Abstract
Background
This study aimed to measure the expression levels of peripheral blood miRNAs in brucellosis and their involvement in the different phases of the brucellosis.
Methods
The expression levels of miRNAs including miR-210, miR-155, miR-150, miR-146a, miR-139-3p, miR-125a-5p, miR-29 and miR-21 were quantified in 57 brucellosis patients subgrouped into acute, under treatment & relapse phase and 30 healthy controls (HCs) using real-time polymerase chain reaction (RT-PCR). The receiver operating characteristic (ROC) analysis curve analysis was performed to find a biomarker for discrimination of different phases of brucellosis.
Results
The expression of miR-155, miR-146a, miR-125a-5p, miR-29, and miR-21 was found to be elevated in the acute brucellosis patients compared to HCs. miR-29 changed in under-treatment patients, while miR-139-3p and miR-125a-5p showed alterations in relapse cases. The ROC curve analysis depicted the potential involvement of miR-21 in the pathogenesis of acute brucellosis.
Conclusion
The expression level of miR-21 is significantly augmented in acute brucellosis and has the potential to be a contributing diagnostic factor for acute infection.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brucellosis, the most common zoonotic disease found globally, is caused by the genus Brucella bacteria with > 500,000 new cases of infections being reported annually [1, 2]. Brucellosis is more prevalent endemically in developing countries, especially in the Mediterranean and the Middle East regions with more than 10 cases per 100,000 population [3, 4]. Among the known ten species of Brucellosis, i.e. Brucella abortus and Brucella melitensis are the 2 most common species observed in Iran and it can not only lead to severe human morbidity but also considerable economic losses in livestock [5, 6]. These intracellular gram-negative bacteria mostly infect domestic animals with humans being incidental hosts. Human brucellosis generally presents itself as an acute febrile illness with symptoms such as fatigue, undulating fever, shivering, perspiration, anorexia, and weight loss [1]. Lack of treatment or delayed diagnosis/misdiagnosis during this phase may cause failure to clear the bacterial infection leading to persistent or chronic disease. Cellular immune responses mediated via various immune cells including T-helper 1 (Th1) cells, macrophages and dendritic cells result in early phase clearance of the intracellular Brucella bacteria during the phagocytosis process [7]. However, the relapse phase of brucellosis occurs mostly due the outstanding ability of Brucella to survive and replicate for prolonged periods within host macrophages by evading the host immune system [5].
MicroRNAs (miRNAs) are small, non-coding RNAs of 17–25 nucleotides in length that can regulate the expression of genes through binding to the 3′‐untranslated region (3'UTR) of their target mRNA. miRNAs have been implicated in several physiological and pathological processes [7]. miRNAs have also been shown to play a crucial role in the pathogenesis of a vast variety of diseases such as cancer, autoimmunity and infectious diseases [8]. Increasing evidence implies that miRNAs play a pivotal role in pathogens’ transmission and in the host response to infection, mostly through targeting of molecules involved in the immune responses [9,10,11,12]. Alternatively, pathogens can also regulate the expression of host miRNAs such as the Brucella can evade the host immune system by modulating miRNAs [13, 14]. Several studies have observed altered expression of miRNAs in the biological fluids of brucellosis [15,16,17]. Therefore, circulating nucleic acids such as miRNAs (cf-miRNAs) are considered as valuable auxiliary diagnostic/prognostic biomarkers due to their easy accessibility and stability as well as their minimal invasive measurement [18]. Hence, evaluation of miRNA expression profiles may provide some promising information for the diagnosis and therapeutic monitoring of Brucellosis.
The aim of the present study was to investigate the expression levels of peripheral blood miRNAs, including miR-210, miR-155, miR-150, miR-146a, miR-139-3p, miR-125a-5p, miR-29 and miR-21 in brucellosis patients and healthy controls (HCs). Studied miRNAs were selected through text-mining to evaluate candidate miRNAs involved in the pathogenesis of brucellosis and to explore the impact of brucellosis on whole blood miRNA expression levels. ROC analysis was also performed to find a non-invasive indicator for distinguishing the brucellosis phases.
Materials and methods
Patients and samples
For this case–control prospective cohort study, 100 patients with brucellosis were recruited from Sina University Hospital (Table 1) and blood samples from 57 of these patients were used to measure the miRNA expression level [19]. Written informed consent from all patients were obtained for using their samples and clinical data. The patients were grouped into three categories: a) 23 patients with acute brucellosis who did not receive antimicrobial therapy and the onset of their disease was less than 3-months, b) 20 patients who were treated with doxycycline (100 mg/BD) and rifampin (600 mg/day) for 4–8 weeks (under-treatment group) and c) 14 cases with relapsed brucellosis (some symptoms reappeared during 3 months to 2 years after initial treatment). Brucellosis diagnosis was confirmed based on the following criteria: a) clinical symptoms such as perspiration, fever, chills, fatigue, anorexia and weight loss, (b) detection of specific antibody titres using standard tube agglutination assay titer (wright titre: 1/80, 2-mercaptoethanol (2ME) test: 1/40 and coombs wright titre: 1/160) [20]. The exclusion criteria were as follows: 1) diagnosis of chronic diseases including autoimmune diseases and cancer; 2) other infectious diseases; 3) receiving of immunosuppressive drugs and antibiotics within 2 weeks before sample collection.
