Abstract
Background
Transmission Assessment Survey (TAS) is the WHO recommended method used for decision-making to stop or continue the MDA in lymphatic filariasis (LF) elimination programme. The WHO has also recommended Molecular Xenomonitoring (MX) of LF infection in vectors as an adjunct tool in settings under post-MDA or validation period. Screening of non-vectors by MX in post-MDA / validation settings could be useful to prevent a resurgence of LF infection, as there might be low abundance of vectors, especially in some seasons. In this study, we investigated the presence of LF infection in non-vectors in an area endemic for LF and has undergone many rounds of annual MDA with two drugs (Diethylcarbamazine and Albendazole, DA) and two rounds of triple drug regimens (Ivermectin + DA).
Methods and results
Mosquitoes were collected from selected villages of Yadgir district in Karnataka state, India, during 2019. A total of 680 female mosquitoes were collected, identified morphologically by species and separated as pools. The female mosquitoes belonging to 3 species viz., Anopheles subpictus, Culex gelidus and Culex quinquefaciatus were separated, pooled, and the DNA extracted using less expensive method and followed by LDR based real-time PCR assay for detecting Wuchereria bancrofti infection in vector as well as non-vector mosquitoes. One pool out of 6 pools of An. subpictus, 2 pools out of 6 pools of Cx. gelidus, and 4 pools out of 8 pools of Cx. quinquefaciatus were found to be positive for W. bancrofti infection by RT-PCR. The infection rate in vectors and non-vectors was found to be 1.8% (95% CI: 0.5–4.2%) and 0.9% (95% CI: 0.2–2.3%), respectively.
Conclusions
Our study showed that non-vectors also harbour W. bancrofti, thus opening an opportunity of using these mosquitoes as surrogate vectors for assessing risk of transmission to humans in LF endemic and post MDA areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To combat one of the most debilitating diseases, lymphatic filariasis (LF), the Global Programme for Elimination of Lymphatic Filariasis (GPELF) was launched in 2000, which involved annual Mass Drug Administration (MDA) with anti-filarial drugs for parasite control and Morbidity Management and Disability Prevention (MMDP) for disease control and management [1]. In 2019, 538.1 million people were treated for lymphatic filariasis (LF) in 38 countries that implemented MDA with 2 drugs Diethylcarbamazine and Albendazole (DA) to populations at risk of the disease [1]. Recently, a combination of 3 drugs viz., Ivermectin, Diethylcarbamazine and Albendazole (IDA) was administered to 45.2 million people in 11 countries [1]. In India, IDA was extended from 4 to 16 districts to treat more than 41 million people as recommended by the World Health Organization [1] The target set by GPELF in 2000 to eliminate LF as a public health problem globally was 2020, but this target was reset to 2030 in alignment with the new Neglected Tropical Diseases (NTD) Road Map [1]. New global estimates suggest a 74% reduction in the number of infected people since the start of GPELF [1].
The GPELF realized that, even when it is succeeded in reducing infection and disease, it would need robust method(s) to know if the transmission was truly interrupted and there was no recrudescence. The immunochromatographic test (ICT) to detect the circulating parasite antigen (CFA) was extremely important in the TAS, because of its ease of use, rapidity and ability to test at any time of day, thereby averting issues with nocturnal periodicity of the principal LF parasite. Still, the GPELF realized that situation may arise towards the ‘endgame’ when supplementary tool(s) might be required to ensure that LF was truly eliminated. This ignited an interest to use infection in vectors, apart from that in humans, as endpoints for monitoring LF elimination [2]. WHO has recommended Molecular Xenomonitoring (MX) as an adjunct tool to the TAS, to make decisions on stopping MDA [3, 4].
Xenomonitoring (MX) is broadly defined as the detection of the pathogen in vectors, which is done conventionally by observing the presence of parasites stages in dissected vectors by microscopy. It is useful for monitoring infection in the community as it is non-invasive and does not require to contact humans. Initially xenomonitoring started in later 1970 in West Africa for assessing onchocerciasis infective larvae in blackflies for calculating annual transmission potential under the Onchocerciasis Control Programme (OCP) [2]. This technique was later adopted to LF vector surveillance and also for malaria surveillance in the communities [2]. Microscopic dissection is labour intensive and also requires well experienced technicians to dissect vectors for identification of larvae in the vectors. Later, PCR tools to detect DNA or RNA of the parasite in vectors, called Molecular xenomonitoring (MX) tools, were developed and found to be highly sensitive methods. A PCR assay developed to amplify Ssp I repeat sequence of 188 bp was found to be highly specific and sensitive to detect W. bancrofti in human blood [5] as well as in pools of vector mosquitoes, Culex pipiens [6]. MX is considered as a proxy for human infection in the community. The development of qPCR assays for detection of W. bancrofti and B. malayi DNA in different vectors has improved significantly since the development of a simple DNA extraction method for high-throughput detection of W. bancrofti in mosquitoes [7].
