Abstract
Background
Type 2 diabetes is characterized by insulin resistance, which manifests mainly in skeletal muscles. SIRT1 has been found to play a role in the insulin signaling pathway. However, the molecular underpinnings of SIRT1’s function in palmitate fatty acid-induced apoptosis still need to be better understood.
Methods
In this research, skeletal muscle cells are treated with palmitate to be insulin resistant. It is approached that SIRT1 is downregulated in C2C12 muscle cells during palmitate-induced apoptosis and that activating SIRT1 mitigates this effect.
Results
Based on these findings, palmitate-induced apoptosis suppressed mitochondrial biogenesis by lowering PGC-1 expression, while SIRT1 overexpression boosted. The SIRT1 inhibitor sirtinol, on the other hand, decreased mitochondrial biogenesis under the same conditions. This research also shows that ROS levels rise in the conditions necessary for apoptosis induction by palmitate, and ROS inhibitors can mitigate this effect. This work demonstrated that lowering ROS levels by boosting SIRT1 expression inhibited apoptotic induction in skeletal muscle cells.
Conclusion
This study’s findings suggested that SIRT1 can improve insulin resistance in type 2 diabetes by slowing the rate of lipo-apoptosis and boosting mitochondrial biogenesis, among other benefits.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Approximately 80% of the body’s glucose elimination, stimulated by insulin, occurs within the skeletal muscle. The skeletal muscle constitutes about 40% of the total body mass [1]. Insulin resistance in skeletal muscle represents a prominent characteristic observed in individuals with type 2 diabetes. Free fatty acids (FFAs) are observed to amass within the skeletal muscle of individuals afflicted with obesity and type 2 diabetes, manifesting as triacylglycerol, diacylglycerol, and ceramides. This accumulation of FFAs has been found to interfere with the regular cellular processes, thereby perturbing normal cell activity [2]. In the context of skeletal muscle, an abundance of (FFAs) induces insulin resistance. It disrupts mitochondrial function, resulting in cellular demise through apoptosis, a phenomenon commonly called “lipo-toxicity.” The variation in toxicity of (FFAs) is widely acknowledged to be influenced by the length and saturation levels of their carbon chains [3]. The lipotoxicity is primarily observed in the long-chain saturated (FFAs), such as palmitate and stearate. Palmitate consistently induces programmed cell death, or apoptosis, in diverse cellular populations. A plethora of in vitro studies have demonstrated that palmitate induces oxidative stress and leads to significant damage to mitochondrial DNA. This damage is closely associated with concurrent impairment of mitochondrial function, apoptosis, and inhibition of insulin signaling in skeletal myotubes [4]. Recent scientific investigations have revealed that muscle mass reduction and consequential ultrastructural impairment observed in rats is a direct consequence of excessive lipid accumulation, thereby leading to compromised functionality of the skeletal muscle tissue [5]. Prior in vitro studies have demonstrated the importance of palmitate in myocyte apoptosis. The activation of the proteasomal system, accompanied by an upregulation in programmed cell death and a downregulation in the regenerative potential of muscle tissue, collectively played a role in this phenomenon [4]. Due to this phenomenon, the inhibition of apoptosis in skeletal muscle can mitigate muscle atrophy and enhance insulin sensitivity by augmentation of mitochondrial function. The depletion of sirt1 exacerbates inflammation, senescence, apoptosis, and endothelial dysfunction [6]. The association between calorie restriction (CR) and SIRT1 acetylase activity has been observed to result in enhanced insulin Phosphoinositide 3-kinases (P13K) stimulation signaling and glucose uptake in the skeletal muscle of rats [7]. Elevated apoptosis and inflammation, both associated with skeletal muscle burns and other related consequences, contribute to insulin resistance and muscle weakening. Inducible Nitric Oxide Synthase (iNOS) is recognized as a crucial inflammatory mediator in stress-induced insulin resistance. In biological systems, Nuclear Factor Kappa B (KB- (NF) and P53 are pivotal regulators of inflammatory responses and programmed cell death, known as apoptosis [8]. Through its interaction with P65NF-KB and P53 transcription factors, SIRT1 exerts regulatory control by inhibiting their activity. Recent scientific investigations have revealed that INOS, also known as inducible nitric oxide synthase, triggers s-nitrosylation of SIRT1 [6]. This biochemical modification renders SIRT1, a protein involved in cellular regulation, inactive. Consequently, this increases the acetylation and activity of KB- (NF) P65 and P53, proteins associated with important cellular functions in various types of cells, including skeletal muscle cells. The augmentation of the inflammatory response and apoptotic changes in inducible nitric oxide synthase (iNOS) could play a role in developing muscle weakness and insulin resistance following burn injuries [9]. The available data provide support for this proposed hypothesis. In the absence of other members of the SIRT1 family, SIRT1 exerts regulatory control over the activity of Peroxisome proliferator-activated receptor-gamma coactivator (PGC-la) within the skeletal system [10]. When the SIRT1 protein is activated, or its production is increased in specific C2C12 mutant cells, there is an observed increase in the expression of PGC-1a mRNA. In contrast, the expression of PGC-la mRNA decreases in skeletal muscle. Indeed, through the process of purification, it has been discovered that SIRT1 serves as the transcriptional activator of PGC-1a. The overexpression of SIRT1 has been observed to have a diminishing effect on the process of myogenesis in C2C12 cells, as well as on the process of adipogenesis in 3T3-L1 cells. By exerting control over the expression of PGC-1 and suppressing the activity of UCP2 protein within the pancreatic cells, SIRT1 ensures the maintenance of glucose homeostasis and the preservation of regular insulin secretion. SIRT1 exerts its inhibitory effects on cell death through three distinct mechanisms [11,12,13].
