Abstract
Modeling a human disease is an essential part of biomedical research. The recent advances in the field of molecular genetics made it possible to obtain genetically modified animals for the study of various diseases. Not only monogenic disorders but also chromosomal and multifactorial disorders can be mimicked in lab animals due to genetic modification. Even human infectious diseases can be studied in genetically modified animals. An animal model of a disease enables the tracking of its pathogenesis and, more importantly, to test new therapies. In the first part of this paper, we review the most common DNA modification technologies and provide key ideas on specific technology choices according to the task at hand. In the second part, we focus on the application of genetically modified mice in studying human diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Presently, many human diseases, both monogenic and multifactorial, are not amenable to treatment. Effective models of various diseases are needed for fundamental studies of their pathogenesis and the search for therapeutic approaches. The simulation of various pathological conditions in animals is gaining momentum in the scientific community. As technology develops, more and more new tools appear to solve these problems. In 2020 the Nobel Prize was awarded for the discovery of the CRISPR/Cas9 system, which has greatly simplified precise genome editing. This review includes a description of both classical and modern genome editing technologies and discusses their applicability in the most interesting animal models of human diseases.
Animals are used in the study of human diseases because of their genetic, anatomical, and often physiological similarities to humans [1]. Laboratory mice are easy to handle, their pregnancy lasts for 3 weeks, and they reach sexual maturity at 4–7 weeks old. In addition, the human and the mouse genomes have 80% homology, and some of their coding sequences are 99% identical. Despite big differences in body size and lifespan, together this makes the mouse the most suitable animal for studying human diseases [1].
In early biomedical studies, new mouse models were created by the process of selective breeding, aimed to produce offspring with the desired traits. Nowadays different genomic DNA modifications can be introduced into the animal genome using several technologies. The modifications, introduced into the germ line cells, can be transmitted to offspring and then to the following generations [2]. The term “genetically modified animal” is a broad term that refers to an animal with a deliberate modification of the genome in contrast to spontaneous mutations [3]. The term “genome-edited animal” is narrower and is usually applied to cases when precise editing tools (e.g., CRISPR/Cas9, TALEN, etc.) are used.
Currently, the most used DNA modification strategies are transgenesis (random insertion of linear DNA bearing an expression unit) and more precise approaches based on the CRISPR/Cas9 system: single-gene knockouts, precise editing when one or several nucleotides are altered, and knock-ins (site-specific insertions). The CRISPR/Cas system is now rapidly evolving and new Cas proteins with novel properties are being discovered and engineered. In this review, we are going to use the CRISPR/Cas9 term because the vast majority of genome-edited animals were obtained using this system.
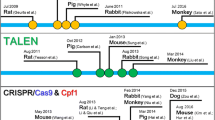
Transcription activator-like effector nucleases (TALEN) system and zinc-finger nucleases (ZFNs) were used for precise genome editing in the recent past, but now they are almost completely superseded by the far more convenient CRISPR/Cas9 system. However, several valuable animal models were created using TALEN and ZFNs. Some models obtained long ago during random large-scale mutagenesis are still in use as well [4, 5].
The components of any of such modification systems can be introduced directly into zygotes or by means of embryonic stem cells (ESC). Direct delivery methods involve DNA/RNA/protein microinjections in the pronuclei or cytoplasm (I) [6, 7], electroporation (II) [8], and even viral delivery (III) (Fig. 1) [9]. Microinjections and electroporation require special equipment and the survival rate of embryos can be limited [8, 10]. There are two principal distinct approaches to embryo electroporation: ex-corporal and in-corporal. In the case of ex-corporal electroporation, embryos are retrieved from mice, placed in a specially constructed chamber that enables the modification of genes, and then electroporation occurs [8]. When in-corporal electroporation (called the i-Gonad method) is used, CRISPR reagents are delivered directly into the pregnant females’ oviducts, then special electrodes are placed near the oviducts and electroporation occurs in vivo, in oviducts themselves [11,12,13,14,15]. Viral delivery requires additional steps to produce the viruses and to ensure a transient way of transgene expression to avoid off-targets. Manipulations with ESCs, on the other hand, have the advantage of preliminary selection and analysis of cells, after which the cells with the desired genotype can be transferred into the early embryos [16, 17]. Application of the ES cells makes it possible to detect and amplify rarer outcomes, however, direct methods are easier and cheaper.
It should be taken into account that the application of the ESC-transfer method or direct methods of delivery into the zygote as a rule leads to the birth of pups with different cells that have different genomes. Thus, in the case of direct methods of delivery, it is explained due to a phenomenon called genetic mosaicism. In ideal conditions, zygote genome editing should occur at the 2n2c stage, resulting in two alleles. However, DNA replication occurs soon after fertilization, before pronuclei fusion, transitioning to the 2n4c stage where genome editing can result in more than two alleles. To reduce the level of mosaicism, a microinjection of a genetic substance may be performed at the early zygote stage or directly into the oocyte before fertilization [18].
When the ESC-transfer method is used, typically chimerism is observed. Pups developed from the embryos that were injected with ES cells [19] or which were aggregated with ES cells [20] contain two subpopulations of cells, those that evolved from ES cells and those that evolved from initial embryos’ cells. To reduce the level of chimerism injection of ES cells could be performed into the tetraploid blastocyst, which is typically obtained by electrofusion of the embryo at the 2-cell stage. Tetraploid cells of the blastocyst predominantly develop into the trophectoderm cells which take place in implantation but not in embryonic tissue formation, and injected diploid ES cells are mainly involved in the formation of embryonic tissues [21].
It should be taken into account that due to mosaicism or chimerism transmission of target mutation in the germline of F0 mouse is a probabilistic process, therefore multiple crossings may be required to obtain a completely transgenic F1 animal. In the worst case when the desired mutation did not present in germline due to mosaicism or chimerism it is required to obtain F0 de novo (see Table 1)
Genome alteration techniques for certain experiments must be chosen based on the final goal to strike a balance between the efficiency and the precision and the experiment’s costs.
In the first part of this review, we are going to briefly discuss molecular biological approaches to genetically modified (GM) animal manufacturing, and in the second part, we will focus on the limitations of genetically modified animals’ usage.
Approaches to genome editing
Historically, the first genome modification approach was random mutagenesis using physical or chemical mutagens [30]. Radiation is usually used to create large-scale mutations, translocations, and multiple deletions [5, 30], whereas chemical compounds (e.g., triethylenemelamine, N-ethyl-N-nitrosourea (ENU)) drive point mutations [4, 31].