The control group included 30 sex and age-matched healthy individuals enrolled from the same geographic region.
Data collection
We employed text-mining techniques to identify a set of miRNAs for investigation, aiming to elucidate the potential roles of miRNAs in the pathogenesis of brucellosis (Table 2). A comprehensive literature review was conducted by using various online databases, such as National Center for Biotechnology Information (NCBI) https://pubmed.ncbi.nlm.nih.gov/advanced/ using the search terms Brucellosis and miRNA: "brucellosis"[MeSH Terms] OR "brucellosis"[All Fields] OR "brucelloses"[All Fields]) AND ("micrornas"[MeSH Terms] OR "micrornas"[All Fields] OR "mirna"[All Fields] OR "mirnas"[All Fields] OR "mirna s"[All Fields]). We also checked the mentioned keywords in google scholar using advanced search to extract relevant literature.
Evaluation of miRNAs expression
Relative quantification of miRNAs expression levels were carried out by real-time polymerase chain reaction (RT-PCR). Total RNA was isolated from whole blood samples of HCs and brucellosis patients’ subgroups using RNXTM-PLUS solution (CinnaGen, Iran). RNA quality and quantity was assessed by measuring 260/280 ratio using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). 5 ng of total RNA was reverse transcribed into cDNA using a first-strand cDNA synthesis kit (Exiqon, Denmark) as per manufacturer’s instructions. Quantitative RT-PCR was carried out using miRCURY LNATM Universal RT microRNA PCR (Exiqon, Denmark) as per the manufacturer’s instructions. The assay was run in duplicate in a LightCycler instrument (LightCycler 96, Roche, Germany). U6 small nuclear RNA (snRNA) was used as a housekeeping gene for data normalization and the expression fold change of each miRNA was calculated using the comparative cycle threshold (CT) 2−∆CT method.
Identification of miR-21 target genes and related pathways
Three widely used miRNA target prediction databases, including TargetScan (http://www.targetscan.org/), miRDB (http://www.mirdb.org/) and miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/) were used to predict potential miR-21 target genes. The predicted gene list from all the above databases were compared using a Venn diagram to extract the overlapping target genes. Furthermore, bioinformatic-analysis based Diana prediction tool was also used for the identification of miR-21-gene interaction. The threshold was set to 0.6. To explore the biological processes linked to the identified potential targets related to miR-21, we employed the Metascape platform for Gene Ontology (GO) analysis (http://metascape.org) [21]. The STRING database (http://string-db.org/) was used to estimate the correlation between the identified target genes, by setting the medium confidence to 0.400 and the important miRNA-gene network was constructed using miRTargetLink 2.0 (https://ccb-compute.cs.uni-saarland.de/mirtargetlink2).
Statistical analysis
The D'Agostino-Pearson omnibus normality test was applied to define the normality of data distribution. Kruskal–Wallis test was used, followed by Dunn's multiple comparisons test for comparisons of miRNA expression levels between the patients’ subgroups and HCs. The receiver operating characteristic (ROC) analysis was performed between patients with acute brucellosis and HCs and the area under the curve (AUC) was measured by calculating sensitivity and specificity. P values equal to or less than 0.05 are considered statistically significant.
Results
Demographics and clinical features of the study population
As previously described [19], there were no remarkable differences for sex and age variables between HCs and patients’ subgroups. 100 patients with brucellosis (63 males and 37 females), with a mean age of 39.89 ± 16.11 years and 30 age-matched HCs (15 males and 15 females), with an average age of 41.07 ± 9.46 years, were enrolled in the present study. 57 samples of patients were used to measure the miRNAs expression levels. The prevalence of brucellosis was higher in males compared to females and more in rural regions than urban areas. Moreover, there were no significant differences in post-diagnosis antibody screening assessments among patients’ subgroups (Table 1).