Method and materials
Cell culture
The C2C12 myoblasts were acquired from the Pasteur Institute of Iran. The myoblasts were cultured at 37 °C, with a CO2 concentration of 5%, in Dulbecco’s Modified Eagle Medium (DMEM) from Gibco, located in Berlin, Germany. The DMEM was supplemented with 10% fetal calf serum (FCS), 2 milligrams of glutamine, and 1% penicillin-streptomycin. The induction of myoblast differentiation into myotubes was facilitated by using DMEM supplemented with 2% horse serum. Following four days post-fusion, the myotubes that had undergone differentiation were employed for the subsequent experimental procedures. Lipid-laden media were generated by combining (FFAs) with bovine serum albumin (BSA) devoid of FFAs. Concisely, sodium palmitate was solubilized in a 50% (v/v) ethanol solution. This solution was then diluted in DMEM, which contained 1% (w/v) fatty-acid-free BSA until reaching the desired concentration. The resulting mixture was incubated at 37 °C for 2 h, with continuous shaking [14]. Two hours before commencing the experiments, myotubes were carefully transferred into a serum-free Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 1% Bovine Serum Albumin (BSA). Subsequently, the cells were incubated either in the presence or absence of 0.5 (mM) palmitate for 24 h. The time intervals and palmitate concentrations were determined using the MTT assay, a commonly employed method in biological research. Cellular exposure to a concentration of 0.5 (mM) palmitate for a duration of 48 h, as well as exposure to concentrations of 0.75 mM and one mM palmitate for durations of 24 and 48 h, respectively, have been observed to lead to significant cell death. However, the specific data illustrating this phenomenon has not been presented. Consequently, a concentration of 0.5 mM palmitate for 24 h has been chosen as the appropriate dosage and timeframe for the ensuing experiments. The addition of inhibitors occurred one hour before the incubation period with palmitate [15].
TUNEL assay
Low-molecular-weight DNA was evaluated to ascertain the presence of DNA fragmentation resulting from apoptosis. This was achieved by employing an in-situ cell death detection fluorescein kit (Roche). Detecting DNA strand breaks can be accomplished by labeling unbound 3′-OH termini with nucleotides marked with fluorescein. The reaction above is mediated by terminal deoxynucleotidyl transferase. After the treatment, the cellular specimens were subjected to a thorough rinsing process using phosphate-buffered saline (PBS). Subsequently, the cells were immobile by exposure to a solution of 4% paraformaldehyde in PBS at room temperature for 20 min. Following that, the cellular membranes underwent permeabilization through exposure to a solution containing 0.2% Triton X-100 in 0.1% sodium citrate for 2 min at a temperature of 4 degrees Celsius. Subsequently, the cellular specimens were exposed to a reaction mixture containing TUNEL, chemically linked to fluorescein. The specimens were then placed in a controlled humidity chamber and incubated at 37 degrees Celsius for 1 h. The TUNEL-positive nuclei were examined and quantified using a fluorescence microscope [16, 17].
Cell survival test or MTT
The MTT assay is a commonly employed colorimetric method that facilitates the measurement of viable cells within a specific culture. The primary objective of this study was to examine the influence of reducing SIRT1 expression on the initiation of apoptosis caused by palmitate fatty acid in C2C12 muscle cells. This investigation was motivated by previous scientific literature demonstrating the apoptotic consequences of palmitate on different types of cells. C2C12 cells were initially exposed to palmitate fatty acid treatment for 24 h, utilizing different concentrations from 0.25 to 1 mM to accomplish the desired goal. The observed induction of significant cell death in C212 cells has been attributed to the presence of palmitate at a concentration of 0.75 mM. This observation holds particular significance within the realm of insulin resistance and type 2 diabetes, wherein the concentrations of plasma fatty acids typically hover around 0.5 (mM). Hence, this particular palmitate concentration exhibits appropriateness for exploring its impacts within this pathological framework. The initiation of programmed cell death, known as apoptosis, was accomplished through the modulation of SIRT1 gene expression, wherein its levels were either reduced or enhanced. In this investigation, C2C12 cells underwent culturing and differentiation protocols in conjunction with untreated control cells exposed to sirtinol and regular C2C12 control cells. Following this, the previously mentioned cells underwent a 24-hour exposure to palmitate fatty acid at a concentration of 0.75 mM. Following the specified incubation period, the cellular organisms were collected and exposed to the MTT assay per the methodology outlined in the Materials and Methods section. Untreated control cells and standard C2C12 control cells were cultured and exposed to differentiation. The previously mentioned cells underwent a 24-hour exposure to palmitate fatty acid at a concentration of 0.75 mM. After the specified incubation period, cellular specimens were collected and underwent MTT analysis following the procedures described in the Materials and Methods section. The results suggest that the untreated RSV control cells experienced a decrease of 55% when exposed to palmitate fatty acid, which was statistically significant with a P-value of less than 0.01. The administration of Sirtinol exhibited a reduction in cellular resilience towards palmitate compared to the control cohort, thereby inducing a 14% augmentation in the cellular mortality rate. As illustrated in Fig. 1, it is evident that the cellular population subjected to RSV treatment displayed a noteworthy decrease of 20% in apoptotic cell demise when juxtaposed with the control cellular population [18]. Resveratrol, also known as 3,5,4′-trihydroxy-trans-stilbene, is a phytoalexin, a natural phenol, and a stilbenoid that is produced by a variety of plants in reaction to damage or disease invasion, including bacteria and fungi. The skin of grapes, blueberries, raspberries, mulberries, and peanuts are among the food sources of resveratrol [19]. NAC functions as a synthetic precursor of intracellular cysteine and glutathione. Its ability to counteract reactive oxygen species (ROS) is due to its capacity to directly scavenge free radicals through the redox potential of thiols, or indirectly by elevating cellular levels of glutathione [20].
Resveratrol is frequently taken as a dietary supplement and has been researched in lab models of human diseases, but there isn’t any solid proof that it lengthens life or significantly affects any human disease.
Caspase-3 and − 9 activity
The activity of caspase-3 and caspase-9 was measured using the Caspase-3/CPP32 colorimetric assay and the Caspase-9/MCH6 colorimetric assay kit (MBL International, Woburn, MA, USA), respectively. The protocols provided by the manufacturer were adhered to for both assays.