In India, all the 339 filariasis endemic districts are covered under LF elimination programme and MDA was stopped in 133 districts based on transmission assessment survey (TAS). With elimination goal around the corner, one can expect more number of endemic districts to move towards post-MDA/validation phases, which requires constant surveillance to ensure that the transmission interruption is sustained. It is to be noted that WHO has recommended MX to be used as a supplementary tool in assessing interruption of transmission [8,9,10,11,12] as it is more sensitive than TAS, particularly for post-MDA/validation surveillance. Generally, for MX of LF vectors known to transmit the filarial parasites are used as they are expected to harbour the parasites. But, our earlier studies have shown that even the mosquitoes in the LF endemic areas, that are not known to transmit the filarial infection (non-vectors) also harbour filarial parasites if they happen to feed on microfilaraemic blood, although they may not or poorly transmit the infection [13]. This opens up a possibility of using such non-vectors as surrogate vectors. Incidentally, the gravid traps used for collecting vector mosquitoes, also collect other mosquitoes which are non-vectors and hence provides an opportunity. Recently, it was reported that Culex tritaeniorhynchus, a vector for Japanese Encephalitis (JE) and a non-vector for LF, was found to harbour filarial parasite DNA [14]. In this study we attempted to assess the infection of filarial parasite by Real-time PCR assay in pools of wild-caught non-vector mosquitoes namely Culex gelidus and Anopheles subpictus collected from Yadgir, a LF endemic district in Karnataka state, India. The findings are discussed on the possibility of non-vectors transmitting LF infection and its implications on LF elimination.
Materials and methods
Study area
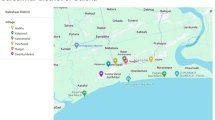
The study areas were villages in two taluks in Yadgir of Yadgir district, Karnataka state, India, having a population of 1,174,271 (as per census of 2011) and known to be endemic for bancroftian filariasis with persistent infection levels according to multicentric trial that was conducted to assess the safety, efficacy and tolerability of IDA against DA. The Mf prevalence ranged between 1.7 and 7.1% in the villages of Yadgir district [15,16,17,18,19]. The mosquito survey was conducted during the month of July 2019, at the time of intermittent rainy season and mosquitoes were collected from 4 wards of Yadgir urban area and four villages in and around Yadgir town (Fig. 1). The main vector for W. bancrofti transmission in this area is Culex quinquefasciatus.
Mosquito collection
Mosquitoes were collected by using modified CDC Gravid traps with hay infusion as oviposition attractant (Subramanian et al. 2017). The traps were set up at 6.00 pm, close to houses, in the selected villages. The traps were setup in dark places near septic tanks, open bathrooms, shrubs without direct sunlight and air movement, avoiding traditional kitchens. The cloth cage was removed 6.00 am next day morning and were transported to the field laboratory for identification and segregation of mosquitoes. The mosquitoes trapped in the cage of the trap were collected using battery operated suction pump.
Mosquito identification, segregation and pooling
The collected mosquitoes were anaesthetized using ether, morphologically identified the species viz., Culex gelidus, Anopheles subpictus and Culex quinquefasciatus using the key [20]. Species wise, female mosquitoes were segregated as fully fed, semi gravid and gravid. These female mosquitoes were pooled (1–25 per pool) species-wise in 2.0 ml microfuge tubes and dried overnight on a dry bath at 95°C before DNA extraction.
DNA extraction
DNA was extracted from pools of dried mosquitoes by TE buffer (pH 8.0) based method [21]. 100 µl of TE buffer with minor modification in which cost-effective steel ball bead (3 mm) was added instead of Zinc Ball bearing beads (4.5 mm) for homogenization using a tissue lyzer (Qiagen II) for 15 min with 25 frequencies followed by keeping the samples in boiling water bath up to 10–11 min. The homogenate was centrifuged at 14,000 rpm for 10 min and the supernatant separated and used for LDR based real time PCR assay.
Real time PCR assay
In our earlier study, DNA obtained through TE-based extraction method was suitable for successful amplification of 188-bp amplicon of W. bancrofti from pools of ten mosquitoes by conventional Ssp I PCR assay [21]. One micro litre of the extracted DNA was used as template in LDR real-time PCR (CFX96 Real time PCR system, Bio-Rad, Germany) as described earlier [22] along with 1 ng (strong positive), 100 pg (moderate positive) and 10 pg (low positive) of genomic DNA samples as positive controls and NTC (water) controls. Real-time PCR reactions were performed with 12.5 µl of Fast start essential Probe Master Mix (Roche) along with 10 μm of “long dispersed repeat” forward primer (LDR1-5′ATTTTGATCATCTGGGAACGTTAATA-3′; reverse primer LDR2-′CGACTGTCTAATCCATTCAGAGTGA) and 10 μm Taq man probe (6FAMATCTGCCCATAGAAATAACTACGGTGGATCTGTAMRA) in a final volume of 25 µl. Cycling parameters used were 50 °C for 2 min, 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The Cycle threshold (Ct) values of samples (Table 1) ranging from 1.0 to 39.0 were considered positive, and samples that failed to reach the fluorescence threshold beyond 39 were considered indeterminate and repeated to confirm the negativity or positivity of those samples [22] (Fig. 2). The positive real-time PCR product of W. bancrofti were analyzed by 2% of agarose gel electrophoresis for the detection and confirmation of LDR specific band of the real-time PCR assay.
