, the prevalence of carbapenem resistance (32.8%) was significantly higher in our quinolone-resistance isolates compared to other studies conducted in Iran (3.2) and Egypt (14%) [5, 6]. However, it should be noted that another study reported a much higher rate of 60% of quinolone-resistant isolates being resistant to carbapenems [2]. In recent years, the emergence of carbapenem-resistant bacteria has become a clinical problem worldwide. These drugs are considered the most potent antibiotics for treating infections caused by MDR bacteria.
Regarding the sources of quinolone-resistance isolates, a substantial proportion (47%) was isolated from urine samples, which aligns with the findings reported by Helmy and Kashef, where the majority of resistant isolates (36%) were obtained from urine [23]. These findings underscore the alarming levels of multidrug resistance among quinolone-resistant isolates and the need for effective infection control measures and antimicrobial stewardship programs to combat the spread of resistance. Furthermore, the high prevalence of carbapenem resistance highlights the urgency of implementing strategies to prevent the emergence and dissemination of carbapenem-resistant bacteria in our healthcare settings.
In this study, we observed that among the 64 quinolones-resistant Gram-negative isolates, Pseudomonas spp. was the most frequently isolated species (68.6%), followed by E. coli (40.6%) and K. pneumoniae (33.3%). This distribution differs slightly from a similar study conducted in Turkey, where P. aeruginosa was the most resistant to quinolones (60.0%), followed by E. coli (38.6%) [24]. Another clinical study reported that K. pneumoniae was the most frequently resistant species (66.7%), followed by E. coli (21.7%) [2]. These variations in species distribution highlight the regional differences in resistance patterns and the importance of local surveillance studies.
Among the quinolones resistance isolates in our study, 51.5% (33/64) were identified as ESBL-producers using the double-disk synergy method. These findings are consistent with the studies conducted by Taha, Omar [5] and Rao et al. [25], which reported that more than half of the Enterobacteriaceae isolates were ESBL-producers. However, in contrast to our findings, Azargun et al. [6] reported that 34.2% of the isolates were ESBL-producers. Furthermore, a study conducted in Morocco reported that 20% (39/188) of Gram-negative isolates were ESBL producers [26]. These variations in ESBL production rates among quinolone-resistant isolates highlight the dynamic nature of resistance patterns and the importance of continuous surveillance and monitoring of resistance mechanisms. Understanding the prevalence and distribution of ESBL-producing strains is crucial for the development of effective treatment strategies and infection control measures.
In the current study, we found that among the ESBL-producing isolates, E. coli accounted for 66.6% (22/33), while K. pneumoniae represented 27.3% (9/33) of the isolates. These findings differ from an Iranian study where 53.5% of ESBL-producing isolates were K. pneumoniae and 33.8% were E. coli [6]. Similarly, in India, 61.4% of E. coli were ESBL producers, while 46.2% of K. pneumoniae isolates exhibited ESBL production [25]. These variations in the distribution of ESBL producing strains can be attributed to several factors such as different regions, the hospitalization period, antibiotic usage patterns, and local antimicrobial resistance policies.
In our study, the most predominant plasmid-mediated quinolone resistance (PMQR) gene was aac(6′)- Ib-cr, which is consistent with the findings of other studies [6, 27]. The high prevalence of the aac(6′)- Ib-cr gene can be attributed to its broad spectrum of activity against both quinolones and aminoglycosides. Similar studies conducted in different regions have also identified aac(6)-Ib-cr as the most prevalent PMQR gene in quinolone-resistant Gram-Negative isolates. For example, Badamchi et al. [28] reported a 24% prevalence of the aac(6)-Ib-cr gene in uropathogenic E. coli, while Ma et al. [29] found an 18.8% prevalence of this gene in E. coli isolates.