Cell viability
Mitochondrial function was assessed to ascertain the quantity of viable cells. The process of MTT reagent reduction to insoluble formazan by cells exhibiting metabolic activity is subsequently accompanied by the solubilization of formazan in the presence of dimethyl sulfoxide (DMSO). After subjecting the wells to a 24-hour exposure of 0.5 mM palmitate, the MTT reagent was applied, and the cells were incubated for 2 h [21]. After the development of formazan crystals, the nutrient-rich liquid in the wells was carefully extracted and subsequently replaced with dimethyl sulfoxide (DMSO). The specimens were later placed in a light-deprived setting for 30 min. The absorbance quantification was performed at a specific wavelength of 540 nm utilizing a microplate reader. The results are expressed as a percentage concerning the untreated control group [22].
Real-time PCR
After the implementation of various treatments, the cellular organisms were gathered, and the total RNA was using an RNeasy mini kit. The reverse transcription process was performed on 1 µg of total RNA using Qiagen reverse transcriptase and random hexamer primers. The Rotor Gene 3000 instrument, developed by Corbett Research in Mortlake, NSW, Australia, performed real-time polymerase chain reaction (PCR). The quantification of gene expression levels was performed by employing QuantiTect primers and QuantiFast SYBR Green PCR Master Mix. The data was normalized about the transcript level of β-actin [23].
Measurements of ROS
Reactive oxygen species (ROS) were quantified within cells using a cell-permeable probe called 2′,7′-dichlorofluorescin diacetate (DCFDA). Following the treatment, the cellular specimens underwent a loading procedure involving the introduction of 10 µM DCFDA in phosphate-buffered saline (PBS) for 30 min. After two rounds of washing with phosphate-buffered saline (PBS), the fluorescence signal was measured using a Spectra MAX Gemini EM plate reader. The plate reader employed an excitation wavelength of 495 nm and an emission wavelength of 525 nm to quantify the fluorescence intensity. All the measurements were normalized according to the protein concentrations [24].
Statistical analyses
The statistical analyses were performed using IBM SPSS 29 software (SPSS, Chicago, IL, USA). The one-way analysis of variance was employed to perform comparisons among all groups to assess potential differences. After attaining statistical significance, the Tukey post hoc test was conducted. Values of p equal to or less than 0.05 were deemed statistically significant. The results are depicted as the mean value accompanied by the standard deviation derived from three independent experiments.
Results
SIRT1 activation improved mitochondrial biogenesis in palmitate-treated C2C12 cells
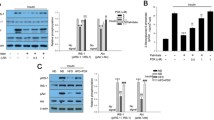
Research findings have elucidated that the presence of Palmitate can induce deleterious effects on the functionality of mitochondria, as observed in various experimental models and living organisms [25]. The objective of the investigation was to evaluate the influence of palmitate on the generation of new mitochondria by measuring the levels of messenger RNA (mRNA) of genes involved in this biological process, such as PGC-1α, NRF-1, and Tfam. The cellular entities were initially exposed to different concentrations of resveratrol (RSV), a compound known to activate SIRT1, for 24 h [26]. The experimental procedure was carried out under the condition of 0.5 (mM) palmitate, a fatty acid compound, and the subsequent analysis involved the utilization of the MTT assay. The choice of 20 mM RSV was determined by analyzing MTT results, which revealed its superior ability to safeguard muscle cells from the detrimental impact of palmitate-induced reduction in cellular viability (Fig. 1A). As depicted in Fig. 1B, the messenger RNA (mRNA) abundance of PGC-1α exhibited a notable decrease of 58% following exposure to a concentration of 0.5 mM palmitate. In contrast, the activation of SIRT1 effectively hindered the decrease in PGC-1α expression induced by palmitate. The findings suggest a notable reduction in the protein abundance of PGC-1α within muscle cells subjected to palmitate treatment. Nevertheless, the administration of RSV exhibited a noteworthy capacity to counterbalance the decline above, as exemplified in Fig. 1C. A 50% and 55% reduction in the expression levels of the Tfam and NRF-1 genes, respectively, was observed following the administration of Palmitate. Nevertheless, the stimulation of SIRT1 exhibited the ability to counteract the diminished expression, as illustrated in Fig. 1D.
The effects of SIRT1 modulation on mitochondrial biogenesis. A MTT result, after treatment with 0.5mM palmitate in the in the presence of different concentrations of resveratrol, *p o 0.01 vs. untreated cells B, C mRNA and protein levels of PGC-1α after treatment with 0.5 mM palmitate for 24 h D mRNA levels of Tfam and NRF-1 in control and treated cells. *p o 0.01 vs. untreated cells, #p o 0.01 vs. control cells. Represented data are from three independent experiments and are means SD
The mechanism involved in SIRT1-mediated improvement of mitochondrial biogenesis
In order to further confirm the role of SIRT1 in the regulation of PGC-1α via SIRT1-mediated mechanisms, the cells were exposed to a combination of palmitate and a specific inhibitor of SIRT1 (sirtinol) at a concentration of 10 mM. The study’s results suggest a notable decrease in the protein abundance of PGC-1α within myotubes upon exposure to sirtinol, As illustrated in Fig. 2A, compared to the cells in the untreated control group. Additionally, the concurrent administration of RSV and sirtinol to myotubes led to a 25% and 35% decline in the expression of the PGC-1α gene and the corresponding protein abundance, as illustrated in Fig. 2B and C. To further elucidate the effects of SIRT1 activation on mitochondrial function, we evaluated the mitochondrial DNA (mtDNA) copy number as an indicator of mitochondrial biogenesis. The research results suggest that the exposure to palmitate and sirtinol led to a notable reduction in the copy number of mitochondrial DNA (mtDNA) within myotubes. Specifically, palmitate was observed to decrease mtDNA copy number by approximately 30%. In contrast, it was observed that RSV exhibited the ability to reinstate the decline in mitochondrial DNA (mtDNA) replication, bringing it back to levels akin to those observed in the control group, as depicted in Fig. 2D.