Although more precise methods have become popular since that time, random mutagenesis still retains one advantage: it can be used for studying human diseases in screening “from phenotype to genotype” research. Screening of animals exposed to mutagens can be used for identification of those with mutations, presumably associated with certain diseases. For example, large-scale mutagenesis and phenotype screening were applied to identify mutant mice with alterations in the nervous system and behavior [32].
Statistically random mutations (alterations, insertions, and deletions), even small and point mutations, in ORFs (open reading frames) quite often result in the frameshifts. Insertions and deletions can cause frameshifts if the number of inserted/deleted nucleotides is not multiple of three or when they affect splicing sites. Alterations can interrupt splicing sites and lead to the frameshifts this way. Consequently, such mutations most often lead to the loss of gene functions, but sometimes translocations and small mutations can lead to the gain of certain gene functions.
Regardless of the method of mutagenesis, as a first step of classification, all genetically modified animals can be divided into two major groups: animals with the loss of a certain gene function and animals with the gain of a certain gene function.
Loss of function
International Knockout Mouse Consortium (IKMC) comprises the Knockout Mouse Project (KOMP), the European Conditional Mouse Mutagenesis Program (EUCOMM), and several others. Their goal includes producing and subsequent phenotype assessment of several thousands of constitutive and conditional knockout mouse strains [33]. Development of IKMC lasted for several decades and currently, the most popular method for knockout animal generating is the ES-mediated CRISPR/Cas9 system. IMPC (International Mouse Phenotyping Consortium) also provides phenotypic data of knockout mouse strains.
The CRISPR/Cas9 system originates from one of the mechanisms of the prokaryotic adaptive immune system, which was adopted by researchers for gene editing purposes. The topic is currently receiving a great deal of attention in the scientific community [34]. New slightly different variants are being developed to enable more thorough coverage of a wider range of tasks, including epigenome editing [35]. The basic principle is that an exogenous nuclease Cas9 cuts the genome at specific sites indicated by sgRNAs (single guide RNA) to generate a double-strand break [36]. After the cut, the resulting double-strand break is then repaired by one of three general DNA repair pathways: the non-homologous end joining (NHEJ) pathway, the homology-directed repair (HDR) pathway or microhomology-mediated end joining (MMEJ). The NHEJ pathway often leads to several nucleotide insertions or deletions leading to frameshifts and thus can be used for knockout generation. MMEJ can be employed to obtain bigger deletions, however are more often used for precise KI [37] (Fig. 2).
As S. pyogenes Cas9 making blunt-ended double-strand breaks is by a wide margin the most harnessed variant, novel variants can be used where SpCas9 drawbacks become critical. First, mutant variants and variants from another species can have different PAMs, which makes it easier to target a specific locus [38, 39]. Another drawback of SpCas9 is its relatively big size (more than 4000 bp and 1300aa). S. aureus Cas9 [39] and Cas12 (Cpf1) are about one-third smaller (about 1000aa), and this makes a difference, for example, for AAV packaging. Cas12 also has a feature important for GE animal manufacturing—it produces “sticky” ends, which facilitate homology-directed repair instead of nonhomologous end joining [40]. Another interesting variant, CasRx, an RNA instead of DNA targeting system, was used to improve HDR efficiency [41].
The efficacy of CRISPR/Cas mediated KO approach is very high and depends mostly on sgRNA selection. The components of the CRISPR/Cas9 system can be injected directly into the zygotes in the RNA/protein form or even as a plasmid intended for transient expression. Also, viral delivery methods can be used. Another interesting approach for gene editing is to execute transgene mice expressing Cas9 [42] and then deliver to the embryos only sgRNA [43].
Before the advance of the CRISPR/Cas9 era, knockouts were often generated in the ESC by gene targeting—an approach based on naturally occurring homologous recombination. There could be some variations, but the general scheme is as follows (see Fig. 2). A genetic structure is made containing two parts of the target locus as homology arms and a selection marker flanked by LoxP sites (or FRT sites) inserted between them instead of the middle part. The structure was delivered to the ES cells and the cells were subjected to chosen selection. To ensure that the insertion is not random, negative selection can be performed as well. Expression cassettes encoding negative selection markers such as DT-A (diphtheria toxin fragment A) or HSV-TK which is toxic in the presence of ganciclovir can be placed outside the homology arms. The surviving cells then are injected into the mouse embryos or used for aggregation chimeras manufacturing, then embryos are transferred to the foster mothers, resulting in the birth of pups. Obtained mice (F0) are propagated and then crossed with Cre or Flp recombinase-expressing strains. After the selection marker excision by the recombinase, a pure line is bred as homozygous if possible, or as heterozygous if not. However, sometimes crossing with deleter strains is omitted. This approach takes much time and is more expensive than CRISPR/Cas9, but many models in use today were made that way [44, 45].
Although schemes implying homologous recombination are now redundant for simple knockout generation, they are still widely used for so-called conditional knockouts. Knockouts of certain genes can be lethal either at the embryonic stages or even in adult animals. In conditional knockout animals, one of the critical exons is flanked by LoxP sites. The exon is considered critical if its length is not divisible by 3 and all the protein isoforms contain this exon [46]. In this case, an inducible and/or tissue-specific Cre-recombinase can produce mouse strains with inducible and/or tissue-specific knockouts. The addition of the CRISPR/Cas9-induced double-strand breaks (DSB) enhances the efficiency of the homologous recombination because these breaks activate DNA damage response pathways, one of which is HDR. In some papers the combination of the CRISPR/Cas9 DSB with the homologous recombination has been shown to enable the production of animals with conditional knockout manipulating zygotes directly, avoiding the need for ES cells [47, 48].
The use of RNA interference may be considered if knockout of a gene is completely impossible even in adult mice or fine-tuning of the gene expression level is required. This mechanism regulates gene expression at the posttranscriptional level and requires short RNA expression. Such expression can be obtained by the random insertion or knock-in of the linear double-stranded DNA expression cassette, containing an RNA promoter, shRNA coding sequence, and a terminator. shRNA (small hairpin RNA) expression can be made inducible with the use of, for example, the TetOn system [49, 50]. The operation of the TetOn/TetOff systems is shown in more detail in Fig. 3.