The aac(6’)-Ib gene encodes aminoglycoside-modifying enzymes responsible for resistance to tobramycin, kanamycin, and amikacin. The aac(6’)-Ib-cr variant gene can confer resistance to both aminoglycosides and fluoroquinolones [28]. The existence of the qnr with aac(6’)-Ib-cr genes further promotes the development of multidrug resistance isolates, as demonstrated in previous studies conducted in China [30]. Considering the widespread use of aminoglycosides and fluoroquinolones for patient treatment in Palestine, it is plausible that the high prevalence of aac(6′)-Ib-cr and its association with multidrug resistance contribute to the emergence of MDR isolates in our study population. These findings underscore the need for judicious use of antibiotics, effective infection control measures, and continuous surveillance of resistance patterns to mitigate the development and spread of MDR bacteria.
In our study, we found that 39% (25/64) of quinolone-resistant isolates carried qnr genes, which is consistent with a previous study [31]. However, this prevalence was lower than reported in two studies from Egypt (60%) and Iran (89.1%) [2, 6], and higher than in an Italian study that reported a prevalence rate of 17% [32]. Among the qnr genes, qnrA was the most dominant, which aligns with the findings of previous studies [5]. The presence of three qnr genes (qnrA, qnrS1 and qnrB) among our isolates has been reported in previous studies [4, 6, 33]. Additionally, three quinolone-resistant isolates in our study harbored two qnr encoding genes, including qnrA with qnrS1 in E. coli strain, qnrS1 with qnrB1 in Pseudomonas, and qnrB1 with qnrA in K. pneumoniae. These findings are consistent with studies from Poland, China and Algeria [34,35,36].
Interestingly, our results revealed that 60% of PMQR genes (aac(6′)-Ib-cr, qnrA, qnrS1 and qnrB1) were found in a significant portion of ESBL-producing isolates (72.7%, 24/33). The presence of these genes in ESBL producers may be due to the coexistence of plasmids carrying ESBL and PMQR genes, which can explain the co-resistance to beta-lactams and fluoroquinolones [36]. Furthermore, we found that most of the qnr-positive Enterobacterales isolates in our study were ESBL producers. Among the qnr-carrier isolates, 33 produced CTX-M, 13 produced TEM-1 and 6 produced SHV-1. Several studies have also reported the association between qnr-positive isolates and ESBL production [4, 7, 8, 26].
CTX-M-15 was the most predominant ESBL type, which is consistent with findings from other studies [6]. In a Palestinian study, CTX-M-15, CTX-M-56, OXA-1, SHV-1, and TEM-1 genes were associated with PMQR genes, including aac(6’)-Ib-cr and qnrB2 in ESBL-positive strains [14]. One significant finding of our study is the significant association between aac(6′)-Ib-cr and qnr with β-lactamase genes (blaCTX−M, blaTEM−1, blaSHV1) in Enterobacterales isolates, which has also been detected in clinical Enterobacterial isolates from Iran, Uruguay, and Togo [4, 6, 37]. Qnr genes are commonly found in plasmids that carry different resistance determinants, including β-lactamase genes [38]. The important association of ESBL-producing isolates with PMQR genes is clinically significant due to the limited therapeutic options for these isolates and may lead to treatment failure and increased mortality rates in patients.
One of the significant results of this study is the detection of class B beta-lactamase genes blaVIM among carbapenem-resistant isolates, specifically in three variants blaVIM−1,blaVIM−2 and blaVIM−28. This is noteworthy because the production of VIM enzymes is associated with a significant decrease in susceptibility to various β-lactam antibiotics, particularly carbapenems, which are crucial for treating multidrug-resistant Gram-negative bacteria. We also observed a significant association between blaVIM and PMQR genes (aac(6′)-Ib-cr, qnrS1), which aligns with the findings of an Italian study where qnrS1 was found in 21 out of 24 VIM-positive isolates [39]. A study conducted in Taiwan demonstrated a high prevalence of qnr (78.6%) among producers of blaIMP, another type of metallo-beta-lactamase [40]. The production of VIM enzymes is indeed associated with significant reductions in susceptibility to different β-lactam antibiotics, especially carbapenems. Carbapenems are crucial drugs for treating multidrug-resistant Gram-negative bacteria, and when these bacteria produce VIM enzymes, it poses a challenge in terms of limited treatment options.