A SIRT1 protein level was quantified by Western blot in cells treated with and without palmitate. *p o 0.01 vs. untreated cells, #p o 0.01 vs. scrambled control cells, and **p o 0.01 vs. GFP control cells. B PGC-1α protein level in the presence of palmitate and sirtinol. *p o 0.01 vs. control cells treated with palmitate, #p o 0.01 vs. knockdown cells treated with only palmitate. C, D PGC-1α protein and mRNA levels in the presence of palmitate and RSV
SIRT1 activation reduces palmitate-induced apoptosis in C2C12 myotubes
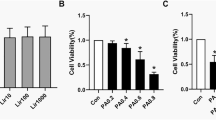
Based on previous studies demonstrating the ability of palmitate to induce apoptosis in various cellular groups, our study aimed to assess the influence of SIRT1 activation on the apoptotic effects of palmitate, specifically in myotubes. The investigation initially exhibited that the incorporation of palmitate in C2C12 cells led to apoptosis, as indicated by the observed 40% cell viability after administering 0.5 mM palmitate to the cells [27, 28] (refer to Fig. 3A). Upon the induction of SIRT1, a notable phenomenon was observed whereby the cellular viability in Resveratrol-treated cells displayed a significant elevation of 50% when compared to the control group, as visually represented in Fig. 3A. Moreover, the administration of Sirtinol elicited a decrease in cellular viability compared to the control group. Further investigations were undertaken to substantiate the manifestation of apoptosis induced by palmitate in myotubes, explicitly emphasizing the programmed cell death process. As depicted in Fig. 3A and B, after cellular exposure to 0.5 mM palmitate, a notable augmentation of approximately 70% and 60% in the enzymatic activity of caspase-3 and − 9, respectively, was observed. Furthermore, palmitate administration resulted in a 48% increase in the population of TUNEL-positive cells, as illustrated in Fig. 3C. Furthermore, a notable decrease in the enzymatic function of caspase-3 and − 9, amounting to 40% and 45% reduction, respectively, was observed in cellular specimens that underwent activation with SIRT1, as visually represented in Fig. 3A and B. Moreover, the activation of SIRT1 led to a reduction of approximately 40% in the quantity of TUNEL-positive cells [29, 30].
Cellular viability was quantified by MTT assay in cells treated with and without palmitate. * p 0.01 vs. control untreated cells, # p vs. palmiate treated control cells. In Figures A and B, 0.5 mM palmitate increased caspase-3 and − 9 enzymatic activity respectively. Palmitate also increased TUNEL-positive cells (Figures D and C). Figures A and B show that SIRT1-activated cells reduced caspase-3 and − 9 activity, respectively. SIRT1 activation reduced TUNEL-positive cells. p o 0.01 vs. control cells
The mechanism by which SIRT1 modulation affects palmitate-induced apoptosis
Previous studies have demonstrated that palmitate can enhance the production of reactive oxygen species (ROS) in various cellular environments. We performed a co-incubation experiment to explore the potential role of reactive oxygen species (ROS) in the mechanism of palmitate-induced apoptosis. C2C12 cells were subjected to a treatment involving simultaneous exposure to 20 mM of the ROS scavenger N-acetylcysteine (NAC) and palmitate for 24 h. The application of (NAC) reduced 2.3 times in the quantity of TUNEL-positive cells compared to the control cells, as illustrated in Fig. 4A. Remarkably, the activation of SIRT1 led to a notable decline of 50% in the generation of reactive oxygen species when cells were exposed to palmitate, as illustrated in Fig. 4B. Moreover, suppressing SIRT1 activity led to a 1.6-fold augmentation in generating reactive oxygen species (ROS) in the cells under normal conditions [31, 32].
A after N-acetylcysteine (NAC) treatment, there were 2.3 fewer TUNEL-positive cells than in the control cells. As shown in Fig. 4B, when cells were treated with palmitate, the activation of SIRT1 resulted in a half reduction in the formation of reactive oxygen species. Additionally, compared to control cells, the SIRT1 suppression led to a 1.6-fold rise in reactive oxygen species (ROS) formation
The activity of caspase enzymes
To validate the initiation of apoptosis in C2C12 cells and further explore the involvement of SIRT1 in this biological process, additional apoptosis assays were conducted, specifically focusing on the quantification of caspase-3 and 9. The experimental conditions for this study were consistent with those employed in the MTT experiment [18] (See Fig. 5).
Following the collection of cellular specimens, subsequent investigations involved the execution of caspase 3 and 9 activity assays, employing the methodology outlined in the Materials and Methods section. As depicted in Fig. 5, the observed caspase 3 and 9 activity levels in the control cells exhibited a notable surge of 62% and 60%, respectively, after administering 0.75 mM palmitate (P < 0.01). The observed augmentation in caspase 3 and 9 activity in cells treated with resveratrol, when exposed to palmitate, exhibited a noteworthy reduction compared to the control cells, with a decrease of 40% and 39%, respectively (P < 0.01). Furthermore, the enzymatic function of caspases 3 and 9 in coexisting cellular populations subjected to RSV and sirtinol resembles the control cellular populations treated with palmitate (P < 0.01).
Results of changes in SIRT1 gene expression on mitochondrial function
As elucidated in the initial segment of this scholarly exposition, mitochondria assume a pivotal role in numerous intracellular phenomena, including but not limited to metabolic activities and cellular differentiation. Mitochondrial dysfunction strongly correlates with compromised insulin signaling pathways while concurrently instigating the initiation of apoptotic cell death pathways. Hence, to explore the involvement of SIRT1 in regulating mitochondrial function, the decision was made to assess the process of mitochondrial biogenesis. The subsequent sections encompass the outcomes of these experiments.
