Abstract
Schizophrenia constitutes a severe psychiatric disorder with detrimental impacts on individuals, their support systems, and the broader economy. Extensive research has revealed a notable association between variations in the Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) gene and an increased susceptibility to schizophrenia.
This study represents the first systematic review of the literature investigating the impact of CTLA-4 polymorphisms and expression on the development and progression of schizophrenia.
Our investigation involved a comprehensive search strategy, using a combination of title, abstract, and MESH terms in four databases, including PubMed, Scopus, Web of Science, and Google Scholar, until August 29th, 2023. The complete texts of the identified records were obtained and rigorously assessed based on predefined exclusion and inclusion criteria. Out of the numerous records, a total of 88 were identified through the databases. 10 studies met the criteria; therefore, their quality was assessed and included in this systematic study. The records were then categorized into polymorphism and expression groups. Our investigation emphasizes an association between rs3087243, rs231779, rs231777, rs16840252, rs5742909, and rs231775 polymorphisms and the development of schizophrenia. The results demonstrate a correlation between CTLA-4 polymorphisms and schizophrenia, compelling the need for further research to thoroughly examine the role of CTLA-4 in schizophrenia and other psychiatric disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a debilitating psychiatric disorder that profoundly affects individuals, support systems, and the overall economic landscape due to its substantial detrimental consequences [1]. Typically, this disorder manifests in late adolescence or early adulthood [2, 3], affecting nearly 1% of the world’s population [3]. The symptoms of schizophrenia encompass hallucinations and delusions (as positive symptoms), alongside withdrawal, alogia, and flattening (as negative symptoms), coupled with cognitive and thinking challenges [3, 4].
The underlying mechanism of schizophrenia is complex and multifactorial, involving a combination of factors [5, 6]. While a family history of psychiatric conditions is recognized as a major risk factor, it is not necessarily a significant causal factor [5]. Furthermore, within schizophrenia, there is notable variation in the severity of symptoms, the course of the disorder, responses to treatment, and its etiology among patients. This inherent heterogeneity gives rise to diverse outcomes in biological investigations [7].
Recently, it has been suggested that autoimmune diseases and severe infections necessitating hospitalization, may contribute to the development of psychiatric disorders like schizophrenia and depression [8,9,10]. Research indicates elevated autoantibody levels and increased autoantibody reactivity in a subgroup of patients with psychiatric disorders, even in the absence of known autoimmune diseases [11, 12]. Moreover, the prevalence of autoimmune diseases is observed to be higher in patients with schizophrenia and other psychotic disorders [9]. An association between elevated C-reactive protein levels and a higher risk of developing late-onset schizophrenia also has been demonstrated in some studies [13, 14].
Numerous studies have demonstrated T-cell-associated dysfunctions, particularly the dysregulation of T-cell-related cytokines, in both schizophrenia and major depressive disorders [15]. In schizophrenia, lower activation of Th1 (decreased levels of Interferon-gamma (IFN-γ)) [16] and higher activation of Th2 (increased levels of interleukin-4 (IL-4), IL-6, and IL-10) [17,18,19] have been identified. However, in major depressive disorders increased levels of pro-inflammatory cytokines such as IL-1β, tumor necrosis factor alpha (TNF-α) and IFN-γ have been reported [20, 21]. These findings highlight the association between immune system dysfunctions and psychiatric disorders [15]. Moreover, genetic predisposition and variants of immune system genes have also been linked to schizophrenia [12, 22].