CTX-M-type enzymes belong to a group of class A ESBLs that are plasmid-mediated [4]. In our isolates, the simultaneous production of CTX-M-type with other plasmid-mediated β lactamase types such as TEM, SHV, VIM is noteworthy. The presence of both ESBLs and VIM-MBL, in the same isolates has been reported in Enterobacteriaceae in Italy [39]. This association suggests a concerning integration and co-localization of diverse resistance genes on individual plasmids or integrons. The concurrent presence of both MBLs and ESBLs in PMQR-positive bacteria highlights the accumulation of critical resistance determinants. This observation raises alarming concerns about the further development and dissemination of MDR strains. The emergence of such extensively resistant strains can result in significant mortality and morbidity, as therapeutic options become severely limited.
In this study, we found class I integrons were found in 31 out of 64 (48%) of the isolates. Similarly, in an Egyptian study, class I integrons were detected in 59 (44%) out of 134 clinical isolates of E. coli [9]. Abbasi, Ghaznavi-Rad [41] detected class 1 integron in 71.4% of quinolone-resistant Salmonella species isolated from diarrheic children in Iran. Integrons are carried by mobile genetics elements within bacterial cells, allowing them to spread to other bacteria. Integrons play a crucial role in carrying and disseminating antimicrobial resistance genes among bacteria through horizontal transfer, which is one of the most significant routes for the distribution of these genes [9].
Five different gene cassettes were found among the 14 intI-1-positive isolates by the sequencing of the amplified variable regions. Three genes of dihydrofolate reductase (dfr family) that encoded for resistance to trimethoprim (dhfr17, dfrA12, dfrA1) and four aminoglycosides resistance genes (aadA1, aadA2, aadA5, and aadA6). The occurrence of cassettes encoded for resistance to trimethoprim and aminoglycosides (dfrA, aadA) in quinolone-resistant isolates has been reported [41,42,43]. Gene cassettes dfrA17-aadA5 and aadA6 were detected in six and four isolates, respectively, which may be due to the transfer of the integrons between bacterial species. The dissemination of integrons is carried out by the cross-transmission of integron-carrying clones from one patient to the other in hospital settings [44]. The two gene cassettes (dfrA17-aadA5, and aadA6) were documented in Gram-negative strains in several regions worldwide [9, 45,46,47]. The spread of these genes in other regions may involve self-transferable plasmids within the host (humans and animals) [48].
Interestingly, we did not detect any PMQR genes in the gene cassettes. Although the presence of integrons did not influence the susceptibility to the tested quinolones in the present study, it is important to note that the prevalence of integrons has played a significant role in the development of MDR bacteria. The quinolones and other antibiotic-resistance genes except dfrA and aadA genes are generally located outside the integrons. The association between quinolone resistance and the presence of an integron is not yet fully understood and has been the subject of ongoing research [48, 49].
Conclusion
The study confirmed a high prevalence of quinolone resistance, ESBL and integrons among the clinical Gram-negative isolates. PMQR determinants were widely reported, with particularly high prevalence of both aac(6’)-Ib and qnrA genes. The important association between ESBL-producing with PMQR genes is clinically significant, as it potentiates the development of MDR phenotypes and severely limits therapeutic options for these isolates. This study also revealed a high prevalence of integrons, often harboring the most dominant gene cassettes conferring resistance to trimethoprim and aminoglycosides. While the integrons did not directly impact quinolone susceptibility (as the resistance genes were not cassette-borne), they played a crucial role in the development of MDR bacteria. To prevent outbreaks of PMQR, integrons and ESBL-producing isolates, it is crucial to implement robust infection control measures and establish continuous antimicrobial resistance surveillance. This can be achieved through the application of an antimicrobial resistance monitoring system to control their dissemination and the linked health hazard. Further studies are needed to study whole genome sequencing and determine plasmids and insertion elements, providing a better understanding of the genetic factors contributing to antimicrobial resistance in these isolates.