Data analysis
The percentage of pools positive (pool positivity rate) for W. bancrofti DNA was calculated as the ratio of number of pools positive for W. bancrofti DNA to total number of pools screened. The prevalence of W. bancrofti parasite DNA (maximum likelihood estimates with 95% confidence interval) was estimated with Pool Screen software 2.0.3 [23, 24]. (Table 1).
Results
A total of 680 mosquitoes were collected and out of this 314 were vectors of filariasis (Cx. quinquefasciatus), and 366 were non-vectors (245 An. subpictus and 121 Cx. gelidus) (Tables 1 and 2). The vector mosquitoes (Cx. quinquefasciatus) formed 46%, while non-vectors (Cx. gelidus and An. subpictus) constituted 52% of the total collections. Majority of the collections were from areas of Yadgir municipality and Baddepalli under Ajlapur Primary Health Centre (PHC), followed by Putpak village under Kandkur Community Health Centre (CHC) (Table 2). Cx. gelidus were collected in more numbers from 4 wards of urban areas of Yadgir municipality, and Cx. quinquefasciatus collected from only 2 wards. On the other hand, majority of collections from Baddepalli were An. subpictus with a small number of Cx. quinquefasciatus collections. High density of Cx. gelidus was observed in urban areas of Yadgir municipality whereas in the semi-urban or surrounding areas, high density of An. subpictus was observed. All collections from Putpak village under Kandkur CHC, Mundargi under Honagera PHC, and Yelheri under Yelheri CHC were Cx. quinquefasciatus.
The mosquitoes collected were grouped into 20 pools. Upon subjecting to RT-PCR assay for W. bancrofti infection, 4 pools out of 8 pools of Cx. quinquefaciatus (pool positivity rate: 50%), 2 pools out of 6 pools of Culex gelidus (33.3%), and 1 pool out of 6 pools of An. subpictus (16.7%) were found to be positive for W. bancrofti infection (Table 2). However, the pool positivity rates were not significantly different (P value = 0.53) and corroborated with the overalapping 95% CI for parasite DNA prevalence 1.77%, (95%CI: 0.54–4.18) in Cx. quinquefasciatus and 1.87%, (95% CI: 0.34–5.29) in Cx. gelidus. The prevalence of W. bancrofti DNA in vectors and non-vectors was found to be 1.8% (95% CI: 0.5–4.2%) and 0.9% (95% CI: 0.2–2.3%) respectively, higher than the provisional threshold of 0.25% with upper limit of 2.3% (Table 1) indicating the risk of on-going transmission.
Discussion
India has targeted LF elimination by 2027, ahead of the global target of 2030 [25]. In view of this, surveillance and monitoring for residual infection in endemic and Post-MDA areas to assess on-going transmission is necessary, either through routine blood surveys in humans and entomological surveys for vectors.
In one of the study sites, Ward 31 of Yadgir, though pools of both the vector mosquito, Cx. quinquefasciatus, and non-vector mosquito, Cx. gelidus were positive for W. bancrofti infection by Real-time PCR there was no significant difference in both pool positivity rates and W. bancrofti parasite DNA rates. This observation indicates the possibility of transmission or circulation of W. bancrofti and/or the susceptibility of non-vectors in these areas. However, their role in transmission needs to be verified by the presence of infective stage (L3) of the parasites, either by dissection or by L3 stage-specific PCR assay. This finding imposes surveillance and monitoring of the local non-vectors also for LF infection.
In India, primary vector of LF is Cx. quinquefaciatus. The development of filarial larvae in vectors having vector competency is related to limitation and facilitation proportionally which implies microfilariae (Mf) developing into L3. This in turn is dependent on the number of Mf actually ingested by the vectors and whether increase or decrease of L3 larvae [26]. Vector parasite relationship limits the transmission capacity.
Most of the vector competency studies are laboratory studies based on the development of B. malayi larvae in mosquitoes [27, 28]. Some studies reported that non -vector transmitting the parasite in the community possibly due to sudden climatic changes which may be adopted to develop parasite larvae in the non-vector mosquitoes acting as vector in some countries [29,30,31,32,33,34,35,36,37].