Expression level of miRNAs in brucellosis patients and healthy controls
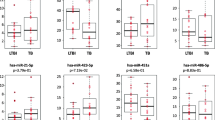
Five out of the eight miRNAs evaluated, miR-155 (P = 0.02), miR-146a (P = 0.03), miR-125a-5p (P = 0.021), miR-29 (P = 0.02), and miR-21 (P = 0.0037) were found to be up-regulated in acute brucellosis compared to HCs (Fig. 1, Table 3). Moreover, miR-21 was also found to be significantly increased in acute brucellosis patients compared to under-treatment (UT) cases (P = 0.02) and patients in relapsing (R) phase (P = 0.037) (Fig. 1H). miR-125a-5p and miR-139-3p were significantly up-regulated in patients with relapse in comparison to HCs (P = 0.049 and P = 0.031, respectively). Among the miRNAs, miR-29 expression is considerably elevated in under-treatment patients compared to HCs (P = 0.04), (Fig. 1G). As illustrated in Fig. 1, the analysis of the expression levels of miR-210 and miR-150 indicated no significant differences among the three groups.
The expression level of miRNAs in the whole blood of brucellosis patients compared to the healthy controls (HCs) (n = 30). The expression level of miRNAs were also compared in patients with acute brucellosis (A) (n = 23), under treatment (UT) (n = 20), and patients with relapse of disease(R) (n = 14). Relative expression levels of miR-210 (A), miR-155 (B), miR-150 (C), miR-146a (D), miR-139-3p (E), miR125a-5p (F), miR-29 (G), and miR-21 (H). Error bars depict the median values based on the Kruskal–Wallis test for multiple comparisons; the results are shown as mean ± SEM (*P < 0.05 and **P < 0.01)
miR-21 as an auxiliary diagnostic marker of acute brucellosis
The assessment of miRNA expressions was evaluated based on their accuracy displayed using the area under the curve (AUC). The analysis of ROC curves for miR‐155, miR‐29 and miR‐21 indicate their potential as biomarkers, with AUC values of 0.71, 0.80 and 0.81 respectively (Fig. 2). Notably, miR-21 expression demonstrated the highest discriminatory capacity as a potential biomarker for distinguishing the acute phase. The ROC curve analysis revealed that the relative expression of miR-21 can effectively differentiate acute brucellosis from HCs, with a specificity of 84% and a sensitivity of 73.6% (P = 0.00083, Fig. 2E).
Receiver operating characteristic (ROC) curve analysis of all the miRNAs. ROC curve was performed to distinguish between acute brucellosis patients (n = 23 s) and healthy controls (HCs) (n = 25). Area under the curve (AUC) and P-value of miR-155 (A), miR-146a (B), miR-125a-5p (C), miR-29 (D), and miR-21 (E) are represented. Among all miRNAs, it is indicated that miR-21 expression had good diagnostic power for distinguishing the acute phase from HCs (P = 0.00083)
miR-21 may target Immunomodulatory molecules during the acute phase of brucellosis
Based on the miRNA expression assessment and ROC analysis, the findings suggest that upregulation of miR-21 levels in whole blood could potentially serve as a robust biomarker in accurately identifying the acute phase of the disease. According to the analysis by the various target prediction databases, miR-21 is involved in the regulation of 384, 469, 625 and 1023 target genes as identified via TargetScan, miRDB, miRTarBase and DIANA tools respectively. The comparative analysis revealed 41 overlapping potential target genes associated with miR-21 Among the predicted target genes, transforming growth factor-β (TGFB) 1, Signal transducer and activator of transcription (STAT) 3, Chemokine ligand (CCL)1, and CCL20 genes are involved in the regulation of the immune system (Fig. 3A) Based on the Diana prediction tool, four additional immune target genes associated with miR-21, including TGFB1, TGFB2, IL-2RA, and IL-10 were identified. Among the predicted target genes, TGFB1, TGFB2, IL-2RA and IL-10 genes with a seed region of 8-mer for TGFB1/2 and 7-mer for IL-2RA and IL-10 genes are involved in the immune responses and Brucella pathogenesis.
The predicted miR-21 target genes with online prediction databases (TargetScan, miRDB, and miRTarBase). 4 immune pathway related genes from the overlapping 41 potential target genes are represented (A). The miRTargetLink 2.0 designed network represents only miRNAs and genes that have the potential roles in the regulation of immune responses for better focus (B). Functional enrichments of genes related to miR-21 according to Metascape. The enrichment score is calculated as the logarithm (base 10) of the P-value. A higher enrichment score indicates a more specific association with the corresponding biological function (C)
Using miRTargetLink 2.0 and selecting strong validated genes linked to miR-21, 132 target was found. Among them, 18 immune related genes was visualized. (Fig. 3B).
The analysis of top-level Gene Ontology biological processes reveals a significant association between genes linked to miR-21 and processes within the immune system (Fig. 3C). Additionally, among immune target genes of miR-21, those were selected which interact with each other and play prominent roles in the immune system. Protein–protein interactions of target genes were investigated using the STRING11.0 database (Supplementary Fig. 1).