Data availability
The datasets generated during and analyzed during the current study are available in this manuscript.
References
Oliveira J, Reygaert WC (2019) Gram negative bacteria
Abd ElSalam M, Gamal D, El-Said M, Salem D, Aitta AA, El-Gamal MS (2018) Prevalence of plasmid-mediated quinolone resistance in multidrug-resistant gram negative bacilli in Egypt. Biomedical Pharmacol J 11(4):1927–1936
Köck R, Siemer P, Esser J, Kampmeier S, Berends MS, Glasner C, Arends JP, Becker K, Friedrich AW (2018) Defining multidrug resistance of gram-negative bacteria in the Dutch–German border region—impact of national guidelines. Microorganisms 6(1):11
Salah FD, Soubeiga ST, Ouattara AK, Sadji AY, Metuor-Dabire A, Obiri-Yeboah D, Banla-Kere A, Karou S, Simpore J (2019) Distribution of quinolone resistance gene (qnr) in ESBL-producing Escherichia coli and Klebsiella spp. in Lomé, Togo. Antimicrob Resist Infect Control 8(1):1–8
Taha SA, Omar HH (2019) Characterization of plasmid-mediated qnrA and qnrB genes among Enterobacteriaceae strains: quinolone resistance and ESBL production in Ismailia, Egypt. Egypt J Med Hum Genet 20(1):1–7
Azargun R, Sadeghi MR, Barhaghi MHS, Kafil HS, Yeganeh F, Oskouee MA, Ghotaslou R (2018) The prevalence of plasmid-mediated quinolone resistance and ESBL-production in Enterobacteriaceae isolated from urinary tract infections. Infect drug Resist 11:1007
Vaziri S, Afsharian M, Mansouri F, Azizi M, Nouri F, Madadi-Goli N, Afshar ZM, Zamanian MH, Alvandi A, Ahmadi K (2020) Frequency of qnr and aac (6’) Ib-cr Genes among ESBL-producing Klebsiella pneumoniae strains isolated from burn patients in Kermanshah, Iran. Jundishapur journal of microbiology 13 (7)
Abdelrahim SS, Hassuna NA, Waly NGFM, Kotb DN, Abdelhamid H, Zaki S (2024) Coexistence of plasmid-mediated quinolone resistance (PMQR) and extended-spectrum beta-lactamase (ESBL) genes among clinical Pseudomonas aeruginosa isolates in Egypt. BMC Microbiol 24(1):175
Abdel-Rhman SH, Elbargisy RM, Rizk DE (2021) Characterization of integrons and quinolone resistance in clinical Escherichia coli isolates in Mansoura City, Egypt. Int J Microbiol 2021
Deng Y, Bao X, Ji L, Chen L, Liu J, Miao J, Chen D, Bian H, Li Y, Yu G (2015) Resistance integrons: class 1, 2 and 3 integrons. Ann Clin Microbiol Antimicrob 14(1):1–11
Abu Taha A, Jaradat A, Shtawi A, Dawabsheh Y (2018) Prevalence and risk factors of extended spectrum beta-lactamase-producing uropathogens among UTI patients in the governmental hospitals of North West Bank: A cross-sectional study. J Infect Dis Preve Med 6(183):2
Elmanama AA, Laham NA, Tayh GA (2013) Antimicrobial susceptibility of bacterial isolates from burn units in Gaza. Burns: J Int Soc Burn Injuries 39(8):1612–1618
Tayh G, Sallem RB, Yahia HB, Gharsa H, Klibi N, Boudabous A, Slama KB (2016) First report of extended-spectrum β-lactamases among clinical isolates of Escherichia coli in Gaza Strip, Palestine. J Global Antimicrob Resist 6:17–21
Hussein AI, Ahmed AM, Sato M, Shimamoto T (2009) Characterization of integrons and antimicrobial resistance genes in clinical isolates of Gram-negative bacteria from Palestinian hospitals. Microbiol Immunol 53(11):595–602