Effect of SIRT1 alteration on the expression of genes involved in mitochondrial biogenesis
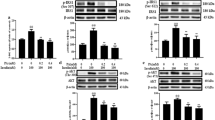
Prior research has demonstrated that palmitate exerts deleterious effects on mitochondrial functionality, both in controlled laboratory conditions (in vitro) and within living organisms (in vivo). To explore the impact of palmitate fatty acid on the process of mitochondrial biogenesis, we conducted an investigation by quantifying the mRNA expression levels of key genes involved in this biological pathway, including NRF-1, PGC-1α, and Tfam, using the real-time polymerase chain reaction (PCR) technique. To examine the impact of SIRT1 gene reduction on PGC-1α expression, the cells were initially subjected to differentiation alongside control cells. Subsequently, they were exposed to a concentration of 0.5 mM palmitate fatty acid for 24 h. Subsequently, the cellular RNA was isolated and subjected to real-time PCR analysis, following the procedures outlined in the Materials and Methods section. The real-time PCR analysis revealed a notable decline of approximately 58% in the quantity of PGC-1α mRNA within the C2C12 cells subjected to 0.5 mM palmitate treatment compared to the control cells (P < 0.01). Interestingly, the expression level of PGC-1α mRNA in the palmitate-treated cells remained unaffected in RSV and did not exhibit any significant deviation from the untreated control cells (Fig. 6). Furthermore, the observed transcriptional activity of the gene mentioned above in myrtle cells subjected to sirtinol treatment exhibited a reduction of 30% in comparison to the control cells (P < 0.01). The findings of this study suggest that, in the context of mitochondrial biogenesis, the presence of palmitate leads to a decrease in the expression of a crucial factor known as PGC-1α. Additionally, it appears that the expression of SIRT1 plays a regulatory role in modulating the activity of this particular factor. Elevated levels of SIRT1 expression exhibit a safeguarding function against the decline in biogenesis induced by palmitate, whereas diminished levels of SIRT1 expression result in a reduction of mitochondrial biogenesis.
Effect of changes in SIRT1 gene expression on PGC-1α protein in normal C2C12 differentiated cells, increase and decrease in SIRT1 expression in palmitate-treated and untreated conditions for 24 h. Data are obtained from three independent experiments that are shown as mean ± standard deviation. *, P < 0.01 in comparison with control cells not treated with palmitate. **, P < 0.01 in comparison with Sirtinol treated cells. #, P < 0.01 compared to control cells
SIRT1 gene expression affects the expression of Tfam and NRF genes
In light of investigating the impact of altering the expression of the SIRT1 gene on the functionality of mitochondria, an assessment was conducted on the expression of two additional genes implicated in the process of mitochondrial biogenesis. The real-time PCR findings indicated a notable decline in Tfam and NRF expression levels in normal cells subjected to a 0.5 mM palmitate treatment, with reductions of approximately 50% and 55%, respectively, compared to control cells (P < 0.01). Conversely, in the presence of the same conditions, the expression levels of these genes in cells derived from RSV-treated patients did not exhibit any significant deviation from those observed in normal cells (Fig. 7). The expression levels of Tfam and NRF genes were observed to decrease in cellular populations subjected to a concentration of 0.75 mM palmitate, and this decrease was further observed in the presence of sirtinol. The experimental findings indicate that the downregulation of the SIRT1 gene in the presence of palmitate treatment leads to a decrease in the expression of genes associated with the mitochondrial biogenesis process, specifically Tfam, and NRF. It is worth mentioning that muscle cells exhibiting heightened levels of SIRT1 demonstrated a 25% and 48% upregulation in the expression of Tfam and NRF genes, respectively [33].
The effect of changing the expression of the SIRT1 gene on the amount of PGC-1α protein
To investigate the impact of SIRT1 reduction on PGC-1α at the protein level, we cultured and differentiated the C2C12 cells with reduced SIRT1 alongside normal cells. Subsequently, these cells were subjected to treatment with 0.5- and 0.75-mM palmitate. In next step, the cellular protein was extracted, and Western blotting was conducted by the prescribed methodology outlined in the materials and methods section.
Effect of ROS on induction of apoptosis by palmitate in normal C2C12 cells under palmitate fatty acid treatment for 24 h. Data are obtained from three independent experiments that are shown as mean ± standard deviation. *, P < 0.05 in comparison with palmitate untreated control cells. #, P < 0.05 compared to cells not treated with NAC. NAC: N-acetylcysteine
The experimental findings indicated a noteworthy decline of 50% in the quantity of PGC-1α protein within the regular C2C12 cells upon exposure to palmitate compared to the control cells (P < 0.01). Under identical experimental conditions, the quantity of PGC-1α protein was assessed in cells treated with palmitate.
The observed cellular characteristics resembled typical C2C12 cells, albeit with a notable reduction of approximately 55% compared to the control cell population. Fascinatingly, the observed quantity of PGC-1α protein within cells subjected to RSV treatment exhibited a notable augmentation of approximately 60% when juxtaposed with cells in the control group (P < 0.01). The experimental findings indicated that the presence of palmitate led to a decrease in the quantity of PGC-1α protein within normal cells. Conversely, the expression of the SIRT1 gene was observed to increase, thereby preventing the decline in PGC-1α protein expression. Furthermore, the observed downregulation of PGC-1α protein expression in cells exhibiting reduced SIRT1 levels showed a statistically significant decrease compared to the control cells (P < 0.01). The findings from this section and the preceding sections collectively underscore the significance of upregulating SIRT1 expression in mitigating the impact of palmitate fatty acid on the process of mitochondrial biogenesis. Conversely, downregulating SIRT1 expression appears to have a diminishing effect on mitochondrial biogenesis. The cumulative data presented herein elucidate the pivotal and fundamental function of SIRT1 in regulating the process of mitochondrial biogenesis within the context of muscle cells.
The mechanisms of SIRT1 gene expression variations on mitochondrial biogenesis
Based on the findings derived from the preceding section, the subsequent section delved into exploring the mechanisms implicated in modulating the expression of SIRT1 about mitochondrial biogenesis.
SIRT1 protein expression changes under SIRT1 activator and inhibitor and palmitate treatment
Prior research has demonstrated that introducing lipids and palmitate leads to a decrease in the manifestation of PGC-1α within the skeletal muscle of humans and C2C12 myotubes. Previous research has additionally demonstrated that S1RT1 serves as a significant upstream regulator of PGC-1α. Hence, the quantification of SIRT1 protein levels is conducted in C2C12 myotubes under the influence of palmitate fatty acid. To conduct this experiment, cellular organisms were cultivated and underwent a differentiation process. Subsequently, they were subjected to a treatment involving a concentration of 0.75 millimolar in the presence of palmitate for 24 h. The cellular entities were gathered after the experimental intervention and subsequently subjected to protein extraction. Subsequently, the protein extract was utilized for the execution of Western blotting analysis [34].