Discussion
Human Brucellosis is a global multisystem disease that presents with varied symptoms causing difficulty in diagnosis. Recently, mounting evidence has indicated the importance of circulating miRNAs as ideal biomarkers for diagnosis of infectious diseases including brucellosis [18]. Early diagnosis of the disease can result in an improved outcome and better management of the disease. These circulating nucleic acids have ample stability mainly owing to their encapsulation in lipid vesicles or the constitution of complexes with various sorts of proteins that conserve them against degradation [18]. Several studies have shown altered expression of miRNAs in biological fluids of brucellosis patients [15, 17]. Zhang C. et al. indicated a significant increase in the expressions of miR-15a-3p, miR-7–2-3p, miR-103b in serum samples of brucellosis patients and suggested miR-103b as a serum biomarker for brucellosis detection [17]. In another study, the expression levels of miR-1238-3p, miR-6069, miR-494 and miR-139-3p were shown to be altered in peripheral blood mononuclear cells (PBMCs) of patients with chronic brucellosis [15].
In this study, the expression levels of eight circulating miRNAs, including miR-210, miR-155, miR-150, miR-146a, miR-139-3p, miR-125a-5p, miR-29 and miR-21 in brucellosis patients, subgrouped into three categories as acute brucellosis (A), under treatment (UT) and relapse (R) brucellosis, were determined and compared with healthy controls (HCs). Studied miRNAs were chosen via text-mining to evaluate candidate miRNAs involved in the diagnosis of brucellosis.
Among our selected miRNAs, the gene expression levels of miR-155, miR-146a, miR-125a-5p, miR-29, and miR-21 were elevated in acute brucellosis compared to HCs.
No significant differences in the expression levels of miR-210 and miR-150 were found among the three patients' subgroups. miR-210 is up-regulated in monocytes and macrophages in response to pathogen interaction and elevated levels of miR-210 in circulating monocytes in humans correlate with the incidence of sepsis [22]. miR-150 influences the function of immune cells and it suppresses the proinflammatory potential of macrophages [23].
Previous studies agree with our findings that expression of miR-155, miR-146a, miR-125a-5p are associated with the acute phase of the disease and inflammatory conditions [24].
miR-155 was found to be elevated by Francisella tularensis, Helicobacter pylori, and in the wild-type Brucellosis-infected human monocyte and macrophages [14, 25, 26]. It has been reported that Omp25 (the main outer membrane protein of Brucella species) inhibits Toll-like receptor (TLR) agonists-induced IL-12 production in monocytes and macrophages through up-regulation of miR-155 [25]. It has also been reported that miR-155 suppresses the production of endotoxin-stimulated TNF-α and decreases the inflammatory responses by targeting Myeloid differentiation primary response 88 (MyD88) and SH-2 containing inositol 5’ polyphosphatase 1 (SHIP-1) during Francisella tularensis infection [23]. Moreover, Helicobacter pylori increases the miR-155 expression in gastric mucosal tissues and epithelial cell lines. This enhancement in miR-155 expression leads to negative regulation of IL-8 production [14]. Taken together, all the results suggest that miR-155 may function as a negative regulator of inflammation during bacterial infection. Hence, it is plausible that the observed post-treatment decrease of miR-155 expression in our study group could potentially be associated with effective management of the infection.
miR-146a negatively regulates the inflammation process by targeting and suppressing the expression of pro-inflammatory genes, such as those involved in the TLR and Nuclear Factor-kappa B (NF-κB) signaling pathways [27]. The expression of miR-146a was high in Brucella-infected cells [28]. This miRNA was found to be up-regulated in heat-killed Candida albicans-infected macrophages as well as via LPS induction [29]. It has been demonstrated that Epstein–Barr virus latent membrane protein 1 (LMP1) can stimulate the expression levels of miR-146a to suppress the interferon response and increase its survival [30].
In our study, the expression level of miR-125a-5p was enhanced in the acute and relapse phase of brucellosis. It has been reported that the expression levels of miR-125a-5p is decreased in FOXP3-positive T cells, and its overexpression was found in effector T cells compared to resting nTreg cells [31]. The expression profile of miRNAs has been evaluated during Mycobacterium avium infection of macrophages and miR-125a-5p was considered to be altered in these immune cells. It is demonstrated that upregulation of miR-125a-5p remarkably elevate autophagy and declined M. avium survival [32]. In another study, it is found that miR-125a-5p can be an impressive inhibitor of the expression of human hepatitis B virus (HBV) surface antigen [33]. These studies suggest that miR-125a-5p has a positive role in the eradication of infection. Alternatively, miR-125a-5p has been shown to impede the activation of classical macrophages (M1), which are crucial for eradicating infections like brucellosis, while promoting the induction of alternative (M2) activation [34]. In another investigation, researchers have discovered that Brucella abortus infection leads to the downregulation of miR-125b-5p within macrophages. Their research has clarified the role of miR-125b-5p in targeting A20, an inhibitor of NF-kB activation, and subsequently, the decrease in the production of TNFα. Therefore, the presence of miR-125b-5p diminishes the survival of B. abortus. A20 promotes B. abortus intracellular growth via inhibition of macrophage activation and their death [35].Nevertheless, further studies are required to clarify the exact role of miR-125a-5p in brucellosis.