Gain of function
The most obvious example of the gain-of-function genome modification is a case when the whole ORF of the gene of interest with some regulatory elements is randomly inserted into the genome as a linear double-stranded DNA. This modification is often referred to as transgenesis (Fig. 4). The regulatory elements can provide inducible, tissue-specific, or inducible tissue-specific expression [6]. The addition of the insulators, sequences that isolate the insertion from its genomic environment, can help to avoid position effects, i.e., the mutual influence of the transgene and the sequences at the place of its insertion [51]. The addition of the homology arms to the structure and the CRISPR/Cas9 directed cuts in the genome allows insertion of the structure precisely into the selected locus, though it makes the process less effective. Precise transgenesis is often referred to as knock-in (Fig. 4). Surprisingly, there are a few limitations regarding the size of the KI sequence. Sequences up to 200 kb were successfully knocked in using single-strand adaptors [52].
Reproduction of SNPs (single nucleotide polymorphism) and other small mutations is a very important type of animal model used in the study of human diseases [53]. They are often found during Genome-Wide Association Studies (GWAS), but without direct experiments, their role in the etiology and the pathogenesis of certain diseases remains unproven and unclear. Small mutations can be reproduced using the CRISPR/Cas9 system and homologous recombination. This approach is most effective when targeted nucleotides lie very close to the selected PAM site (the element in the sequence required for CRISPR/Cas9 targeting). The desired outcome can be obtained by the microinjections of a synthetic single-stranded oligodeoxynucleotide (ssODN) as an HDR matrix and a plasmid structure transiently expressing Cas9 protein and sgRNA into the zygotes, bypassing the need for ESC. However, for a direct approach, it is important to choose genes which knockout is not lethal, because HDR is less effective than NHEJ [54, 55], and in the majority of the embryo’s cells NHEJ would cause different frameshifts and only a small part would contain target mutation. (Fig. 4).
As HDR efficiency in mouse embryos (as well as in other species) is rarely higher than 5% [56], there is a branch of research focused on improving the efficiency of precise KIs. One possible approach lies in the plane of manipulation with DSB repair pathways inhibiting, most often chemically, NHEJ and promoting HDR [57]. Besides chemical inhibition, as RNA can be easily delivered to the embryo along with the components of the editing system, RNA interference-based methods are suitable for gene suppression in embryos (for example, KU70 and KU80 NHEJ proteins). Also, mRNA of the protein promoting HDR (e.g. Rad50 and Rad51) can be delivered this way. These approaches are thoroughly reviewed in [55] and [58]. Interestingly, RNA targeting Cas variant, CasRx was efficiently used to destroy mRNA (Rad52, Ku70, and Polq) coding proteins involved in undesired repair pathways to promote HDR [41].
Another approach involves improved design of the template for recombination. For example, it was shown that chemical modifications of ssODN [59], chemical prevention of template concatemerization [60], or biotin-streptavidin linking of Cas protein and DNA template [61] can increase HDR efficiency. Different template designs can also be related to employing different DNA repair pathways. MMEJ—a less studied DSB repair pathway, which requires much shorter homology arms (about 20 bp against 800 bp for HDR) (Fig. 5), not only makes template cloning much easier but also increases KI rate in mouse embryos and other clinically relevant cell types [56]. Its characteristic feature is also the design of the template plasmid which includes sgRNA recognition sites at the 5’ and 3’ ends of homology arms so that circular plasmid is processed to the linear template already in the embryo (Fig. 5) [62]. Homology-mediated end joining (HMEJ) technique combining HDR long homology arms and MMEJ template design appeared to be even more efficient (Fig. 5) [56]. Combi-CRISPR strategy successfully combined HDR and NHEJ options by adding an adjacent intronic sgRNA both to the template and to the editing mix (Fig. 5) [63].
Another potentially important aspect of HDR efficiency is the cell cycle stage and corresponding chromatin state. As HDR is restricted to late S/G2 stages, it was proposed that postponing microinjections from the standard pronucleus stage to the 2-cell stage can favor HDR. This hypothesis was successfully confirmed by Gu et al. [64]
Several diseases are shown to be caused by chromosomal rearrangement. Besides inborn genetic disorders, chromosomal rearrangements play an important role in tumor formation (for example, Philadelphia translocation in leukemias) [65]. These cases can also be regarded as “gain of function” examples. The targeted rearrangement of chromosome regions can be achieved by LoxP site insertion in specific positions. In this case, rearrangement can occur upon induction of Cre recombinase [66]. Depending on the LoxP orientation, deletions, duplications, and inversions may be produced (reviewed in [67]) (Fig. 6).
Similarly, chromosome rearrangements can be generated using the CRISPR/Cas9 system. Simultaneously cleaved chromosomes can be repaired incorrectly, which leads to the formation of chimeric chromosomes and gene fusions. This approach enabled the reproduction of CD74-ROS1, EML4-ALK, and KIF5B-RET rearrangements occurring in lung cancer in mouse models [68, 69].
Viral delivery
Viral delivery of the components of the genome-editing systems represents an attractive though understudied topic in the field of genome-modified animals. Of all the viral vectors used in laboratories, adeno-associated viral (AAV) vectors seem to be the most promising for the transduction of zygotes. Likely because of their small size (18–26 nm against 80–130 nm for lenti- and retro-viruses, and 70–105 nm for adenoviruses [70]), AAVs, unlike other viral types [71] can penetrate freely through zona pellucida (Fig. 7). The genome of AAVs consists of a single strand DNA molecule, which is probably the best way to deliver the HDR matrix. AAVs do not integrate into the genome and sustain only transient transgene expression, which enables the avoidance of off-target effects and leads to better biosafety characteristics [70].
AAVs were used to deliver HDR matrix into the murine and rat zygotes [27] and successfully transduced bovine zygotes [26, 27]. The main drawback of AAVs—their limited packaging capacity (< 4.5 kb)—makes it difficult to deliver the most common Cas variant—SpCas9—ORF along with the sgRNA expression unit. Nevertheless, newly emerged Cas variants with shorter ORFs solve this problem [70]. Alternatively, genome editing effectors that induce double-strand breaks could be delivered by microinjections or electroporation.
Selection of the optimal way of transgenesis depends on many factors: available equipment, personnel, and the type of required transgene. Thus, getting the knockouts by NHEJ can be achieved easily by the injection of a genetic substance into the cytoplasm or pronuclei, while insertion of long site-specific structures requiring HDR may be difficult; the better way in this case may be production, selection, and further injection of transgene ESC into the blastocysts to obtain chimeras.