As depicted in Fig. 8, applying palmitate results in a 34% decrease in the abundance of SIRT1 protein within normal cells (P < 0.01). The protein expression levels in cells subjected to RSV treatment exhibited a statistically significant increase of 32% compared to the control cells (P < 0.01). In order to assess the impact of sirtinol on the expression of SIRT1 protein in, C2C12 cells were cultivated and underwent differentiation. The cells were then treated with sirtinol and were gathered 24 h after initiating cell differentiation. The SIRT1 protein exhibited a notable reduction of 29% in cells treated with sirtinol compared to the control cells (P < 0.01). Additionally, the presence of both RSV and sirtinol further contributed to an increase in the abundance of this protein in comparison to the sirtinol treated cells.
Effect of palmitate on the amount of SIRT1 protein in differentiated normal C2C12 cells treated with RSV and sirtinol under conditions treated and untreated with palmitate fatty acid for 24 h. Data are obtained from three independent experiments that are shown as mean ± standard deviation. *, P < 0.01 in comparison with control cells not treated with palmitate. **, p < 0.01 compared to Sirtinol treated cells. #, P < 0.01 in comparison with 0.75 mM palmitate-treated cells
Results of PGC-1α protein measurements in the presence of Sirtinol
To study more closely and to ensure the role of SIRT1 in the regulation of apoptosis through PGC-1α, myotube cells were treated simultaneously with 0.75 mM palmitate and a specific inhibitor of SIRT1, 20 µM sirtinol [35].
Results of protein assay and expression of PGC-1 α gene in C2C12 cells in the presence of RSV
This experiment validated the findings acquired from the preceding section’s experiment. The cells under investigation were cultured and subjected to differentiation alongside the control cells to conduct this experiment. The cellular specimens were subsequently exposed to a distinct activator known as SIRT1, Resveratrol, at a concentration of 20 µM over 24 h. After cellular treatment, the cellular entities were gathered and subjected to the experimental protocol outlined in the Materials and Methods section. Subsequently, real-time PCR and Western blotting techniques were employed to analyze the collected samples.
The mechanism of the effect of SIRT1 expression changes on induction of apoptosis
Prior research has demonstrated that the application of palmitate to various cellular entities elicits the generation of reactive oxygen species (ROS) within said cellular entities. Hence, to unravel the intricate involvement of reactive oxygen species (ROS) in the initiation of apoptosis, the initial approach involved the utilization of a ROS inhibitor, specifically N-acetylcysteine (NAC). For experimental purposes, following the cultivation and differentiation of C2C12 cells, said cells were subjected to a combined treatment involving the administration of 0.75 millimolar concentration of palmitate fatty acid and 20 micromolar concentration of N-acetylcysteine. After treatment administration, the cells were subjected to the TUNEL test.
Discussion
The excessive deposition of fatty acids in peripheral non-adipose tissues, including the heart, pancreas, liver, and skeletal muscle, is a characteristic feature of lipotoxicity. An overabundance of lipids leads to apoptotic cell death and a reduced functional tissue mass. These outcomes can potentially exacerbate cellular dysfunction. In the context of pancreatic beta cells, it has been observed that elevated levels of (FFAs) can impede insulin production and diminish insulin gene expression [36, 37], and ultimately result in programmed cell death, known as apoptosis. Elevated concentrations of unbound fatty acids have been linked with diminished capacity for insulin-facilitated glucose uptake. Cardiac steatosis leads to the manifestation of cardiomyopathy and a subsequent decline in contractile ability. The promotion of apoptosis in pericytes, an early occurrence in the advancement of diabetic retinopathy, has been noted to be facilitated by saturated fatty acids. Furthermore, the previous research findings and the findings obtained in our study provide substantiation for the impact of lipo-apoptosis on the cellular functionality of skeletal muscle. While the understanding of the impact of lipo-apoptosis on skeletal muscle decline in individuals with type 2 diabetes remains limited, existing evidence indicates a potential involvement of insulin resistance and saturated fatty acids in the process of skeletal muscle loss [38]. Based on these observations, it can be inferred that therapeutic interventions targeting inhibiting lipotoxic events may potentially yield beneficial outcomes in mitigating muscle loss among individuals with diabetes. Hence, the primary aim of this study was to ascertain the potential of sirt1 in impeding the occurrence of lipo-apoptosis within the skeletal muscle cells. In this investigation, we have unveiled that the activation of sirt1 can impede lipo-apoptosis within skeletal muscle cells. This phenomenon is achieved through a mechanism that entails the diminishment of ceramide levels and the mitigation of oxidative stress. This was accomplished by illustrating that the activation of sirt1 can inhibit lipo-apoptosis [39, 40]. Our initial step involved investigating the pivotal role of sirt1 in the intricate process of mitochondrial formation and exploring the plausible underlying mechanisms at play. Prior studies have elucidated that the introduction of lipids through infusion and the presence of palmitate adversely impact the expression of PGC-1 in skeletal muscle and C2C12 myotubes. PGC-1, short for Peroxisome proliferator-activated receptor gamma coactivator 1, plays a pivotal role in governing the process of mitochondrial biogenesis. Consistent with the earlier studies, our investigation revealed that palmitate suppresses