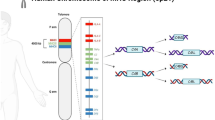
The Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) gene, consisting of four exons, is located on the long arm of chromosome 2q33 [23, 24] and has been proposed as a potential candidate gene associated with susceptibility to autoimmune diseases (Fig. 1) [23, 25, 26]. CTLA-4, as an inhibitory molecule, plays a vital role in establishing and maintaining tolerance to self-antigens and regulates the activation of T-cells [15, 27]. It has been found that genetic variations within the CTLA-4 gene may reduce the inhibitory effect on T cells, leading to the onset of various systemic and autoimmune diseases. Examples include insulin-dependent diabetes mellitus (IDDM) and Hashimoto thyroiditis observed in patients with rheumatoid arthritis. Additionally, reduced levels of CTLA-4 have been observed in individuals with autism and Alzheimer’s disease [28, 29]. Additionally, several studies have linked CTLA-4 gene polymorphisms to an increased risk of schizophrenia, a relationship that will be explored in this study. Although several systematic reviews have affirmed the association between the immune system and schizophrenia [29], to our knowledge, none have specifically investigated the role of CTLA-4 expression and polymorphisms in this psychiatric disorder. Therefore, to gain a comprehensive understanding of the roles of CTLA-4 in schizophrenia, we will conduct a systematic review of the literature, examining the influence of CTLA-4 on the development and pathogenesis of schizophrenia, encompassing both gene expression and polymorphisms.
Methods
Literature search strategy
The current systematic review was done according to PRISMA guidelines. A comprehensive search was conducted utilizing a combination of title, abstract, and MESH terms across four databases: PubMed, Scopus, Web of Science, and Google Scholar, up until August 29th, 2023. All selected articles were in English. Additionally, we included references from textbooks and articles to identify potentially eligible studies (Table 1).
Inclusion and exclusion
We included studies investigating the effect of or association between CTLA-4 antigen and schizophrenia in humans. Additionally, studies examining schizophrenia in conjunction with other psychiatric disorders, such as major depressive disorder and bipolar disorder, were included. No restrictions were imposed on age, ethnicity, duration, gender, or sample size. Since our study investigates the complete role of CTLA-4 in schizophrenia, we included studies related to both polymorphisms (genetics) and expressions (transcriptomics). Notably, this review excludes books, animal studies, and review articles, except for a specific letter directly pertinent to our subject, which is included to enhance the quality of the results. The results from our database search were integrated into Endnote, and duplicate studies were systematically removed. Irrelevant studies were removed during the scanning phase when two independent researchers decided on the matching studies until August 29th, 2023.
Data extraction
Two authors pooled together the results and extracted the data following our protocol, and in case of disagreement, a third author was asked. We extracted the name of the first author, publication year, country of the study, race, sample sizes, including case and control groups, and the results, which were either expressions or polymorphisms.
Quality assessment
We used a predefined checklist to assess the eligibility of studies. Apart from those doing the data extraction, two other researchers assessed the quality of studies using Joanna Briggs Institute’s (JBI) critical appraisal of evidence Effectiveness tool. The tool has eleven yes/no questions and assesses the study eligibility for systematic reviews [30].
Results
Study selection and characteristics
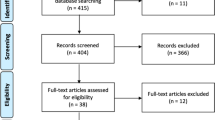
Throughout our search, we obtained a total of 88 studies. Following a thorough duplication check, 38 studies were eliminated due to duplication. Subsequently, the remaining 50 articles underwent assessment based on their titles and abstracts, leading to the selection of 11 studies as the final results after a comprehensive review of both the abstract and full text. One study was excluded as it initially existed as a poster by Frydecka but was later published as a journal article; consequently, only the latter was included in our study. Finally, 10 studies met our criteria, and their quality was assessed before being incorporated into the systematic review.
Figure 2 shows the flowchart of our systematic search. The identified studies were subsequently categorized into two groups: polymorphism (n = 8) and expression (n = 3), with the study conducted by Kordi-Tamandani [31] falling into both categories. These 10 studies span the publication years from 2001 to 2020. Most studies were conducted in Asia, particularly East Asia, such as Japan, China, Korea, Indonesia, and Iran (Tables 2 and 3), however, one study was conducted in Australia [32]. The only European country investigating the role of CTLA-4 on schizophrenia was Poland [33,34,35]. The ethnicities predominantly represented in the studies were Caucasian, Chinese, Korean, and Iranian (Tables 2 and 3). In terms of diagnostic tools, one study utilized DSM-V [36], five used DSM-IV [15, 31, 33, 37, 38], one employed DSM-III [32], and two studies utilized both DSM-IV and ICD10 [34, 35]. The diagnostic approach in one study was unspecified [39] (Tables 2 and 3). Two studies investigated the role of CTLA-4 in three disorders, including schizophrenia, bipolar disorder, and major depressive disorders [15, 36].