Disease models
An animal model of a disease enables the study of its pathogenesis and, more importantly, in some cases, to test new therapies. Several types of disorders, not only monogenic but also chromosomal and multifactorial disorders can be mimicked in lab animals by genetic modification. Even human-specific infectious diseases can be studied in genetically modified animals.
Monogenic disorders are the most natural field for the transgenic animal models application as they are relatively easy to reproduce and the causal relationship between the genotype and the phenotype is obvious. The models representing amyotrophic lateral sclerosis, Duchenne muscular dystrophy, and Lesch-Nyhan disease are of great interest.
Multifactorial disorders such as cancer, cardiovascular diseases, obesity and diabetes, autoimmune diseases, etc. rarely demonstrate Mendelian inheritance but have genetic components that may be revealed, for example, using GWAS. In this case, animal models can serve as a tool to prove the role of certain genes in the disease’s etiology and pathogenesis.
Moreover, although GM models do not ideally mimic corresponding human multifactorial disorders, they can be helpful in preclinical studies of potential therapies.
The most significant models of human diseases performed on mice are given in Table 2.
Concerns
Although many human diseases can be easily reproduced in mice, the application of mice as a model has certain limitations. As has been mentioned above, the human and mouse genomes have 80% homology but 20% are not identical, resulting in different lifespan and body structure. Thus, many age-related human diseases are not observed in mice because the mice's lifespan usually is limited to 1–2 years. Other potential concerns about the application of mice as a models will be considered below.
Genetic background
When planning an experiment for modeling human pathological conditions in mice, it should be considered that the genetic background of the model can affect its phenotypic manifestations [95]. This is especially important for the manifestation of various neurodegenerative diseases’ symptoms. It is known that different inbred mice lines are prone to certain pathologies. For example, DBA/2 J(D2) mice are prone to diseases related to nerve cell death, in particular, to glaucoma [96] and hearing loss [97]. As a result, DBA/2 J.APPswePSEN1de9 model of Alzheimer’s disease with overexpression of human PSEN1 and APP genes with Alzheimer-related mutations on the DBA/2 genetic background has more pronounced phenotypic manifestations than the same model on the C57BL/6 genetic background [83].
It has also been shown that mice with knockout of the Cln3 gene encoding a lysosomal protein, a model of the neurodegenerative juvenile Batten disease, have different manifestations on two genetic backgrounds: 129S6/SvEv and C57BL/6 J. It was shown that Cln3−/− has more pronounced symptoms on the 129S6/SvEv genetic background, which makes these mice the most suitable for the development of therapeutic approaches [98].
A similar effect is observed in the myodystrophy mouse model (e.g. MDX): in MDX mice, in which a mutation in the Dmd gene results in the premature stop-codon in exon 23, symptoms appear more clearly on the DBA/2 J genetic background than in the same model on the C57Bl/6 line [99].
Mouse models of SMA (spinal muscular atrophy), also demonstrate that the severity of symptoms depends on the genetic background. Mice carrying a mutation in the splicing region of exon 7 of the mouse Smn gene (Smn2B/2B) were created and then transferred to the C57BL/6(BL6) and FVB genetic backgrounds and crossed with Smn−/−. It was shown that Smn2B/− mice on the FVB background have a shorter lifespan than the same model on the C57BL/6 background (median lifespan was 19 days and 25 days, respectively). No difference was found in the level of Smn gene expression between these strains [100].
In a model of familial amyotrophic lateral sclerosis (fALS) with the G93A mutation in the hSOD1 gene, the most severe symptoms develop in ALR, NOD.Rag1KO, SJL, and C3H mice. Less severe phenotypic manifestations are observed in hybrid B6xSJL mice and, finally, the mildest manifestations were observed in the inbred mouse strains B6, B10, BALB/c, and DBA [101].
Comparative analysis of genetic pathologies and their manifestations in different lines of animals will allow us not only to obtain an animal model that is closest in phenotype to human pathology but also to find concomitant genes that affect the severity of the phenotypic manifestation with the same mutation, which will enable full exploration of the pathogenesis and possible therapeutic approaches [102].
Atherosclerosis
Another type of diseases that are difficult to model by creating genetically modified animals is atherosclerosis. Atherosclerosis is a condition when the wall of the artery develops abnormalities, called lesions. These lesions may lead to blood vessel narrowing due to the buildup of atheromatous plaque.
Wild-type mice do not develop atherosclerosis due to their very short lifespan compared to humans. Several genetically modified atherosclerosis mouse strains are used to study the fundamental aspects of atherosclerosis development, but their use as models for the development of therapeutic approaches is not always possible due to the peculiarities of the development of atherosclerotic lesions. The most well-known model of atherosclerosis is the apolipoprotein E gene knockout mouse, ApoE−/− [79, 80], which has a significant increase in plasma cholesterol levels compared to wild-type animals. The formation of atherosclerotic vascular lesions in these mice occurs even on a standard diet. However, it must be considered that the APOE protein is multifunctional and is involved in many processes, such as inflammation, proliferation, and migration of smooth muscle cells. Therefore, ApoE knockout can lead to the formation of atheromas unrelated to the lipid profile of blood plasma [103].
Another extremely popular atherosclerosis model is the Ldlr gene knockout, which encodes the low-density lipoprotein receptor. LDLR is a glycoprotein on the cell surface of hepatocytes, which plays a key role in the endocytosis of low-density lipoproteins; patients with mutations in this gene develop hereditary hypercholesterolemia with an increased level of low-density lipoproteins in blood plasma and the development of atherosclerotic vascular damage [104].
During the testing of drugs intended for the treatment of atherosclerosis and its consequences on Ldlr−/− mice, it turned out that the level of cholesterol in the blood plasma in these animals is not reduced as effectively as in humans [82], moreover, these animals do not have a human-specific complication of atherosclerosis, atherosclerotic plaque rupture, and thrombus formation [81].
The reasons why mouse models of atherosclerosis do not fully represent the phenotype typical for humans are not well understood. Potentially, it depends on the life span, blood flow, or structure of blood vessels.