miR-139-3p was increased only in patients in the relapse phase of brucellosis compared with other groups. A recent study by Budak et al. demonstrated a decrease in miR-139-3p expression in PBMCs of brucellosis patients and its relation to the chronicity of the disease. They also demonstrated miR-139-3p relation to various mechanisms and cellular pathways including bacterial invasion of epithelial cells pathways, cytokine-cytokine receptor association, T cell receptor signaling pathway and chemokine signaling pathway [15]. A probable explanation for this discrepancy may be differences in the methodology, study groups and their clinical status.
miR-29 expression was increased in under-treatment patients. As reported by Steiner et al. miR-29 suppresses IFN-γ production following targeting of T-bet suggesting that miR-29 can regulate improper expression of IFN-γ [36]. Moreover, our previous report on these patients indicated that 4–8 weeks of treatment with doxycycline (100 mg/BD) and rifampin (600 mg/day) reduces the abundance of Th1 and secretion of IFN-γ [19]. The decrease in serum level of IFN-γ after the treatment of brucellosis patients has also been reported previously [37]. Taken together, it is possible that the downregulation of IFN-γ production correlates with overexpression of miR-29 in the under-treatment patients.
We found that miR-21 was remarkably increased in acute brucellosis patients in comparison to other subgroups. In a study conducted by Singh et al., high expression of bta-miR-21-5p was reported in the PBMCs of water buffaloes infected with Brucella [16].
It has been demonstrated that pathogens are able to exploit host miRNAs to escape from the immune response [9]. The survival capability of bacteria inside the macrophages was seen in the relapse phase and chronic infection of brucellosis via altering macrophage’s activities including inhibition of apoptosis [5, 38]. Studies have shown that miR-21 can directly target and regulate the expression of B-cell lymphoma-2 (Bcl-2). Overexpression of miR-21 leads to a decrease in the levels of Bcl-2 protein within the cells. The downregulation of Bcl-2 by miR-21 has been implicated in promoting cell survival and resistance to apoptosis [39].
He et al. revealed the downregulation of Bcl-2 as an anti-apoptotic protein in Brucella-infected RAW264.7 cells which are generally used because of their similarity to primary macrophages [28, 40]. In another study, it has been shown that miR-21 promotes M. tuberculosis survival and apoptosis while diminishing the secretion of inflammatory cytokines (IL-1β, IL-6, and TNF-α) by targeting Bcl-2 and TLR4 [41].
It has been shown that Omp25 leads to overexpression of miR-21 in monocytes and macrophages. The enhancement of miR-21-5p negatively regulates IL-12 production by targeting 3'UTR of IL12A and IL12B, resulting in the inhibition of the expression of IL-12 p35 and p40 subunits [25]. IL-12 is produced in the initiation of the infection process and is involved in starting the immune response against Brucella. It promotes the activation of macrophages and facilitates the connection between innate and adaptive immune responses to eliminate Brucella infection [42]. Therefore, an increase in miR-21 expression seems to result in immune dysfunction during Brucella infection [25].
Considering miRNAs, crucial role in the regulation of the immune system, we aimed to identify immune target genes of miR-21 associated with Brucella infection. Through bioinformatics analysis, we observed that miR-21 specifically targets TGFB1, TGFB2, IL-2RA, IL-10, STAT3, CCL1, and CCL20 genes. In our comprehensive bioinformatics analysis, we found TGFB1 to be a shared target gene across all datasets, indicating its significant association with miR-21. It has been shown that the decline in the miR-21 expression leads to an elevated expression of Foxp3, TGF-β1 and circulating Treg cells frequency [43]. Studies have indicated that the activity of regulatory cells can suppress the immune responses against pathogens in brucellosis [44]. It is also reported that the enhanced activity of TGF-β1 is associated with suppressed T cell responses and prolongation of disease in patients with chronic brucellosis [45]. Th1 cells have a central role in immunity to brucellosis and the shift of responses from Th1 to Th2 through IL-4 and IL-10 production may result in the severity of infection [44]. Therefore, it can be suggested that enhanced expression of miR-21 can beneficially modulate the immune responses by selectively targeting genes associated with negative regulation of immune responses. Studies have shown that TGFB1 and TGFB2 are involved in the negative regulation of macrophage cytokine production thereby modulating immune responses [46]. Hence, miR-21 could play a regulatory role in this pathway and be effective in the eradication of infection through suppression of TGFB1 and TGFB2 mRNA expression.
It has been shown that suppression of miR-21 with antisense oligonucleotides significantly enhanced the expression of IL-10 in B cells. This indicates that miR-21 directly regulates the production of IL-10 [47]. Studies showed that B. abortus infection triggers the production of IL-10 and polymorphisms of IL-10 gene have been associated with elevated susceptibility to human brucellosis [48].