Kaur J, Chopra S, Sheevani, Mahajan G (2013) Modified double disc synergy test to detect ESBL production in urinary isolates of Escherichia coli and Klebsiella pneumoniae. J Clin Diagn Research: JCDR 7(2):229–233
Dashti AA, Jadaon MM, Abdulsamad AM, Dashti HM (2009) Heat treatment of bacteria: a simple method of DNA extraction for molecular techniques. Kuwait Med J 41(2):117–122
Rocha-Gracia R, Ruiz E, Romero-Romero S, Lozano-Zarain P, Somalo S, Palacios-Hernández JM, Caballero-Torres P, Torres C (2010) Detection of the plasmid-borne quinolone resistance determinant qepA1 in a CTX-M-15-producing Escherichia coli strain from Mexico. J Antimicrob Chemother 65(1):169–171
Jouini A, Vinué L, Slama KB, Saenz Y, Klibi N, Hammami S, Boudabous A, Torres C (2007) Characterization of CTX-M and SHV extended-spectrum β-lactamases and associated resistance genes in Escherichia coli strains of food samples in Tunisia. J Antimicrob Chemother 60(5):1137–1141
Slama KB, Sallem RB, Jouini A, Rachid S, Moussa L, Sáenz Y, Estepa V, Somalo S, Boudabous A, Torres C (2011) Diversity of genetic lineages among CTX-M-15 and CTX-M-14 producing Escherichia coli strains in a Tunisian hospital. Curr Microbiol 62(6):1794–1801
El-Badawy MF, Alrobaian MM, Shohayeb MM, Abdelwahab SF (2019) Investigation of six plasmid-mediated quinolone resistance genes among clinical isolates of pseudomonas: a genotypic study in Saudi Arabia. Infect Drug Resist 12:915
Wang A, Yang Y, Lu Q, Wang Y, Chen Y, Deng L, Ding H, Deng Q, Zhang H, Wang C (2008) Presence of qnr gene in Escherichia coli and Klebsiella pneumoniae resistant to ciprofloxacin isolated from pediatric patients in China. BMC Infect Dis 8(1):1–6
Moreno E, Prats G, Sabaté M, Pérez T, Johnson JR, Andreu A (2006) Quinolone, fluoroquinolone and trimethoprim/sulfamethoxazole resistance in relation to virulence determinants and phylogenetic background among uropathogenic Escherichia coli. J Antimicrob Chemother 57(2):204–211
Helmy OM, Kashef MT (2017) Different phenotypic and molecular mechanisms associated with multidrug resistance in Gram-negative clinical isolates from Egypt. Infect drug Resist 10:479
Bastopcu A, Yazgi H, Uyanik MH, Ayyildiz A (2008) Evaluation of quinolone resistance in gram negative bacilli isolated from community-and hospital-acquired infections. Eurasian J Med 40(2):58
Rao SP, Rama PS, Gurushanthappa V, Manipura R, Srinivasan K (2014) Extended-spectrum beta-lactamases producing Escherichia coli and Klebsiella pneumoniaei: a multi-centric study across Karnataka. J Lab Physicians 6(01):007–013
Bouchakour M, Zerouali K, Claude JDPG, Amarouch H, El Mdaghri N, Courvalin P, Timinouni M (2010) Plasmid-mediated quinolone resistance in expanded spectrum beta lactamase producing enterobacteriaceae in Morocco. J Infect Developing Ctries 4(12):779–803
Karah N, Poirel L, Bengtsson S, Sundqvist M, Kahlmeter G, Nordmann P, Sundsfjord A, Samuelsen Ø (2010) Plasmid-mediated quinolone resistance determinants qnr and aac (6′)-Ib-cr in Escherichia coli and Klebsiella spp. from Norway and Sweden. Diagn Microbiol Infect Dis 66(4):425–431