Two published studies [105, 106] aimed to evaluate the effects of the human apolipoprotein AI overexpression in db/db mice (db/db mice carry mutation-producing type 2 diabetes [84, 85]). Elevating the APO-AI level or mimicking it with other peptides is one of the modern therapeutic approaches supposed to improve HDL (high-density lipoprotein) atheroprotective functions. The researchers bred transgenic APO-AI mice [107] with the overexpression of the human apolipoprotein A-I (APOA-I) gene with db/db mice. Mendez-Lara et al. [105] demonstrated in this research that the overexpression of APO-AI in db/db mice enhanced the anti-atherogenic properties of high-density lipoprotein (HDL). However, the overexpression of APO-AI also exacerbated weight gain and the fatty liver phenotype in mice; due to these side effects the use of APO-AI-mediated or HDL-based therapies is not recommended in humans suffering from obesity. This study illustrates how experiments with GM animals can help to predict adverse side effects of certain therapies.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints, typically resulting in warm, swollen, and painful joints.
In the case of rheumatoid arthritis, genetically modified mouse models elucidated different aspects of the pathogenesis such as the roles of CD4 + T− and B-cells, proinflammatory cytokines, and autoantigens. The main problem of such models is that due to the relatively low homology level between animal and human immune targets, most of the potentially effective therapeutics tested in model animals are not effective in humans. To overcome this problem, humanized animal models were proposed (reviewed in [108]). Humanized animal models imply transgenic expression of human molecules, such as HLA class II, RA-associated synovial autoantigens, and/or an autoantigen-specific T cell receptor, in immunocompetent mice. Being a step forward, these models partially share their main drawback with classical models: inflammation is driven by the murine immune system.
Cancer
There are two main distinct purposes for using genetically modified mouse models in cancer research. The first is to identify the genes with sequence alterations or expression level changes between tumor and normal tissue which are responsible for tumor formation, growth, and metastasis. The second is to produce models for preclinical studies of newly emerging therapies.
Knockouts and knock-ins of specific genes are often used to determine their role in cancer. However, constitutive knockouts and knock-ins are often unsuitable because they may be lethal or change the phenotype too much or their effect may be even neutralized by the developmental compensation [109].
The use of highly specific promoters can partially solve this problem. For example, a pancreatic cancer model was obtained by overexpression of the SV40 T antigen under the control of the insulin promoter [110]. But Cre and Tet systems offer a more precise level of control over transgene expression. The APC mice that spontaneously develop colorectal adenomas were obtained by infection of mice harboring floxed 14th exon of the APC (Adenomatous polyposis coli) gene with Cre-expressing adenovirus with gastrointestinal tropism [111]. Expression of the c-Myc gene under TetOff control demonstrated that activation of the expression leads to the lymphoma formation and the expression switching off leads to its regression [112].
Chromosome disorders
Although homology levels in murine and human genomes are high, multiple genetic differences do not allow extrapolation of data obtained from mice to humans. Thus, the application of mouse models in the investigation of human chromosome aneuploidies is limited because of the divergence between human and mouse genomes. For example, mouse homologs of the human genes from the part of the human chromosome 21 responsible for Down syndrome are divided between mouse chromosomes 10, 16, and 17. The Down syndrome mouse models obtained through microcell-mediated chromosome transfer and similar techniques in embryonic stem cells include Tc1 (with part of human chromosome 21) [113], T65Dn and Ts1Cje (with the part of mouse chromosome 16 containing about 75% of the involved genes) and Dp(10)1Yey/ + , Dp(16)1Yey/ + , Dp(17)1Yey/ + (with the parts of mouse chromosomes 10, 16 and 17) (reviewed in [114]). All these models had phenotypes similar but not identical to Down syndrome. At the same time, they made a significant contribution to the study of its pathogenesis and helped to prove that it is the increased gene copy number that causes the phenotype. These models were also used in the works that demonstrated the possibility of the therapy of cognitive disorders in patients with Down syndrome. It is worth noting separately a study [115] that revealed that the Ets2 gene from chromosome 21 contributed the most to the antitumor protection described for patients with Down syndrome.
In contrast with aneuploidy, mouse models of large deletions (0.5–10 megabase) closely mimic the effects of similar mutations in the human genome. These models greatly contributed to the molecular understanding of such disorders as Prader–Willi syndrome, Angelman syndrome, DiGeorge syndrome, Williams–Beuren syndrome, etc. They helped to identify specific genes responsible for the phenotype and to distinguish between the effects of the copy number alteration and the changes in the genomic environment.
Infectious diseases
Despite significant healthcare progress, viruses remain one of the greatest threats to public health. On the one hand, many viruses like HIV are still lethal and incurable despite being well studied; and on the other hand, novel viruses appear periodically, like the SARS-CoV-2, Zika virus, H1N1 swine flu, etc. Surprisingly, mouse models can be relatively easily generated even for infectious diseases that do not normally affect mice. The cause of such different susceptibility to viruses often lies in small distinctions in surface molecules (receptors), variations in immune response, and other processes important for viral cell cycle progression. This difference can be amended by humanization—transgenesis aimed at the expression of selected human genes. Ideally, this expression must be inducible (not to affect embryonic development) and tissue-specific (to mimic natural viral tropism more accurately) (reviewed in [116]).
Coronaviruses
The global SARS-CoV-2 pandemic that broke out at the end of 2019 challenged the scientific community. The first animal models suitable for SARS-CoV-2 studies had already been developed due to the SARS-CoV-1 outbreak in the early 2000s [117]. In this model, the human ACE2 receptor required for SARS-CoV entry into the cells was expressed under the control of the constitutive CMV promoter [118] or tissue-specific promoters: mouse Ace2 promoter [119], the Krt18 promoter [117], Hfh4/FoxJ1 lung ciliated epithelial promoter [120], or even under the control of an inducible and tissue-specific system [121]. All these mice developed symptoms similar but not identical to COVID-19 in humans. Some of these strains developed encephalitis [122,123,124]. The symptoms differed much in severity from 100% lethality [123] to slight transient body weight loss [125]. In one of the studies [126], K18hACE2 mice developed such COVID-19 conditions distinctive for humans as anosmia and thrombosis.
Therefore, there are examples of mouse models when overexpression of a single gene is sufficient to make an animal susceptible to disease. However, there is a hypothesis that TMPRSS2 [127] expression is also required for proper SARS-CoV-2 cell entry and more precise modeling.
Poliovirus
In some cases, expression of the viral entry molecules in target tissues is not enough to make mice susceptible to the virus. Thus, an interesting example [128] of a two-step model is the mouse model for oral poliovirus infections. Transgenic mice expressing the human PVR (poliovirus receptor) gene could be infected by PV, but the virus could not replicate in the digestive tract, which is an important feature of the poliovirus. As it was shown that the mouse interferon system prevents replication, knock-out mice without alpha/beta interferon receptors (Ifnar) were obtained. In these mice, the virus replicated successfully, but the severity of the disease was higher than in immunocompetent humans. Likely the third step—introduction of the human IFNAR gene—is required to obtain a more precise model. However, these models made a valuable contribution to poliovirus research.