Limited laboratory and field studies were carried out to find B. malayi infection in non-vector mosquitoes by dissection but not much was reported on the development of L3 larvae in the experiment. These reports have shown that B. malayi DNA was detected in Cx. pipiens and other Culex species [27, 28]. Various field studies have proved non-vectors are as competent as the vectors in transmitting B. malayi and W. bancrofti [14]. Studies elsewhere also reported that the field collected mosquitoes of various species viz., Cx. tritaeniorhynchus and Cx.gelidus as a JE vector in India [38]. In Indonesia, B. malayi DNA was detected in Cx. tritaeniorhynchus by PCR assay [14]. This was not previously considered primary vector of B. malayi, but this result suggested that these mosquitoes might play a role in transmitting B. malayi in the Balangan district, Indonesia [14]. Anopheles gambiae and An. funestus are main vectors in Ghana and a recent report showed that An. melas could also transmit W. bancrofti microfilaraemia at low level [29]. Mansonia species viz., Ma. africana, Ma. uniformis, which are non-vectors were found to harbour larvae of W. bancrofti larvae upon dissection and confirmed by PCR assay [30] and there was a first report in Ghana and in West Africa since in Guinea [30]. The study reported that Anopheles species had genetic differentiation and insecticide resistance influencing the vector density in West Africa [31]. It was found that Culex quinquefaciatus a non-vector harboured W. bancrofti larvae in Nigeria [32, 33].
Earlier report indicated that second and third stage (infective) larvae, one each were found in wild caught An. subpictus mosquitoes collected from Indonesia which were identified by dissection [34] An. subpictus was reported to be the most important vector of W. bancrofti on Flores, an island near Alor [35, 36] as has been seen in Yadgir. This mosquito which was earlier considered as a local non-vector has been found to naturally pick up LF infection, which also could lead to active transmission in the study area. In a similar study reported the presence of W. bancrofti DNA in non-vectors such as Cx. quinquefasciatus, Ae. aegypti, Ae.(Finalaya) spp and Ae.upolensis in American Samoa [39]. Spacio-temporal effect influences the adaptability behaviour in LF vectors Anopheles species of An. arabiensis, An. gambiae, An. merus, An. melas, and An. funestus. in Saharan Africa [40]. MX has been observed as a potential predictor of human filarial infection transmitted by different filarial vectors in wide-range of environmental situations [41, 42].
While Mf prevalence of 1% was found with the presence of both the species of parasites B. malayai and W. bancrofti, xenomonitoring revealed infection of vectors with parasites indicating ongoing transmission 5 years Post-MDA in Thailand [43]. Elsewhere, MX showed diurnally sub-periodic W. bancrofti infection in Aedes niveus post nine rounds of MDA in Nancowry Islands and Andaman Nicobar Islands [44]. Another study that monitored resting and feeding behaviour of vectors showed co-infection of W. bancrofti and Plasmodium falciparum in the vectors of Burkin Faso suggesting the possibility of an integrated surveillance [45]. MX detected W. bancrofti in vector mosquitoes in the city of São Luís and hence the risk of transmission in the community of Brazil [46].
As both vector and non-vectors are influenced by seasonal climatic changes, a sudden surge in vector density of these species could trigger adoptability of parasite development which could lead to higher chances of picking up LF infection from infected individuals. Hence, it is necessary to have non-vectors also under vector surveillance, especially in the endgame of elimination [29,30,31,32,33,34,35,36,37,38].
Recently MX tool expanded to other vector species similar to our study that showed vector species Anopheles to be positive for W. bancrofti infection in Country Kenya [47], Cx. quinquefasciatus in Tanzania [48], Anopheles in Coastal Kenya [49]. The assessment of various collection methods and different region LF vectors were explained [50]. Recently as per guidelines of WHO Comparative assessment of national protocol and mini-TAS were assessed in Indian settings of two non-endemic districts of Odisha. In this study MX, a non invasive method to assess LF transmission in community was found to be cost effective compared to mini-TAS [51].
This is a preliminary study in Yadgir that has made an attempt to see the possible role of local vectors and non-vectors in the transmission of LF in an endemic area. In Yadgir, in spite of several rounds of MDA the expected decline in the infection levels in humans were not achieved and this could be due to many reasons like sub-optimal coverage, high densities of the vector and local non-vectors, persisting high Mf density in infected individuals, lack of awareness about MDA etc. Added to these, this finding of the risk of non-vectors circulating parasite of LF is really a cause of concern, particularly for the local health authorities and as such for the country, in achieving the LF elimination. Studies in many countries indicated that non-vectors are acting as vectors for transmitting filariasis infection [14, 30, 31]. This finding sounds an alert that particularly in high endemic areas, routine surveillance of vector mosquitoes alone may not be sufficient and regular/frequent monitoring of local non-vectors for LF infection, will help the programme to take appropriate vector control measures to bring down the man-vector contact, particularly when there is an on-going MDA programme in place. The findings in this study also emphasize the need for the combination of both vector control alongside of MDA which will reduce the mosquito (both vector and non-vector) density thereby reducing transmission to the human hosts.