PGC-1 expression in myotubes. However, the activation of sirt1 counteracts this reduction, whereas its inhibition leads to a decrease in PGC-1 expression. This discovery aligns with the observations made in previous studies. Furthermore, our investigation revealed that the activation of sirt1 exhibited a safeguarding effect on the decline of ATP levels observed in muscle cells. The discovered results provide substantiation for the hypothesis positing that the regulation of sirt1 exerts an influence on the cellular type’s mitochondrial function [41, 42]. By utilizing diverse methodologies, we successfully demonstrated that the activation of sirt1 within muscle cells effectively impedes the occurrence of mitochondrial-induced apoptosis. As expected, the inhibition of sirt1 resulted in the activation of apoptosis in muscle cells. This discovery aligns with the research that demonstrated the acceleration of cellular processes culminating in apoptosis in hepatic stellate cells upon inhibition of sirt1, indicating that the suppression of sirt1 expedited the process. Additionally, our findings indicate that including the reactive oxygen species (ROS) scavenger NAC in both cell types subjected to palmitate treatment reduced palmitate-induced apoptosis. This observation highlights ROS’s pivotal involvement in facilitating cellular demise within these specific cell populations [43]. The results of our study offer compelling evidence supporting the notion that the manipulation of sirt1 in skeletal muscle cells induces significant alterations in the process of apoptosis. Apoptosis, also known as programmed cell death, is a biological phenomenon characterized by cells regulated and controlled demise. When the activity of Sirt1 is suppressed in muscle tissue, the activity of caspases is enhanced, resulting in an elevation in the production of reactive oxygen species (ROS) by mitochondria. Decreased levels of reactive oxygen species (ROS) are correlated with heightened levels of the SIRT1 protein and diminished levels of caspase activation, which are subsequently linked to elevated levels of PGC-1 expression and enhanced mitochondrial biogenesis in muscle cells. The activation of Sirt1, a key protein involved in cellular processes, results in a cascade of events that ultimately leads to decreased reactive oxygen species (ROS) production. This reduction in ROS levels has been observed to significantly impact the rate of apoptosis, a programmed cell death, specifically in muscle cells. When considered collectively, these observations imply that the activation of sirt1 may hold potential therapeutic value in addressing lipotoxicity-induced diabetic complications, specifically within the context of muscle cell physiology. It is better to carry out the results in this study as well. NAC, RSV can be used as one of the molecules that have a high potential in apoptosis and also improve insulin resistance in animal and laboratory models as a complement to the preclinical study [44,45,46].
Data availability
Not applicable.
References
Dela F, Ingersen A, Andersen NB, Nielsen MB, Petersen HH, Hansen CN et al (2019) Effects of one-legged high‐intensity interval training on insulin‐mediated skeletal muscle glucose homeostasis in patients with type 2 diabetes. Acta Physiol 226(2):e13245
Park SS, Seo YK (2020) Excess Accumulation of lipid impairs insulin sensitivity in skeletal muscle. Int J Mol Sci. 21(6)
Su Z, Nie Y, Huang X, Zhu Y, Feng B, Tang L et al (2019) Mitophagy in hepatic insulin resistance: therapeutic potential and concerns. Front Pharmacol 10:1193
Lipke K, Kubis-Kubiak A, Piwowar A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States—Current View of Knowledge. Cells. 2022;11(5):844
Römer A, Linn T, Petry SF (2021) Lipotoxic impairment of mitochondrial function in β-Cells: a review. Antioxid (Basel Switzerland). 10(2)
Hwang J-w, Yao H, Caito S, Sundar IK, Rahman I (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61:95–110
Zhou S, Tang X, Chen HZ (2018) Sirtuins and insulin resistance. Front Endocrinol 9:748
Antuña E, Cachán-Vega C, Bermejo-Millo JC, Potes Y, Caballero B, Vega-Naredo I et al (2022) Inflammaging: implications in Sarcopenia. Int J Mol Sci 23(23):15039
Saini R, Singh S (2019) Inducible nitric oxide synthase: an asset to neutrophils. J Leukoc Biol 105(1):49–61
Nakazawa H, Chang K, Shinozaki S, Yasukawa T, Ishimaru K, Yasuhara S et al (2017) iNOS as a driver of inflammation and apoptosis in Mouse Skeletal Muscle after burn Injury: possible involvement of Sirt1 S-Nitrosylation-mediated acetylation of p65 NF-κB and p53. PLoS ONE 12(1):e0170391
Park DR, Kim JS, Kim CK (2014) The effect of SIRT1 protein knock down on PGC-1α acetylation during skeletal muscle contraction. J Exerc Nutr Biochem 18(1):1–7
Gurd BJ (2011) Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Applied physiology, nutrition, and metabolism = physiologie appliquee, nutrition et metabolisme. 36(5):589–597
Mendoza M, Mendoza M, Lubrino T, Briski S, Osuji I, Cuala J et al (2023) Arginine methylation of the PGC-1α C-Terminus is temperature-dependent. Biochemistry 62(1):22–34
Rodgers BD, Wiedeback BD, Hoversten KE, Jackson MF, Walker RG, Thompson TB (2014) Myostatin stimulates, not inihibits, C2C12 myoblast proliferation. Endocrinology 155(3):670–675
Ronzoni FL, Aliberti F, Scocozza F, Benedetti L, Auricchio F, Sampaolesi M et al (2022) Myoblast 3D bioprinting to burst in vitro skeletal muscle differentiation. J Tissue Eng Regen Med 16(5):484–495
Darzynkiewicz Z, Huang X, Okafuji M (2006) Detection of DNA strand breaks by flow and laser scanning cytometry in studies of apoptosis and cell proliferation (DNA replication). Methods in molecular biology. (Clifton NJ) 314:81–93
Hooker DJ, Mobarok M, Anderson JL, Rajasuriar R, Gray LR, Ellett AM et al (2012) A new way of measuring apoptosis by absolute quantitation of inter-nucleosomally fragmented genomic DNA. Nucleic Acids Res 40(15):e113