Non-translatable mice models
As mentioned above, sometimes it is not possible to create an adequate model of human pathology based on genetically modified mice. Usually, this is due to the difference in the structure of human and mouse genomes, the low homology of the corresponding proteins, the mechanisms of regulation that have changed in the course of evolution, and several physiological differences, such as, for example, lifespan. One of the most striking examples of the inability to adequately reproduce the symptoms of human disease in mice is the attempt to create a model of the Lesch–Nyhan syndrome (LNS) (repeated in numerous studies). This failure is particularly noteworthy because LNS is an inherited monogenic disease caused by mutations (including point mutations) in the HPRT gene.
Lesch–Nyhan disease
This hereditary disease is associated with a deficiency of the enzyme hypoxanthine phosphoribosyl transferase (HPRT) and at the biochemical level is manifested in impaired purine metabolism, hyperuricemia, and hyperproduction of uric acid. This pathology is characterized by a spectrum of neurological manifestations, such as dystonia, choreoathetosis, impaired cognitive abilities, and aggressive behavior with self-harm. Lesch–Nyhan syndrome belongs to the group of orphan diseases, the prevalence is 1–9 cases per 1,000,000 people. The enzyme hypoxanthine phosphoribosyl transferase is responsible for the recirculation of purines and converts hypoxanthine to inosine monophosphate and guanine to guanosine monophosphate in the presence of phosphoribosyl pyrophosphate [129]. The gene encoding hypoxanthine phosphoribosyl transferase, HPRT1, is located on the X chromosome and is inherited as a monogenic recessive trait. Simulation of the syndrome in animals is a promising approach to the development of treatment for this disease. The first attempt to create a mouse model for this pathology was made in 1987. Then mice with hypoxanthine phosphoribosyltransferase deficiency were generated, but they exhibited no phenotypic traits. To date, several models of Lesch–Nyhan syndrome have been created, both with gene knockout and with point mutations [129, 130]; however, they are all asymptomatic and are mainly used for studying the metabolism [131]. There is a hypothesis that the difference between mice and humans with HPRT1 mutations and knockouts is associated with inactivation of the Prtfdc1 gene in mice (an HPRT1 paralog with unknown functions). Overexpression of this gene in mice resulted in neurological symptoms similar to those in humans [132, 133].
Thus, the use of genetically modified animals as models of human diseases undoubtedly makes a significant contribution to understanding the pathogenesis of various conditions but has a limited scope of application that must be considered when planning experiments.
Alzheimer’s disease
Currently, Alzheimer’s disease (AD) is one of the most intensively studied diseases. At the same time, despite all the efforts, there’s no effective cure, the exact causes of the disease onset are unknown, and causal relationships between different aspects of pathogenesis are still being discussed. Although in some cases AD is genetically determined and these cases are characterized by early onset, most often AD develops as a multifactorial disease among patients older than 60 years, when there is only partial genetic predisposition.
Since 1995, many genetically modified mouse strains have been created for the study of AD, and new ones continue to be created [134]. Specific and easily detectable features of AD pathogenesis are the formation of extracellular beta-amyloid plaques and intracellular neurofibrillary tangles, consisting of a hyperphosphorylated form of tau protein, in the brain. For a long time, the focus of AD research has been directed to the study of these processes. The first transgenic mice obtained for this purpose in 1995 were mice expressing the mutant APP (amyloid precursor protein) gene [135]. They were followed by strains with other variants of the APP gene carrying mutations specific for patients with early onset of genetically determined AD, as well as animals with overexpression of mutant forms of the PSEN1 and PSEN2 genes with the same specificity, which encode proteins included in the gamma-secretase complex, responsible for proteolysis of the APP protein. These mutant forms cause the predominant formation of the Aβ42 isoform, which is prone to transition to a pathological conformation, as opposed to other isoforms. Crossing these strains with each other made it possible to obtain mice with an extremely early onset of AD, which seemed convenient for research (reviewed in [136]). However, such models have a significant defect. Genetically determined cases of early AD onset make up a small percentage of all AD cases, and such animals are poorly suited for studying the etiology and pathogenesis of other cases [137].
In addition to the models based on the mutations that are directly associated with the occurrence of AD with the early onset, several models reproducing allelic variants associated with the increased risk of developing AD in old age have also been generated. Allelic variants of the APOE gene are associated with the risk of AD. The ε4 allelic variant significantly increases the risk, while the ε2 variant reduces it, and the ε3 variant is neutral. A similar relationship was observed in mice expressing human variants ε3 and ε4 [138, 139]. Another gene with allelic variants associated with the increased risk of AD is the TREM2 gene (triggering receptor expressed on myeloid cells 2), which encodes a receptor that controls, among other processes, inflammation in the microglia. Mice expressing corresponding human TREM2 variants have also been generated and show similar phenotypes. To a certain extent, the last two classes of models can serve as models of AD with late-onset [137].
However, genetically modified animals as a model of AD also showed a more significant drawback. It turned out that drugs that lead to improvements (primarily the dissolution of amyloid plaques) in model mice are ineffective in humans [140].
Recent studies show that beta-amyloid plaques are sometimes found in asymptomatic people. It has been suggested that the immediate cause of symptoms development is not the formation of plaques and neurofibrillary tangles, but the death of cholinergic neurons [136, 141], which is not fully observed in model animals, in contrast to the deposition of amyloid plaques and the formation of neurofibrillary tangles.
This case clearly illustrates that a certain level of etiology and pathogenesis understanding is necessary to create a model of a disease, and models that reproduce only some elements of pathogenesis may not be very useful.
Conclusion
As genetic engineering progresses, the number of molecular tools for genome editing increases, allowing it to modify and edit genes surgically, to knock-out and knock-in single or multiple genes.
Genetically modified animals, especially mice, can contribute to the research of human disease, as various animal strains can be developed relatively fast and easily to track the pathogenesis of disease and to evaluate the involvement of certain genes. Mouse models proved to be a useful tool for discovering targets for therapeutic drugs.
However, it is important to be realistic about the limitations of animal models. Sometimes due to the large differences between mice and humans, certain preclinical treatments in animal models cannot be translated to human clinical trials. The most problematic fields in this respect are behavior, immune system, and chromosomal disorders. On the other hand, mouse models can turn out to be useful in unexpected circumstances—for example, for research of viruses that do not affect wild-type mice.