Conclusion
The findings of this study indicated the presence of LF infection in vector and non-vector mosquitoes and hint at the risk of transmission in the community. Microscopy is the gold standard but dissection and observation of parasite larvae in mosquitoes is difficult to differentiate from mosquito muscles, and hence trained and experienced personnel is needed to avoid false negative which may lead to wrong decision in the process of elimination. MX over comes these short comings and serves a complimentry tool for assessing LF transmission in human by processing large number of mosquito samples using less expensive method of DNA extraction. The cost of DNA extraction is cheaper and is about US dollar 0.21 /per pool to detect infection in pools of vector and local non-vector mosquitoes in the endemic / resurgence in post-MDA area. The present study has hinted at the probable changes in transmission dynamics of vector and therefore on the need for the future surveillance to be focused on both vector and non-vector and also competency study, particularly in areas where the infection is persistent after many rounds of MDA. The antigen based assays are less sensitive than molecular assay. The MX could be the potential surveillance tool for vector surveillance and may also offer early signal for the parasite circulation in the community. This study suggests that vector control for seasonal vector abundance and reinforcement of surveillance activities may be helpful for LF elimination programme.
Data availability
Extracted DNA and supporting data for the real time PCR assay are stored properly. The raw data used for the analysis during this study are available upon reasonable request.
Abbreviations
- TAS:
-
Transmission Assessment Survey
- WHO:
-
World Health Organization
- Mf:
-
Microfilariae
- LDR:
-
Long dispersed repeat
- CFA:
-
Circulating filarial antigen
- RT-PCR:
-
Real time Polymerase Chain Reaction
- MX:
-
Molecular Xenomonitoring
- NTD:
-
Neglected Tropical Diseases
- DA:
-
Diethyl carbamazine and Albendazole
- MDA:
-
Mass Drug Administration
- ICT:
-
Immuno Chromatographic Test
- OCP:
-
Onchocerciasis Control Programme
- DNA:
-
Deoxyribonucleic acid
- RNA:
-
Ribonucleic acid
- PCR:
-
Polymerase Chain Reaction
- IDA:
-
Ivermectin, Diethylcabamazine and Albendazole
- GPELF:
-
Global Programme for Elimination of Lymphatic Filariasis
- MMDP:
-
Morbidity Management and Disability Prevention
- CDC:
-
Center for Disease Control and Prevention
References
Cameron MM, Ramesh A (2021) The use of molecular xenomonitoring for surveillance of mosquito-borne diseases. Philosophical Trans Royal Soc B 376(1818):20190816. https://doi.org/10.1098/rstb.2019.0816
WHO (2011) Monitoring and epidemiological assessment of mass drug administration in the global programme to eliminate lymphatic filariasis: a manual for national elimination programmes. Geneva: World Health Organization. Available: http://apps.who.int/iris/bitstream/10665/44580/1/9789241501484_eng.pdf. Accessed 14 January 2014
WHO (2013) Practical entomology in the global programme to eliminate lymphatic filariasis: a handbook for national elimination programmes. Geneva: World Health Organization. Available: http://apps.who.int/iris/bitstream/10665/87989/1/9789241505642_eng.pdf. Accessed 14 January 2014
Zhong M, McCarthy J, Bierwert L, Lizotte-Waniewski M, Chanteau S, Nutman TB, Ottesen EA, Williams SA (1996) A polymerase chain reaction assay for detection of the parasite Wuchereria bancrofti in human blood samples. Am J Trop Med Hyg 54(4):357–363. https://doi.org/10.4269/ajtmh.1996.54.357
Chanteau S, Luquiaud P, Failloux AB, Williams SA (1994) Detection of Wuchereria bancrofti larvae in pools of mosquitoes by the polymerase chain reaction. Trans R Soc Trop Med Hyg 88(6):665–666. https://doi.org/10.1016/0035-9203(94)90219-4
Vasuki V, Subramanian S, Hoti SL, Jambulingam P (2012) Use of a simple DNA extraction method for high-throughput detection of filarial parasite Wuchereria bancrofti in the vector mosquitoes. Parasitol Res 111(6):2479–2481. https://doi.org/10.1007/s00436-012-3026-3