MiR-21 can directly target and downregulate negative regulators of STAT3, which is a transcription factor that plays a crucial role in immune responses and inflammation, leading to increased STAT3 activity. Wang et al., demonstrated that STAT3 is a direct target of miR-21 in macrophages. The presence of miR-21, along with the lipid mediator prostaglandin E2 (PGE2), plays a significant role in determining the polarization of macrophages. Silencing of the STAT3 gene dysregulates PGE2-mediated expression of M2 genes in miR-21-/- macrophages [49].
Based on our analysis, another target for miR-21 is CCL20, which acts as a chemotactic factor for lymphocytes. It has been observed that miR-21 can directly target and regulate the expression of CCL20. This regulation of CCL20 expression by miR-21 may impact immune cell recruitment and inflammatory processes [50].
Altogether, all the predicted immune pathway related miR-21 target genes have been shown in several studies to be involved in modulation of infection induced immunity however their functional linkage with miR-21 requires further exploration.
Additionally, ROC curve analysis demonstrated that the corresponding AUC was 0.81, suggesting that miR-21 can be considered as a potential auxiliary indicator for acute brucellosis detection.
In summary, we investigated the expression profile of eight relevant miRNA in Brucellosis patients and observed that the expression of miR-21 is increased in patients in the relapse phase of brucellosis. Based on extensive literature survey, we hypothesize that miR-21 up-regulation could be correlated with Brucella's ability to survive inside the macrophages. miR-21 could be considered a potential therapeutic target for brucellosis. Inhibiting miR-21 could potentially disrupt the mechanisms that allow Brucella to survive inside macrophages by altering the regulation of key genes such as Bcl-2, TGFB1, TGFB2, and STAT3. In addition, suppression of miR-21 might prevent the downregulation of inflammatory cytokines like IL-1β, IL-6, and TNF-α, which are crucial for an effective immune response against intracellular infections. Furthermore, suppression of miR-21 could potentially reduce the production of IL-10, which has been associated with susceptibility to brucellosis. This could help in controlling the immunosuppressive effects of this cytokine. Since miR-21 downregulates Bcl-2, therapeutic strategies aimed at reducing miR-21 levels might promote apoptosis in infected macrophages, leading to better clearance of the bacteria. However, further studies and experiments are essential to validate this hypothesis and to evaluate the potential target genes of miR-21 and its relationship with the development of brucellosis.
Conclusion
We evaluated the circulating levels of eight miRNAs in patients with acute, under-treatment and relapse brucellosis, and found that miR-21 is remarkably increased in acute brucellosis patients. ROC curve analysis indicated that miR-21 may be a potential auxiliary biomarker for the diagnosis of acute brucellosis. Monitoring miR-21 levels could aid in the early detection and diagnosis of the disease. As described above, sufficient evidence exists that describe the role of the currently investigated miRNAs, including miR-21 in immune modulation and inflammation during Brucellosis. However, the mechanism of action of miRNAs in Brucella pathogenesis requires additional investigation. In addition, further studies are required to validate the potential immune pathway related targets of miR21, including TGFB1, TGFB2, IL-2RA, IL-10, STAT3, CCL1, and CCL20 genes and their relationship with development and progression of human brucellosis. Hence, characterization of the role of miRNAs in host-Brucella interactions could serve as the foundation for the development of prognostic and therapeutic strategies.
Data availability
All data generated or analyzed during this study are included in this published article.