Tian H, Zhao H, Mei X, Li D, Lin J, Lin S et al (2021) Resveratrol inhibits LPS-induced apoptosis in VSC4.1 motoneurons through enhancing SIRT1-mediated autophagy. Iran J Basic Med Sci 24(1):38–43
Liu K, Zhou R, Wang B, Mi M-T (2014) Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials123. Am J Clin Nutr 99(6):1510–1519
Halasi M, Wang M, Chavan TS, Gaponenko V, Hay N, Gartel AL (2013) ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem J 454(2):201–208
Ghasemi M, Turnbull T, Sebastian S, Kempson I (2021) The MTT assay: utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int J Mol Sci. 22(23)
Buranaamnuay K (2021) The MTT assay application to measure the viability of spermatozoa: a variety of the assay protocols. Open Veterinary Journal 11(2):251–269
Roth R, Madhani HD, Garcia JF (2018) Total RNA isolation and quantification of specific RNAs in fission yeast. Methods in molecular biology. (Clifton NJ) 1721:63–72
Mo Y, Wan R, Zhang Q (2012) Application of reverse transcription-PCR and real-time PCR in nanotoxicity research. Methods in molecular biology. (Clifton NJ) 926:99–112
Dludla PV, Silvestri S, Orlando P, Mazibuko-Mbeje SE, Johnson R, Marcheggiani F et al (2020) Palmitate-induced toxicity is associated with impaired mitochondrial respiration and accelerated oxidative stress in cultured cardiomyocytes: the critical role of coenzyme Q(9/10). Toxicology in vitro: an international journal published in association with BIBRA. 68:104948
Chuang YC, Chen SD, Hsu CY, Chen SF, Chen NC, Jou SB (2019) Resveratrol promotes mitochondrial Biogenesis and protects against Seizure-Induced neuronal cell damage in the Hippocampus following Status Epilepticus by activation of the PGC-1α signaling pathway. Int J Mol Sci. 20(4)
Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T (2011) MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res 92(1):75–84
Yang HY, Chen JY, Huo YN, Yu PL, Lin PZ, Hsu SC et al (2022) The role of Sirtuin 1 in Palmitic Acid-Induced endoplasmic reticulum stress in Cardiac myoblasts. Life (Basel Switzerland). 12(2)
Li S, Li H, Yang D, Yu X, Irwin DM, Niu G et al (2017) Excessive autophagy activation and increased apoptosis are Associated with Palmitic Acid-Induced Cardiomyocyte insulin resistance. J Diabetes Res 2017:2376893
Zang Y, Fan L, Chen J, Huang R, Qin H (2018) Improvement of lipid and glucose metabolism by Capsiate in Palmitic Acid-treated HepG2 cells via activation of the AMPK/SIRT1 Signaling Pathway. J Agric Food Chem 66(26):6772–6781
Xu T, Song Q, Zhou L, Yang W, Wu X, Qian Q et al (2021) Ferulic acid alleviates lipotoxicity-induced hepatocellular death through the SIRT1-regulated autophagy pathway and independently of AMPK and akt in AML-12 hepatocytes. Nutr Metabolism 18(1):13
Vázquez-Mosquera ME, Fernández-Moreno M, Cortés-Pereira E, Relaño S, Dalmao-Fernández A, Ramos-Louro P et al (2021) Oleate prevents palmitate-Induced mitochondrial dysfunction in Chondrocytes. Front Physiol. 12
Diaz-Vegas A, Sanchez-Aguilera P, Krycer JR, Morales PE, Monsalves-Alvarez M, Cifuentes M et al (2020) Is mitochondrial dysfunction a Common Root of Noncommunicable Chronic diseases? Endocr Rev. 41(3)
Wang SJ, Zhao XH, Chen W, Bo N, Wang XJ, Chi ZF et al (2015) Sirtuin 1 activation enhances the PGC-1α/mitochondrial antioxidant system pathway in status epilepticus. Mol Med Rep 11(1):521–526
Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X et al (2007) SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metabol 6(4):307–319
Schaffer JE (2003) Lipotoxicity: when tissues overeat. Curr Opin Lipidol 14(3):281–287
Brookheart RT, Michel CI, Schaffer JE (2009) As a matter of fat. Cell Metab 10(1):9–12
Turcotte LP, Fisher JS (2008) Skeletal muscle insulin resistance: roles of fatty acid metabolism and Exercise. Phys Ther 88(11):1279–1296
Chen M-Y, Meng X-F, Han Y-P, Yan J-L, Xiao C, Qian L-B (2022) Profile of crosstalk between glucose and lipid metabolic disturbance and diabetic cardiomyopathy: inflammation and oxidative stress. Front Endocrinol. 13
Flierl A, Schriner SE, Hancock S, Coskun PE, Wallace DC (2022) The mitochondrial adenine nucleotide transporters in myogenesis. Free Radic Biol Med 188:312–327
Gomes AP, Duarte FV, Nunes P, Hubbard BP, Teodoro JS, Varela AT et al (2012) Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochimica et Biophysica Acta (BBA) -. Mol Basis Disease 1822(2):185–195
Chang E, Kim Y, Vitamin D (2019) Ameliorates Fat Accumulation with AMPK/SIRT1 activity in C2C12 skeletal muscle cells. Nutrients 11(11):2806
Cao Y, Zhang M, Li Y, Lu J, Zhou W, Li X et al (2022) O-GlcNAcylation of SIRT1 protects against cold stress-Induced skeletal muscle damage via amelioration of mitochondrial homeostasis. Int J Mol Sci 23(23):14520
Pardo PS, Boriek AM (2011) The physiological roles of Sirt1 in skeletal muscle. Aging 3(4):430–437
Pahlavani HA (2022) Exercise-induced signaling pathways to counteracting cardiac apoptotic processes. Front Cell Dev Biology. 10
Alipourfard I, Bakhtiyari S, Gheysarzadeh A, Di Renzo L, De Lorenzo A, Mikeladze D, Khamoushi A (2021) The key role of Akt protein kinase in metabolic-inflammatory pathways cross-talk: TNF-α down-regulation and improving of insulin resistance in HepG2 cell line. Curr Mol Med 21(3):257–264. https://doi.org/10.2174/1566524020666200427102209
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Gh. T. planned and supervised the research. N.S. and N. N. performed the analysis of the study. Gh. T. and Z. R. interpreted the results and wrote the draft. I. A. worked out the technical details edited and submitted the manuscript as the correspondence. All authors have reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taheripak, G., Sabeti, N., Najar, N. et al. SIRT1 activation attenuates palmitate induced apoptosis in C2C12 muscle cells. Mol Biol Rep 51, 354 (2024). https://doi.org/10.1007/s11033-024-09250-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09250-w