Rao RU, Nagodavithana KC, Samarasekera SD, Wijegunawardana AD, Premakumara WD, Perera SN, Settinayake S, Miller JP, Weil GJ (2014) A comprehensive assessment of lymphatic filariasis in Sri Lanka six years after cessation of mass drug administration. PLoS Negl Trop Dis 8(11):e3281. https://doi.org/10.1371/journal.pntd.0003281
Rao RU, Samarasekera SD, Nagodavithana KC, Dassanayaka TDM, Punchihewa MW, Ranasinghe USB, Weil GJ (2017) Reassessment of areas with persistent lymphatic filariasis nine years after cessation of mass drug administration in Sri Lanka. PLoS Negl Trop Dis 11(10):e0006066. https://doi.org/10.1371/journal.pntd.0006066
Rao RU, Samarasekera SD, Nagodavithana KC, Goss CW, Punchihewa MW, Dassanayaka TDM, Ranasinghe USB, Mendis D, Weil GJ (2018) Comprehensive Assessment of a Hotspot with Persistent Bancroftian Filariasis in Coastal Sri Lanka. Am J Trop Med Hyg 99(3):735–742. https://doi.org/10.4269/ajtmh.18-0169
Subramanian S, Jambulingam P, Chu BK, Sadanandane C, Vasuki V, Srividya A, Mohideen AbdulKader MS, Krishnamoorthy K, Raju HK, Laney SJ, Williams SA, Henderson RH (2017) Application of a household-based molecular xenomonitoring strategy to evaluate the lymphatic filariasis elimination program in Tamil Nadu, India. PLoS Negl Trop Dis 11(4):e0005519. https://doi.org/10.1371/journal.pntd.0005519
Subramanian S, Jambulingam P, Krishnamoorthy K, Sivagnaname N, Sadanandane C, Vasuki V, Palaniswamy C, Vijayakumar B, Srividya A, Raju HKK (2020) Molecular xenomonitoring as a post-MDA surveillance tool for global programme to eliminate lymphatic filariasis: field validation in an evaluation unit in India. PLoS neglected tropical diseases PLoS. Negl Trop Dis 14(1):e0007862. https://doi.org/10.1371/journal.pntd.0007862
Paily KP, Hoti SL, Balaraman K (2006) Development of lymphatic filarial parasite Wuchereria bancrofti (Spirurida: Onchocercidae) in mosquito species (Diptera: Culicidae) fed artificially on microfilaremic blood. J Med Entomol 43(6):1222–1226. https://doi.org/10.1603/0022-2585(2006)43[1222:dolfpw]2.0.co;2
Supriyono S, Tan S (2020) DNA of Brugia malayi detected in several mosquito species collected from Balangan District, South Borneo Province, Indonesia. Vet World 13(5):996–1000. https://doi.org/10.14202/vetworld.2020.996-1000
Jambulingam, P., Kuttiatt, V. S., Krishnamoorthy, K., Subramanian, S., Srividya, A.,Raju, H. K. K., … Weil, G. J. (2021). An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India.PLoS Negl Trop Dis 15(2):e0009069. https://doi.org/10.1371/journal.pntd.0009069
Kuttiatt VS, Somani RK, Swaminathan S, Krishnamoorthy K, Weil GJ, Purushothaman J (2020) Frequency and clinical significance of localized adverse events following mass drug administration for lymphatic filariasis in an endemic area in South India. Am J Trop Med Hyg 102(1):96. https://doi.org/10.4269/ajtmh.19-0532
Shivalingaiah AH, Ravikumar K, Gurupadaswamy SM (2019) Evaluation of coverage and compliance to mass drug administration for lymphatic filariasis elimination in two endemic districts of Karnataka. Int J Community Med Public Health 6(8):3583–3587. https://doi.org/10.18203/2394-6040.ijcmph20193492
Weil GJ, Bogus J, Christian M, Dubray C, Djuardi Y, Fischer PU, DOLF IDA Safety Study Group (2019) The safety of double-and triple-drug community mass drug administration for lymphatic filariasis: a multicenter, open-label, cluster-randomized study. PLoS Med 16(6):e1002839
Krentel, A., Basker, N., Beau de Rochars, M., Bogus, J., Dilliott, D., Direny, A.N., … Weil, G. J. (2021). A multicenter, community-based, mixed methods assessment of the acceptability of a triple drug regimen for elimination of lymphatic filariasis.PLoS Negl Trop Dis 15(3):e0009002
Barraud PJ (1934) The Fauna of British India, including Ceylon and Burma. Diptera. Family Culieldae. Tribes Megarhinini and Culicini. The Fauna of British India, including Ceylon and Burma. Diptera, vol 5. Vol. 5. Family Culieldae. Tribes Megarhinini and Culicini.