References
de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG (2015) Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol 185:1505–1517
Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV (2006) The new global map of human brucellosis. Lancet Infect Dis 6:91–99
Golshani M, Buozari S (2017) A review of brucellosis in Iran: epidemiology, risk factors, diagnosis, control, and prevention. Iran Biomed J 21:349
Abedi A-S, Hashempour-Baltork F, Alizadeh AM et al (2020) The prevalence of Brucella spp. in dairy products in the middle east region: a systematic review and meta-analysis. Acta Trop 202:105241
Hosseini SM, Abbasalipourkabir R, Jalilian FA et al (2019) Doxycycline-encapsulated solid lipid nanoparticles as promising tool against Brucella melitensis enclosed in macrophage: a pharmacodynamics study on J774A. 1 cell line. Antimicrob Resist Infect Control 8:1–12
Zowghi E, Ebadi AG, Yarahmadi M (2008) Isolation and identification of Brucella organisms in Iran. Arch Clin Infect Dis 3:185–188
Kazemi S, Mirzaei R, Sholeh M et al (2021) microRNAs in human brucellosis: a promising therapeutic approach and biomarker for diagnosis and treatment. Immun Inflamm Dis 9:1209–1218
Long H, Wang X, Chen Y, Wang L, Zhao M, Lu Q (2018) Dysregulation of microRNAs in autoimmune diseases: pathogenesis, biomarkers and potential therapeutic targets. Cancer Lett 428:90–103
Drury RE, O’Connor D, Pollard AJ (2017) The clinical application of microRNAs in infectious disease. Front Immunol 8:1182
Zhang D, Yi Z, Fu Y (2019) Downregulation of miR-20b-5p facilitates Mycobacterium tuberculosis survival in RAW 264.7 macrophages via attenuating the cell apoptosis by Mcl-1 upregulation. J Cell Biochem 120:5889–5896
Lee DY, Jeyapalan Z, Fang L et al (2010) Expression of versican 3′-untranslated region modulates endogenous microRNA functions. PLoS ONE 5:e13599
Zabaglia LM, Sallas ML, Santos MPD et al (2018) Expression of miRNA-146a, miRNA-155, IL-2, and TNF-α in inflammatory response to Helicobacter pylori infection associated with cancer progression. Ann Hum Genet 82:135–142
Castañeda-Ramírez A, González-Rodríguez D, Hernández-Pineda JA, Verdugo-Rodríguez A (2015) Blocking the expression of syntaxin 4 interferes with initial phagocytosis of Brucella melitensis in macrophages. Can J Vet Res 79:39–45
Xiao B, Liu Z, Li B-S et al (2009) Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis 200:916–925
Budak F, Bal SH, Tezcan G, Akalın H, Goral G, Oral HB (2016) Altered expressions of miR-1238–3p, miR-494, miR-6069, and miR-139–3p in the formation of chronic brucellosis. J Immunol Res 2016:1–11
Singh J, Dhanoa JK, Choudhary RK et al (2020) MicroRNA expression profiling in PBMCs of Indian water Buffalo (Bubalus bubalis) infected with Brucella and Johne’s disease. ExRNA 2:1–13
Zhang C, Fu Q, Ding M et al (2019) Comprehensive analysis of differentially expressed serum microRNAs in humans responding to Brucella infection. Ann Transl Med 7:301
Condrat CE, Thompson DC, Barbu MG et al (2020) miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells 9:276
Rahmanpour M, Keramat F, Jourghasemi S et al (2019) Direct correlation between Th1 and Th17 responses in immunity to Brucella infection. Microbes Infect 21:441–448
Eini P, Keramat F, Hasanzadehhoseinabadi M (2012) Epidemiologic, clinical and laboratory findings of patients with brucellosis in Hamadan, west of Iran. J Res Health Sci 12:105–108
Zhou Y, Zhou B, Pache L et al (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10:1523
Virga F, Cappellesso F, Stijlemans B et al (2021) Macrophage miR-210 induction and metabolic reprogramming in response to pathogen interaction boost life-threatening inflammation. Sci Adv 7:eabf0466
Bandyopadhyay S, Long ME, Allen L-AH (2014) Differential expression of microRNAs in Francisella tularensis-infected human macrophages: miR-155-dependent downregulation of MyD88 inhibits the inflammatory response. PLoS ONE 9:e109525
Rezaeepoor M, Pourjafar M, Tahamoli-Roudsari A, Basiri Z, Hajilooi M, Solgi G (2020) Altered expression of microRNAs may predict therapeutic response in rheumatoid arthritis patients. Int Immunopharmacol 83:106404
Cui B, Liu W, Wang X et al (2017) Brucella Omp25 upregulates miR-155, miR-21-5p, and miR-23b to inhibit interleukin-12 production via modulation of programmed death-1 signaling in human monocyte/macrophages. Front Immunol 8:708
Cremer T, Ravneberg D, Clay C, Piper-Hunter M, Marsh C (2009) MiR-155 induction by F. novicida but not the virulent F. tularensis results in SHIP down-regulation and enhanced pro-inflammatory cytokine response. PLoS ONE 4:e8508
Taganov KD, Boldin MP, Chang K-J, Baltimore D (2006) NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci 103:12481–12486
Zheng K, Chen D-S, Wu Y-Q et al (2012) MicroRNA expression profile in RAW264. 7 cells in response to Brucella melitensis infection. Int J Biol Sci 8:1013
Monk CE, Hutvagner G, Arthur JSC (2010) Regulation of miRNA transcription in macrophages in response to Candida albicans. PLoS ONE 5:e13669