Systematic review
The investigation into the role of CTLA-4 was conducted in two stages. Initially, we explored CTLA-4 mRNA expression, and subsequently, we studied CTLA-4 gene polymorphisms.
CTLA-4 expression
Three studies investigated the expression level of CTLA-4 in schizophrenia [31, 36, 38]. Cai et al. investigated the mRNA expression levels of membrane CTLA-4 (mCTLA-4) and soluble CTLA-4 (sCTLA-4) in peripheral blood mononuclear cells (PBMC) derived from first-episode schizophrenia patients and healthy controls [38]. Their findings revealed a significant reduction in sCTLA-4 expression among schizophrenic patients, while the difference in mCTLA-4 mRNA expression between the control and patient groups remained insignificant (p-values of 0.009 and 0.769, respectively).
In another study conducted by Miyamoto et al., a lower CTLA-4 mRNA expression was detected in the peripheral blood sample of patients with schizophrenia compared to the healthy control [36]. Conversely, in the study by Kordi-Tamandani et al., CTLA-4 exhibited higher expression levels compared to the control group [31]. A comprehensive list of study characteristics is provided in Table 2.
CTLA-4 polymorphisms
Eight studies investigated the polymorphism of the CTLA-4 gene and its association with schizophrenia, as shown in Table 3. All these studies focused on single nucleotide polymorphisms (SNPs), and two of them were conducted by the same author [33, 34]. Polymorphism of the CTLA-4 gene at position 49 of exon 1 (A/G, rs231775) was the most studied polymorphism (Fig. 1) [15, 32,33,34,35, 37]. One study showed a significant association between polymorphisms of rs231775 and the affective symptom domain in schizophrenia [34], suggesting a potential link to the development of the disorder [37]. rs231779, rs231777, and rs16840252 were also found to be associated with the disease in the Chinese Han population [15]. Additionally, in Caucasian populations, the promoter region SNP − 318 C/T (rs5742909) and the 3´-UTR SNP (rs3087243) demonstrated nominally significant associations with schizophrenia, particularly in males [32]. Kordi-Tamandani studied CTLA-4 -318 C/T polymorphism and found the CT genotype had a protective role in terms of schizophrenia risk in Iranian population [31] which was contrary to the results from Sumirtanurdin study which was conducted in Asia [39]. Moreover, Frydecka showed that T allele frequency in the rs5742909 is associated with higher chances of schizophrenia development and is related to worse clinical outcomes [34]. Thus, the polymorphisms that seem to play a role in schizophrenia development, according to the search results, seem to be: rs3087243, rs231779, rs231777, rs16840252, rs5742909, rs231775.
Discussion
Chronic, low-grade inflammation and T-cell dysfunction have been implicated as risk factors for schizophrenia [38, 40]. The regulatory role of CTLA-4 in T-cell activation and proliferation is crucial, as its deficiency can result in the dysregulation of immune responses and the onset of autoimmunity [38, 41, 42]. On chromosome 2q33, the CTLA-4 gene encodes two distinct isoforms through alternative splicing: the membrane-bound CTLA-4 (mCTLA4) and soluble CTLA-4 (sCTLA4) [38]. While mCTLA-4 mRNA comprises four exons, sCTLA-4 mRNA lacks exon 3 (transmembrane domain) and consists of three exons [38, 43]. Although studies suggest a potential link between CTLA-4 gene variants and susceptibility to schizophrenia, the exact nature of the relationship between CTLA-4 and schizophrenia remains incompletely understood [38].
In this study, the gathered data was divided into two parts: the first part elucidates studies focusing on CTLA-4 expression, while the second part delves into the polymorphisms of the CTLA-4 gene. Two studies reported a significant reduction in CTLA-4 levels [36, 38].