Vasuki V, Hoti SL, Sadanandane C, Jambulingam P (2003) A simple and rapid DNA extraction method for the detection of Wuchereria bancrofti infection in the vector mosquito, Culex quinquefasciatus by Ssp I PCR assay. Acta Trop 86(1):109–114. https://doi.org/10.1016/s0001-706x(02)00267-x
Rao RU, Atkinson LJ, Ramzy RM, HelmyH, Farid HA, Bockarie MJ, Susapu M, Laney SJ, Williams SA, Weil GJ (2006) A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. Am J Trop Med Hyg 74(5):826–832
Katholi CR, Unnasch TR (2006) Important experimental parameters for determining infection rates in arthropod vectors using pool screening approaches. Am J Trop Med Hyg 74(5):779–785
Katholi CR, Toé L, Merriweather A, Unnasch TR (1995) Determining the prevalence of Onchocerca Volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J Infect Dis 172(5):1414–1417. https://doi.org/10.1093/infdis/172.5.1414
Ministry of Health and Family Welfare (India). Ministry of Health & Family Welfare launches nationwide (LF); 2023 [Cited 2023 June 11]. [Internet]. https://pib.gov.in/PressReleasePage.aspx?PRID=1898214
Subramanian S, Krishnamoorthy K, Ramaiah KD, Habbema JD, Das PK, Plaisier AP (1998) The relationship between microfilarial load in the human host and uptake and development of Wuchereria bancrofti microfilariae by Culex quinquefasciatus: a study under natural conditions. Parasitology 116(Pt 3):243–255. https://doi.org/10.1017/s0031182097002254
Erickson SM, Fischer K, Weil GJ, Christensen BM, Fischer PU (2009) Distribution of Brugia malayi larvae and DNA in vector and non-vector mosquitoes: implications for molecular diagnostics. Parasit Vectors 2(1):56. https://doi.org/10.1186/1756-3305-2-56
Fischer P, Erickson SM, Fischer K, Fuchs JF, Rao RU, Christensen BM, Weil GJ (2007) Persistence of Brugia malayi DNA in vector and non-vector mosquitoes: implications for xenomonitoring and transmission monitoring of lymphatic filariasis. Am J Trop Med Hyg 76(3):502–507
AmuzuH, Wilson MD, Boakye DA (2010) Studies of Anopheles gambiae s.l (Diptera: Culicidae) exhibiting different vectorial capacities in lymphatic filariasis transmission in the Gomoa district. Ghana Parasit Vectors 3:85. https://doi.org/10.1186/1756-3305-3-85
Ughasi J, Bekard HE, Coulibaly M, Adabie-Gomez D, Gyapong J, Appawu M, Wilson MD, Boakye DA (2012) Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasit Vectors 5:89. https://doi.org/10.1186/1756-3305-5-89
de Souza DK, Koudou B, Kelly-Hope LA, Wilson MD, Bockarie MJ, Boakye DA (2012) Diversity and transmission competence in lymphatic filariasis vectors in West Africa, and the implications for accelerated elimination of Anopheles-transmitted filariasis. Parasit Vectors 5:259. https://doi.org/10.1186/1756-3305-5-259
Anosike JC, Nwoke BE, Ajayi EG, Onwuliri CO, Okoro OU, Oku EE, Asor JE, Amajuoyi OU, Ikpeama CA, Ogbusu FI, Meribe CO (2005) Lymphatic filariasis among the Ezza people of Ebonyi State, eastern Nigeria. Ann Agric Environ Med 12(2):181–186
Agi PI, Ebenezer A (2009) Observations on filarial infection in Amassoma community in the Niger Delta. Nigeria J Appl SCI Environ Manag 13(1)
Atmosoedjono S, Dennis DT (1977) Anopheles Aconitus and An. Subpictus naturally infected with Wuchereria bancrofti in Flores. Indonesia Mosq News 37(3)
Hoedojo, Partono F, Atmosoedjono S, Purnomo, Teren T (1980) A study on vectors of bancroftian filariasis in West Flores, Indonesia. Southeast Asian J Trop Med Public Health 11(3):399–404
Lee VH, Atmosoedjono S, Dennis DT, Suhaepi A, Suwarta A (1983) The anopheline (Diptera: Culicidae) vectors of malaria and bancroftian filariasis in Flores Island, Indonesia. J Med Entomo 20(5):577–578. https://doi.org/10.1093/jmedent/20.5.577
Dorkenoo MA, de Souza DK, Apetogbo Y, Oboussoumi K, Yehadji D, Tchalim M, Etassoli S, Koudou B, Ketoh GK, Sodahlon Y, Bockarie MJ, Boakye DA (2018) Molecular xenomonitoring for post-validation surveillance of lymphatic filariasis in Togo: no evidence for active transmission. Parasit Vectors 11(1):52. https://doi.org/10.1186/s13071-017-2611-9
Ramesh D, Muniaraj M, Samuel PP, Thenmozhi V, Venkatesh A, Nagaraj J, Tyagi BK (2015) Seasonal abundance & role of predominant Japanese encephalitis vectors Culex Tritaeniorhynchus & Cx. Gelidus Theobald in Cuddalore district, Tamil Nadu. Indian J Med Res 142(Suppl 1):S23–S29. https://doi.org/10.4103/0971-5916.176607
Schmaedick M A, Koppel A L, Pilotte N, Torres M, Williams S A, Dobson S L, … Won K Y (2014). Molecular xenomonitoring using mosquitoes to map lymphatic filariasis after mass drug administration in American Samoa. PLoS Negl Trop Dis 8(8):e3087. https://doi.org/10.1371/journal.pntd.0003087