Cameron JE, Yin Q, Fewell C et al (2008) Epstein-barr virus latent membrane protein 1 induces cellular MicroRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol 82:1946–1958
Li D, Kong C, Tsun A et al (2015) MiR-125a-5p decreases the sensitivity of treg cells toward IL-6-mediated conversion by inhibiting IL-6R and STAT3 expression. Sci Rep 5:14615
Wang Y, Chen C, Xu X-d et al (2020) Levels of miR-125a-5p are altered in mycobacterium avium-infected macrophages and associate with the triggering of an autophagic response. Microbes Infect Microbes Infect 22:31–39
Potenza N, Papa U, Mosca N, Zerbini F, Nobile V, Russo A (2011) Human microRNA hsa-miR-125a-5p interferes with expression of hepatitis B virus surface antigen. Nucleic Acids Res 39:5157–5163
Banerjee S, Cui H, Xie N et al (2013) miR-125a-5p regulates differential activation of macrophages and inflammation*. J Biol Chem 288:35428–35436
Liu N, Wang L, Sun C, Yang L, Sun W, Peng Q (2016) MicroRNA-125b-5p suppresses brucella abortus intracellular survival via control of A20 expression. BMC Microbiol 16:171
Steiner David F, Thomas Molly F, Hu Joyce K et al (2011) MicroRNA-29 regulates T-box transcription factors and Interferon-γ production in helper T cells. Immunity 35:169–181
Akbulut H, Celik I, Akbulut A (2007) Cytokine levels in patients with brucellosis and their relations with the treatment. Indian J Med Microbiol 25:387
Gross A, Terraza A, Ouahrani-Bettache S, Liautard J-P, Dornand J (2000) In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect Immun 68:342–351
Li Y, Sun G, Wang L (2022) MiR-21 participates in LPS-induced myocardial injury by targeting Bcl-2 and CDK6. Inflamm Res 71:205–214
He Y, Reichow S, Ramamoorthy S et al (2006) Brucella melitensis triggers time-dependent modulation of apoptosis and down-regulation of mitochondrion-associated gene expression in mouse macrophages. Infect Immun 74:5035–5046
Zhao Z, Hao J, Li X, Chen Y, Qi X (2019) MiR-21-5p regulates mycobacterial survival and inflammatory responses by targeting Bcl-2 and TLR4 in mycobacterium tuberculosis-infected macrophages. FEBS Lett 593:1326–1335
Sathiyaseelan J, Goenka R, Parent M et al (2006) Treatment of Brucella-susceptible mice with IL-12 increases primary and secondary immunity. Cell Immunol 243:1–9
Li S, Fan Q, He S, Tang T, Liao Y, Xie J (2015) MicroRNA-21 negatively regulates treg cells through a TGF-β1/Smad-independent pathway in patients with coronary heart disease. Cell Physiol Biochem 37:866–878
Rafiei A, Ardestani SK, Kariminia A, Keyhani A, Mohraz M, Amirkhani A (2006) Dominant Th1 cytokine production in early onset of human brucellosis followed by switching towards Th2 along prolongation of disease. J Infect 53:315–324
Elfaki MG, Al-Hokail AA (2009) Transforming growth factor β production correlates with depressed lymphocytes function in humans with chronic brucellosis. Microbes Infect 11:1089–1096
Maheshwari A, Kelly DR, Nicola T et al (2011) TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140:242–253
Wang H, Xu W, Shao Q, Ding Q (2017) miR-21 silencing ameliorates experimental autoimmune encephalomyelitis by promoting the differentiation of IL-10-producing B cells. Oncotarget 8:94069
Xavier MN, Winter MG, Spees AM et al (2013) CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog 9:e1003454
Wang Z, Brandt S, Medeiros A et al (2015) MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generation. PLoS ONE 10:e0115855
Yao T, Lin Z (2012) MiR-21 is involved in cervical squamous cell tumorigenesis and regulates CCL20. Biochim Biophys Acta Mol Basis Dis 1822:248–260
Acknowledgements
We acknowledge the support from Hamadan University of Medical Science.
Funding
This study was funded by Hamadan University of Medical Sciences (Grant Number: # 9607044220), Hamadan, Iran.
Author information
Authors and Affiliations
Contributions
MR: Investigation, conducting of the experiments, analysis, writing. FK: Brucellosis diagnosis and introducing patients. SJ: Investigation, conducting of the experiments. MR: Investigation, conducting of the experiments. AL: Editing and analysis. MH: Project administration, funding acquisition GS: Supervision, Review, Editing.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of Hamadan University of Medical Sciences (No: IR.UMSHA.REC.1396.441).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11033_2023_9193_MOESM1_ESM.tif
Supplementary file1 (TIF 97 KB)—The network was designed using the STRING11.0 database. Red nodes represent proteins with a negative regulatory role in cytokine production by macrophage. Blue nodes represent related molecules that have roles in the negative regulation of cytokines. Green, yellow, and purple nodes were used for indicating molecules that participate in negative regulation of immune response, immune effector process, and inflammatory responses, respectively.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rezaeepoor, M., Keramat, F., Jourghasemi, S. et al. MicroRNA -21 expression as an auxiliary diagnostic biomarker of acute brucellosis. Mol Biol Rep 51, 264 (2024). https://doi.org/10.1007/s11033-023-09193-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09193-8