However, a study conducted by Kordi-Tammandani et al. presented a contrary finding, revealing a substantial increase in CTLA-4 mRNA expression among individuals with schizophrenia [31]. Despite Cai’s exploration of expression levels related to specific polymorphisms, it did not conclude a direct connection between polymorphisms and the risk of schizophrenia [38]. Consequently, it was only incorporated into the expression section. The increased expression levels in Kordi’s study were extremely significant. The difference in results observed within these studies can be due to different study samples. Interestingly, the observed alteration in expression was influenced by gene polymorphisms, highlighting the importance of investigating CTLA-4 gene polymorphisms within populations affected by schizophrenia [44].
The single nucleotide polymorphisms (SNPs) of the CTLA-4 gene produced controversial results. Several studies identified several CTLA-4-related SNPs, and among them, rs3087243, rs231779, rs231777, rs16840252, rs5742909, and rs231775 were reported in different studies to be associated with schizophrenia.
The SNP CTLA-4 + 49 A/G (rs231775), located in the exon 1 of CTLA-4, has been implicated in the development of schizophrenia in several studies [34, 37]. This polymorphism has also been examined in the context of various disorders, contributing to conditions such as systemic lupus erythematosus (SLE), thyroiditis, and malignancies [45,46,47,48]. However, investigations into its role in other psychiatric disorders, including depression, have not revealed a significant association [49]. On the other hand, rs231777, rs231779, and rs16840252 demonstrated a notable association with schizophrenia in the Chinese Han population [15]. Nevertheless, the studies exploring these SNPs were limited, necessitating further investigation. Furthermore, the rs5742909 or -318 C/T SNP within the promoter region has been consistently associated with schizophrenia and poor clinical outcomes in multiple studies [31, 32, 34].
In males, rs3087243 demonstrated a significant association with schizophrenia in Caucasian populations [32]. Notably, this SNP has also been implicated in malignancies such as breast, hepatic, kidney, and skin cancer [50].
Several studies attempted to investigate the polymorphisms among schizophrenic population, but the results are inconclusive. A fit of SNPs were found, but their association to schizophrenia differed among studies. This can be explained mainly by the different study population and racial backgrounds, which can highly attribute to genome differences among patients. Large multi-center and multi-national studies are highly needed to illustrate the association between different SNPs and genetic variations and schizophrenia.
In summary, our results imply a connection between multiple polymorphisms in the CTLA-4 gene and schizophrenia. This association is noteworthy not only in the context of schizophrenia but also extends to several other disorders, with a notable presence in neoplasia and immune-related conditions.
Limitations and future directions
To the best of our knowledge, this is the first systematic review of the role of CTLA-4 in the development and pathogenesis of schizophrenia, which is accompanied by certain limitations. First, it cannot be definitively claimed that the authors have thoroughly studied all published works on the subject of the review. The authors might miss some pertinent works. Second, our review was based on a small number of studies, especially with regard to those investigating the expression of CTLA-4 in schizophrenia. Thus, future studies must evaluate CTLA-4 expression to clarify its contribution to schizophrenia. Third, the included studies did not mention covariates like insulin-dependent diabetes mellitus, Hashimoto thyroiditis, Graves’ disease, celiac disease, systemic lupus erythematosus and other autoimmune diseases that might influence CTLA-4 polymorphism or expression, so there can be a confounding bias. Finally, the results are rather heterogeneous. Since high statistical heterogeneity has an essential role in compromising the level of reliability of the results, we could not quantitatively review the studies. Therefore, future studies with homogeneous data are required to access quantitative results and conduct meta-analyses. Since the results have shown that CTLA-4 polymorphisms are associated with schizophrenia, we highly encourage future researchers to evaluate the role of other inhibitory receptors, such as PD-1 and LAG-3, in psychiatric disorders.
Data availability
No Data associated with the manuscript.