Sinha A, Kumar S, Dayal D, Yadav V, Pramanik A, Chaubey KK, Kumar S (2023) Elimination of lymphatic filariasis: where do we stand so far? Asian Pac J Trop Med 16(9):385–399. https://doi.org/10.4103/1995-7645.380729
Pryce J, Reimer LJ (2021) Evaluating the diagnostic test accuracy of molecular xenomonitoring methods for characterizing community burden of lymphatic filariasis. Clin Infect Dis 72(Supplement3):S203–S209. https://doi.org/10.1093/cid/ciab197
Pryce J, Unnasch TR, Reimer LJ (2021) Evaluating the diagnostic test accuracy of molecular xenomonitoring methods for characterising the community burden of Onchocerciasis. PLoS Negl Trop Dis 15(10):e0009812. https://doi.org/10.1371/journal.pntd.0009812
Meetham P, Kumlert R, Gopinath D, Yongchaitrakul S, Tootong T, Rojanapanus S, Padungtod C (2023) Five years of post-validation surveillance of lymphatic filariasis in Thailand. Infect Dis Poverty 12(1):113. https://doi.org/10.1186/s40249-023-01158-0
Premkumar A, Shriram AN, Krishnamoorthy K, Subramanian S, Vasuki V, Vijayachari P, Jambulingam P (2020) Molecular xenomonitoring of diurnally subperiodic Wuchereria bancrofti infection in Aedes (Downsiomyia) niveus (Ludlow, 1903) after nine rounds of Mass Drug Administration in Nancowry Islands, Andaman and Nicobar Islands, India. PLoS Negl Trop Dis 14(10):e0008763 https://doi.org/10.1371/journal.pntd.0008763
Coulibaly S, Sawadogo SP, Hien AS, Nikièma AS, Sangaré I, Rabila B, … Dabiré RK (2021)Malaria and lymphatic filariasis co-transmission in endemic health districts in Burkina Faso. Adv. entomol 9(4):155–175. https://doi.org/10.4236/ae.2021.94014
Araújo TAD, Lima de Albuquerque A, Melo DCTVD, Santos EMDM, Oliveira ALSD, Ayres CFJ, Oliveira CMFD (2023) Detection of Wuchereria bancrofti in the city of São Luís, state of Maranhão, Brazil: New incursion or persisting problem? PLoS Negl Trop Dis 17(1):e0011091. https://doi.org/10.1371/journal.pntd.0011091
Kinyatta N, Wachira D, Githae R, Lusweti J, Ingonga J, Ichugu C, Kamau L (2023) Detection of Wuchereria bancrofti in human blood samples and mosquitoes in Matayos, Busia County-Kenya. Sci Rep 13(1):19420. https://doi.org/10.1038/s41598-023-46329-z
Lupenza E, Gasarasi DB, Minzi OM (2021) Lymphatic filariasis, infection status in Culex quinquefasciatus and Anopheles species after six rounds of mass drug administration in Masasi District, Tanzania. Infect Dis Poverty 10:1–11. https://doi.org/10.1186/s40249-021-00808-5
Njenga SM, Kanyi HM, Mwatele CM, Mukoko DA, Bockarie MJ, Kelly-Hope LA (2022) Integrated survey of helminthic neglected tropical diseases and comparison of two mosquito sampling methods for lymphatic filariasis molecular xenomonitoring in the River Galana area, Kilifi County, coastal Kenya. PLoS ONE 17(12):e0278655. https://doi.org/10.1371/journal.pone.0278655
Bhuvaneswari A, Shriram AN, Raju KHK, Kumar A (2023) Mosquitoes, Lymphatic Filariasis, and Public Health: A Systematic Review of Anopheles and Aedes Surveillance Strategies. Pathogens 12(12):1406. https://doi.org/10.3390/pathogens12121406
Panda BB, Krishnamoorthy K, Das A, Jain HK, Dixit S, Rahi M, … Bal M (2023) Mini-TAS as a confirmatory mapping tool for remapping areas with uncertain filarial endemicity to exclude/include for mass drug administration: A report from field validation in India. PLoS One 18(11):e0293641. https://doi.org/10.1371/journal.pone.0293641
Acknowledgements
The Authors are grateful to Dr. P. Jambulingam, Former Director, ICMR-VCRC, Puducherry for his constant encouragement for the study. Authors also thank Mr. Krishna Raj, Mr. P. Shanthakumar and Mrs. M. Shabura for their technical assistance and support. We extend our gratitude to the health authorities in Yadgir.
Funding
This work supported by ICMR-VCRC, Puducherry.
Author information
Authors and Affiliations
Contributions
Conceptualization: R.B and V.V Methodology, formal analysis and investigation: R.B and V.V Writing—original draft preparation: R.B,V.V, A.S and SLH Writing—review and editing: R. B, A K, P R A. A.S,S.S, Supervision: V.V, A.S and S.S, Mapping-KHKR.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable, oral permission obtained from head of the residence for keeping Gravid trap for mosquito collection outside of the residence.
Consent for publication
Not applicable.
Consent for publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramalingam, B., Venkatesan, V., Abraham, P.R. et al. Detection of Wuchereria bancrofti DNA in wild caught vector and non-vector mosquitoes: implications for elimination of lymphatic filariasis. Mol Biol Rep 51, 291 (2024). https://doi.org/10.1007/s11033-024-09256-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09256-4