Data availability
No data is associated with the manuscript.
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- APP:
-
Amyloid precursor protein
- AAV:
-
Adeno-associated virus
- BMMC:
-
Bone marrow mononuclear cell
- Cu/Zn-SOD:
-
Cu/Zn superoxide dismutase-1
- DMD:
-
Duchenne muscular dystrophy
- DSB:
-
Double-strand breaks
- ESC:
-
Embryonic stem cells
- FUS:
-
Fused in sarcoma
- GM:
-
Gene modified
- GWAS:
-
Genome-wide association studies
- HBV:
-
Hepatitis B virus
- HDL:
-
High-density lipoprotein
- LDLR:
-
Low-density lipoprotein receptor
- HDR:
-
Homology directed repair
- HLA:
-
Human leukocyte antigens
- HPRT:
-
Hypoxanthine phosphoribosyl transferase
- HPV:
-
Human papillomaviruses
- LNS:
-
Lesch–Nyhan syndrome
- KI:
-
Knock-in
- KO:
-
Knock-out
- NHEJ:
-
Non-homologous end joining
- NOD:
-
Non-obese diabetes
- sgRNA:
-
Single guide RNA
- siRNA:
-
Small interfering RNA
- SNP:
-
Single nucleotide polymorphism
- ORFs:
-
Open reading frames
- PV:
-
Poliovirus
- PVR:
-
Poliovirus receptor
- RA:
-
Rheumatoid arthritis
- SLE:
-
Systemic lupus erythematosus
- SMA:
-
Spinal muscular atrophy
References
Breschi A, Gingeras TR, Guigó R (2017) Comparative transcriptomics in human and mouse. Nat Rev Genet 18(7):425–440. https://doi.org/10.1038/nrg.2017.19
Averina OA, Vysokikh MY, Permyakov OA, Sergiev PV (2020) Simple recommendations for improving efficiency in generating genome-edited mice. Acta Nat 12(1):42–50. https://doi.org/10.32607/actanaturae.10937
Ormandy EH, Dale J, Griffin G (2011) Genetic engineering of animals: ethical issues, including welfare concerns. Canadian Vet J 52(5):544–50
Hrabé de Angelis MH, Flaswinkel H, Fuchs H, Rathkolb B, Soewarto D, Marschall S, Heffner S, Pargent W, Wuensch K, Jung M, Reis A, Richter T, Alessandrini F, Jakob T, Fuchs E, Kolb H, Kremmer E, Schaeble K, Rollinski B, Roscher A, Peters C, Meitinger T, Strom T, Steckler T, Holsboer F, Klopstock T, Gekeler F, Schindewolf C, Jung T, Avraham K, Behrendt H, Ring J, Zimmer A, Schughart K, Pfeffer K, Wolf E, Balling R (2000) Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nat Genet 25(4):444–447. https://doi.org/10.1038/78146
Nolan PM, Peters J, Strivens M, Rogers D, Hagan J, Spurr N, Gray IC, Vizor L, Brooker D, Whitehill E, Washbourne R, Hough T, Greenaway S, Hewitt M, Liu X, McCormack S, Pickford K, Selley R, Wells C, Tymowska-Lalanne Z, Roby P, Glenister P, Thornton C, Thaung C, Stevenson JA, Arkell R, Mburu P, Hardisty R, Kiernan A, Erven A, Steel KP, Voegeling S, Guenet JL, Nickols C, Sadri R, Nasse M, Isaacs A, Davies K, Browne M, Fisher EM, Martin J, Rastan S, Brown SD, Hunter J (2000) A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat Genet 25(4):440–443. https://doi.org/10.1038/78140
Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH (1980) Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci USA 77(12):7380–7384. https://doi.org/10.1073/pnas.77.12.7380
Lin TP (1966) Microinjection of mouse eggs. Science (New York) 151(3708):333–337. https://doi.org/10.1126/science.151.3708.333
Kim YS, Kim GR, Park M, Yang SC, Park SH, Won JE, Lee JH, Shin HE, Song H, Kim HR (2020) Electroporation of AsCpf1/RNP at the zygote stage is an efficient genome editing method to generate knock-out mice deficient in leukemia inhibitory factor. Tissue Eng Regen Med 17(1):45–53. https://doi.org/10.1007/s13770-019-00225-8
Yoon Y, Wang D, Tai PWL, Riley J, Gao G, Rivera-Pérez JA (2018) Streamlined ex vivo and in vivo genome editing in mouse embryos using recombinant adeno-associated viruses. Nat Commun 9(1):412. https://doi.org/10.1038/s41467-017-02706-7
Nakagawa Y, Sakuma T, Sakamoto T, Ohmuraya M, Nakagata N, Yamamoto T (2015) Production of knockout mice by DNA microinjection of various CRISPR/Cas9 vectors into freeze-thawed fertilized oocytes. BMC Biotechnol 15:33. https://doi.org/10.1186/s12896-015-0144-x
Gurumurthy CB, Sato M, Nakamura A, Inui M, Kawano N, Islam MA, Ogiwara S, Takabayashi S, Matsuyama M, Nakagawa S, Miura H, Ohtsuka M (2019) Creation of CRISPR-based germline-genome-engineered mice without ex vivo handling of zygotes by i-GONAD. Nat Protoc 14(8):2452–2482. https://doi.org/10.1038/s41596-019-0187-x
Melo-Silva CR, Knudson CJ, Tang L, Kafle S, Springer LE, Choi J, Snyder CM, Wang Y, Kim SV, Sigal LJ (2023) Multiple and consecutive genome editing using i-GONAD and breeding enrichment facilitates the production of genetically modified mice. Cells. https://doi.org/10.3390/cells12091343
Ohtsuka M, Sato M, Miura H, Takabayashi S, Matsuyama M, Koyano T, Arifin N, Nakamura S, Wada K, Gurumurthy CB (2018) i-GONAD: a robust method for in situ germline genome engineering using CRISPR nucleases. Genome Biol 19(1):25. https://doi.org/10.1186/s13059-018-1400-x
Sato M, Miyagasako R, Takabayashi S, Ohtsuka M, Hatada I, Horii T (2020) Sequential i-GONAD: an improved in vivo technique for CRISPR/Cas9-based genetic manipulations in mice. Cells. https://doi.org/10.3390/cells9030546
Takahashi G, Gurumurthy CB, Wada K, Miura H, Sato M, Ohtsuka M (2015) GONAD: genome-editing via oviductal nucleic acids delivery system: a novel microinjection independent genome engineering method in mice. Sci Rep 5:11406. https://doi.org/10.1038/srep11406
Jin SL, Latour AM, Conti M (2005) Generation of PDE4 knockout mice by gene targeting. Methods Mol Biol (Clifton) 307:191–210. https://doi.org/10.1385/1-59259-839-0:191
Misra RP, Bronson SK, Xiao Q, Garrison W, Li J, Zhao R, Duncan SA (2001) Generation of single-copy transgenic mouse embryos directly from ES cells by tetraploid embryo complementation. BMC Biotechnol 1:12. https://doi.org/10.1186/1472-6750-1-12
Lamas-Toranzo I, Galiano-Cogolludo B, Cornudella-Ardiaca F, Cobos-Figueroa J, Ousinde O, Bermejo-Álvarez P (2019) Strategies to reduce genetic mosaicism following CRISPR-mediated genome edition in bovine embryos. Sci Rep 9(1):14900 https://doi.org/10.1038/s41598-019-51366-8.