References
Moreno-Küstner B, Martin C, Pastor L (2018) Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS ONE 13(4):e0195687
Gomes FV, A.A.J (2017) .S.b. Grace, adolescent stress as a driving factor for schizophrenia development—a basic science perspective. 43(3):486–489
Gogtay N et al (2011) Age of onset of schizophrenia: perspectives from structural neuroimaging studies. 37(3):504–513
Mosolov SN (2022) and P.A.J.F.i.p. Yaltonskaya, Primary and secondary negative symptoms in schizophrenia : p. 2317
Mortensen PB, Pedersen MG, Pedersen CB (2010) Psychiatric family history and schizophrenia risk in Denmark: which mental disorders are relevant? Psychol Med 40(2):201–210
Javitt DC (2007) Glutamate and schizophrenia: phencyclidine, N-methyl‐d‐aspartate receptors, and dopamine–glutamate interactions. Int Rev Neurobiol 78:69–108
Müller N et al (2000) The immune system and schizophrenia: an integrative view. Ann N Y Acad Sci 917(1):456–467
Benros ME, Mortensen PB, Eaton WW (2012) Autoimmune diseases and infections as risk factors for schizophrenia, vol 1262. Annals of the New York Academy of Sciences, pp 56–66. 1
Benrós ME, Mortensen PBJI (2015) and P.F.B.R.t.T. Interventions, The role of infections and autoimmune diseases for schizophrenia and depression: findings from large-scale epidemiological studies : p. 107–135
Benros ME et al (2013) Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry 70(8):812–820
Laske C et al (2008) Autoantibody reactivity in serum of patients with major depression, schizophrenia and healthy controls. Psychiatry Res 158(1):83–86
Pathmanandavel K et al (2013) Autoantibodies and the immune hypothesis in psychotic brain diseases: challenges and perspectives 2013
Wium-Andersen MK, Ørsted DD, Nordestgaard BG (2014) Elevated C-reactive protein associated with late-and very-late-onset schizophrenia in the general population: a prospective study. Schizophr Bull 40(5):1117–1127
Metcalf SA et al (2017) Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: a prospective birth cohort study. Brain Behav Immun 59:253–259
Liu J et al (2011) CTLA-4 confers a risk of recurrent schizophrenia, major depressive disorder and bipolar disorder in the Chinese Han population. Brain Behav Immun 25(3):429–433
Rothermundt M et al (1996) Production of cytokines in acute schizophrenic psychosis. Biol Psychiatry 40(12):1294–1297
Mittleman BB et al (1950) Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease Journal of immunology (Baltimore, Md.: 1997. 159(6): p. 2994–2999
Lin A et al (1998) The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res 32(1):9–15
Cazzullo CL et al (1998) Cytokines production in chronic schizophrenia patients with or without paranoid behaviour. Prog Neuropsychopharmacol Biol Psychiatry 22(6):947–957
Das R et al (2021) Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res 295:113568
Maes M et al (2009) The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 24:27–53
Consortium C- (2014) D.G.o.t.P.G., Biological insights from 108 schizophrenia-associated genetic loci. Nature 511(7510):421–427
Nisticò L et al (1996) The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Hum Mol Genet 5(7):1075–1080
Garcia-Perez JE et al (2019) CTLA4 message reflects pathway disruption in monogenic disorders and under therapeutic blockade 10: p. 998
Ligers A et al (2001) CTLA-4 gene expression is influenced by promoter and exon 1 polymorphisms, vol 2. Genes & Immunity, pp 145–152. 3
Kristiansen O et al (2000) CTLA-4 in autoimmune diseases–a general susceptibility gene to autoimmunity? 1(3):p170–p184
Romo-Tena J, Gómez-Martín D, Alcocer-Varela J.J.A.r. (2013) CTLA-4 and autoimmunity: new insights into the dual regulator of tolerance. 12(12):1171–1176