Badamchi A, Javadinia S, Farahani R, Solgi H, Tabatabaei A (2019) Molecular detection of plasmid mediated Quinolone resistant genes in uropathogenic E coli from Tertiary Referral Hospital in Tehran Iran. Archives Pharmacol Ther 1(1):19–24
Ma J, Zeng Z, Chen Z, Xu X, Wang X, Deng Y, Lü D, Huang L, Zhang Y, Liu J (2009) High prevalence of plasmid-mediated quinolone resistance determinants qnr, aac (6′)-Ib-cr, and qepA among ceftiofur-resistant Enterobacteriaceae isolates from companion and food-producing animals. Antimicrob Agents Chemother 53(2):519–524
Wang M, Jacoby GA, Mills DM, Hooper DC (2009) SOS regulation of qnrB expression. Antimicrob Agents Chemother 53(2):821–823
Tohamy ST, Aboshanab KM, El-Mahallawy HA, El-Ansary MR, Afifi SS (2018) Prevalence of multidrug-resistant Gram-negative pathogens isolated from febrile neutropenic cancer patients with bloodstream infections in Egypt and new synergistic antibiotic combinations. Infect Drug Resist 11:791
Richter SN, Frasson I, Bergo C, Manganelli R, Cavallaro A, Palù G (2010) Characterisation of qnr plasmid-mediated quinolone resistance in Enterobacteriaceae from Italy: association of the qnrB19 allele with the integron element ISCR1 in Escherichia coli. Int J Antimicrob Agents 35(6):578–583
Shinu P, Bareja R, Nair AB, Mishra V, Hussain S, Venugopala KN, Sreeharsha N, Attimarad M, Rasool ST (2020) Monitoring of Non-β-Lactam Antibiotic Resistance-Associated genes in ESBL producing enterobacterales isolates. Antibiotics 9(12):884
Michalska AD, Sacha PT, Ojdana D, Wieczorek A, Tryniszewska E (2014) Prevalence of resistance to aminoglycosides and fluoroquinolones among Pseudomonas aeruginosa strains in a University Hospital in Northeastern Poland. Brazilian J Microbiol 45:1455–1458
Yang H, Hu L, Liu Y, Ye Y, Li J (2016) Detection of the plasmid-mediated quinolone resistance determinants in clinical isolates of Acinetobacter baumannii in China. J Chemother 28(5):443–445
Touati A, Brasme L, Benallaoua S, Gharout A, Madoux J, De Champs C (2008) First report of qnrb-producing Enterobacter cloacae and qnra-producing Acinetobacter baumannii recovered from Algerian hospitals. Diagn Microbiol Infect Dis 60(3):287–290
Vignoli R, García-Fulgueiras V, Cordeiro NF, Bado I, Seija V, Aguerrebere P, Laguna G, Araújo L, Bazet C, Gutkind G (2016) Extended-spectrum β-lactamases, transferable quinolone resistance, and virulotyping in extra-intestinal E. Coli in Uruguay. J Infect Developing Ctries 10(01):43–52
Nordmann P, Poirel L (2005) Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J Antimicrob Chemother 56(3):463–469
Aschbacher R, Doumith M, Livermore DM, Larcher C, Woodford N (2008) Linkage of acquired quinolone resistance (qnrS1) and metallo-β-lactamase (bla VIM-1) genes in multiple species of Enterobacteriaceae from Bolzano, Italy. J Antimicrob Chemother 61(3):515–523
Wu J-J, Ko W-C, Tsai S-H, Yan J-J (2007) Prevalence of plasmid-mediated quinolone resistance determinants QnrA, QnrB, and QnrS among clinical isolates of Enterobacter cloacae in a Taiwanese hospital. Antimicrob Agents Chemother 51(4):1223–1227
Abbasi E, Ghaznavi-Rad E (2021) Quinolone resistant Salmonella species isolated from pediatric patients with diarrhea in central Iran. BMC Gastroenterol 21(1):1–6