Khandaker GM et al (2015) Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2(3):258–270
van Mierlo HC et al (2020) The association between schizophrenia and the immune system: review of the evidence from unbiased ‘omic-studies’. Schizophr Res 217:114–123
Alrashed MM et al (2022) MiR-624-5p enhances NLRP3 augmented gemcitabine resistance via EMT/IL-1β/Wnt/β-catenin signaling pathway in ovarian cancer. J Reprod Immunol, : p. 103488
Kordi-Tamandani DM et al (2013) Evaluation of polymorphism, hypermethylation and expression pattern of CTLA4 gene in a sample of Iranian patients with schizophrenia. Mol Biol Rep 40(8):5123–5128
Jones AL et al (2009) CTLA-4 single-nucleotide polymorphisms in a caucasian population with schizophrenia. Brain Behav Immun 23(3):347–350
Frydecka D et al (2013) The role of genetic variations of immune system regulatory molecules CD28 and CTLA-4 in schizophrenia. Psychiatry Res 208(2):197–198
Frydecka D et al (2015) CTLA4 and CD28 gene polymorphisms with respect to Affective Symptom Domain in Schizophrenia. Neuropsychobiology 71(3):158–167
Mak M et al (2018) Polymorphisms in immune-inflammatory response genes and the risk of deficit schizophrenia. Schizophr Res 193:359–363
Miyamoto K et al (2020) CTLA4 mRNA expression in blood is lower in schizophrenia, but not in affective disorders. Asian J Psychiatr 52:102112
Jun T-Y et al (2002) Polymorphism of CTLA-4 gene at position 49 of exon 1 may be associated with schizophrenia in the Korean population. Psychiatry Res 110(1):19–25
Cai L et al (2020) Association of the soluble CTLA4 with schizophrenia: an observational study. J Bio-X Res 3(03):116–122
Sumirtanurdin R et al (2019) Single-nucleotide polymorphism of CTLA-4 (rs5742909) in correlation with Schizophrenia Risk factor. J Pharm Bioallied Sci 11(Suppl 4):S605–s610
Walunas TL et al (1994) CTLA-4 can function as a negative regulator of T cell activation. Immunity 1(5):405–413
Hosseini A et al (2020) CTLA-4: from mechanism to autoimmune therapy. 80:p106221
Wang XB et al (2002) Abnormal expression of CTLA-4 by T cells from patients with myasthenia gravis: effect of an AT-rich gene sequence. J Neuroimmunol 130(1–2):224–232
Oaks MK et al (2000) A native soluble form of CTLA-4. Cell Immunol 201(2):144–153
Wong HK et al (2006) Increased expression of CTLA-4 in malignant T cells from patients with Mycosis Fungoides – cutaneous T-Cell lymphoma. J Invest Dermatology 126(1):212–219
Devaraju P et al (2014) The CTLA4 + 49 A/G (rs231775) polymorphism influences susceptibility to SLE in South Indian tamils. Tissue Antigens 83(6):418–421
Liu Z et al (2015) Association between CTLA-4 rs231775 polymorphism and hepatocellular carcinoma susceptibility. Int J Clin Exp Pathol 8(11):15118–15122
Williams JL, Klein RS (2017) Blood-brain barrier dysfunction during Central Nervous System Autoimmune diseases. Blood Brain Barrier Inflamm, : p. 175–186
Mewes C et al (2018) The CTLA-4 rs231775 GG genotype is associated with favorable 90-day survival in caucasian patients with sepsis. Sci Rep 8(1):15140
Jun TY et al (2001) Polymorphism of CTLA-4 gene for major depression in the Korean population. J Neuropsychiatry Clin Neurosci 55(5):533–537
Van Nguyen S et al (2021) Cytotoxic T-lymphocyte antigen-4 (CTLA-4) gene polymorphism (rs3087243) is related to risk and survival in patients with colorectal cancer in vivo. 35(2):969–975
Acknowledgements
The images presented in this paper were prepared using Adobe Illustrator 2023 software.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fayedeh, F., Khorashadizadeh, S., Yousefi, M. et al. CTLA-4 expression and polymorphisms in Schizophrenia; a systematic review of literature. Mol Biol Rep 51, 431 (2024). https://doi.org/10.1007/s11033-024-09299-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09299-7