Seo KW, Lee YJ (2019) Prevalence and characterization of plasmid mediated Quinolone Resistance genes and class 1 Integrons among Multidrug-Resistant Escherichia coli isolates from Chicken meat. J Appl Poult Res 28(3):761–770
Moura A, Henriques I, Ribeiro R, Correia A (2007) Prevalence and characterization of integrons from bacteria isolated from a slaughterhouse wastewater treatment plant. J Antimicrob Chemother 60(6):1243–1250
Leverstein-van Hall MA, Box AT, Blok HE, Paauw A, Fluit AC, Verhoef J (2002) Evidence of extensive interspecies transfer of integron-mediated antimicrobial resistance genes among multidrug-resistant Enterobacteriaceae in a clinical setting. J Infect Dis 186(1):49–56
Tayh G, Al Laham N, Ben Yahia H, Ben Sallem R, Elottol AE, Ben Slama K (2019) Extended-Spectrum β-Lactamases among Enterobacteriaceae Isolated from Urinary Tract Infections in Gaza Strip, Palestine. BioMed Research International 2019: 4041801
Naas T, Poirel L, Nordmann P (1999) Molecular characterisation of In51, a class 1 integron containing a novel aminoglycoside adenylyltransferase gene cassette, aadA6, in Pseudomonas aeruginosa. Biochim Biophys Acta 1489(2–3):445–451
Hossain S, Dahanayake PS, De Silva BCJ, Wickramanayake M, Wimalasena S, Heo GJ (2019) Multidrug resistant Aeromonas spp. isolated from zebrafish (Danio rerio): antibiogram, antimicrobial resistance genes and class 1 integron gene cassettes. Lett Appl Microbiol 68(5):370–377
Leverstein-van Hall MA, Blok M, Donders HET, Paauw AR, Fluit A, Verhoef AC J (2003) Multidrug Resistance among Enterobacteriaceae is strongly Associated with the Presence of integrons and is Independent of species or isolate origin. J Infect Dis 187(2):251–259
Mooij MJ, Schouten I, Vos G, Van Belkum A, Vandenbroucke-Grauls CMJE, Savelkoul PHM, Schultsz C (2005) Class 1 integrons in ciprofloxacin-resistant Escherichia coli strains from two Dutch hospitals. Clin Microbiol Infect 11(11):898–902
Sáenz Y, Briñas L, Domínguez E, Ruiz J, Zarazaga M, Vila J, Torres C (2004) Mechanisms of resistance in multiple-antibiotic-resistant Escherichia coli strains of human, animal, and food origins. Antimicrob Agents Chemother 48(10):3996–4001
Funding
This study was funded by the Tunisian Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Contributions
Gh.T. designed the study, performed the experimental work (the microbiological and molecular tests), collected the data, analyzed and interpreted the data and drafted the manuscript. I.F. and M.B.S. contributed to final writing and editing the manuscript. A.B. participated in the project design and contributed to final writing and editing the manuscript. K.B.S. designed and supervised the study. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Conflicts of interest
The authors declare that they have no conflicts of interest. All authors read and approved the final version of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tayh, G., Fhoula, I., Said, M.B. et al. Prevalence and characterization of quinolone resistance and integrons in clinical Gram-negative isolates from Gaza strip, Palestine. Mol Biol Rep 51, 855 (2024). https://doi.org/10.1007/s11033-024-09721-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09721-0