Jiang J, Zhang L, Zhou X, Chen X, Huang G, Li F, Wang R, Wu N, Yan Y, Tong C, Srivastava S, Wang Y, Liu H, Ying QL (2016) Induction of site-specific chromosomal translocations in embryonic stem cells by CRISPR/Cas9. Sci Rep 6:21918. https://doi.org/10.1038/srep21918
Sumiyama K, Matsumoto N, Garçon-Yoshida J, Ukai H, Ueda HR, Tanaka Y (2018) Easy and efficient production of completely embryonic-stem-cell-derived mice using a micro-aggregation device. PLoS ONE 13(9):e0203056. https://doi.org/10.1371/journal.pone.0203056
Wen D, Saiz N, Rosenwaks Z, Hadjantonakis AK, Rafii S (2014) Completely ES cell-derived mice produced by tetraploid complementation using inner cell mass (ICM) deficient blastocysts. PLoS ONE 9(4):e94730. https://doi.org/10.1371/journal.pone.0094730
Chu VT, Weber T, Graf R, Sommermann T, Petsch K, Sack U, Volchkov P, Rajewsky K, Kühn R (2016) Efficient generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6 zygotes. BMC Biotechnol 16:4. https://doi.org/10.1186/s12896-016-0234-4
Tanimoto Y, Mikami N, Ishida M, Iki N, Kato K, Sugiyama F, Takahashi S, Mizuno S (2022) Zygote microinjection for creating gene cassette knock-in and flox alleles in mice. J Vis Exp. https://doi.org/10.3791/64161
Qin W, Wang H (2019) Delivery of CRISPR-Cas9 into mouse zygotes by electroporation. Methods Mol Biol (Clifton) 1874:179–190. https://doi.org/10.1007/978-1-4939-8831-0_10
Takemoto T (2020) Zygote electroporation for CRISPR/Cas9 delivery to generate genetically modified mice. Methods Mol Biol (Clifton) 2050:121–126. https://doi.org/10.1007/978-1-4939-9740-4_13
Krivonogova AS, Bruter AV, Makutina VA, Okulova YD, Ilchuk LA, Kubekina MV, Khamatova AY, Egorova TV, Mymrin VS, Silaeva YY, Deykin AV, Filatov MA, Isaeva AG (2022) AAV infection of bovine embryos: novel, simple and effective tool for genome editing. Theriogenology 193:77–86. https://doi.org/10.1016/j.theriogenology.2022.09.007
Mizuno N, Mizutani E, Sato H, Kasai M, Ogawa A, Suchy F, Yamaguchi T, Nakauchi H (2018) Intra-embryo gene cassette Knockin by CRISPR/Cas9-mediated genome editing with adeno-associated viral vector. Science 9:286–97. https://doi.org/10.1016/j.isci.2018.10.030
Leidy-Davis T, Cheng K, Goodwin LO, Morgan JL, Juan WC, Roca X, Ong ST, Bergstrom DE (2018) Viable mice with extensive gene humanization (25-kbp) created using embryonic stem cell/blastocyst and CRISPR/Zygote injection approaches. Sci Rep 8(1):15028. https://doi.org/10.1038/s41598-018-33408-9
Ma Y, He L, Xiang L, Zhang J, Wang J, Zhu W, Cao W, Zhu Y, Gao M, Zhou F, Liu Z (2021) Efficiency comparison of B6(Cg)-Tyr(c-2j) /J and C57BL/6NTac embryos as hosts for the generation of knockout mice. Transgenic Res 30(3):275–281. https://doi.org/10.1007/s11248-021-00248-9
Snell GD (1935) The Induction by X-rays of hereditary changes in mice. Genetics 20(6):545–567. https://doi.org/10.1093/genetics/20.6.545
Yin H, Zhang T, Wang H, Hu X, Hou X, Fang X, Yin Y, Li H, Shi L, Su YQ (2021) Echinoderm microtubule associated protein like 1 is indispensable for oocyte spindle assembly and meiotic progression in mice. Front Cell Dev Biol 9:687522. https://doi.org/10.3389/fcell.2021.687522
Vitaterna MH, Pinto LH, Takahashi JS (2006) Large-scale mutagenesis and phenotypic screens for the nervous system and behavior in mice. Trends Neurosci 29(4):233–240. https://doi.org/10.1016/j.tins.2006.02.006
Austin CP, Battey JF, Bradley A, Bucan M, Capecchi M, Collins FS, Dove WF, Duyk G, Dymecki S, Eppig JT, Grieder FB, Heintz N, Hicks G, Insel TR, Joyner A, Koller BH, Lloyd KC, Magnuson T, Moore MW, Nagy A, Pollock JD, Roses AD, Sands AT, Seed B, Skarnes WC, Snoddy J, Soriano P, Stewart DJ, Stewart F, Stillman B, Varmus H, Varticovski L, Verma IM, Vogt TF, von Melchner H, Witkowski J, Woychik RP, Wurst W, Yancopoulos GD, Young SG, Zambrowicz B (2004) The knockout mouse project. Nat Genet 36(9):921–924. https://doi.org/10.1038/ng0904-921
Strzyz P (2020) CRISPR-Cas9 wins Nobel. Nat Rev Mol Cell Biol 21(12):714. https://doi.org/10.1038/s41580-020-00307-9
Gemberling MP, Siklenka K, Rodriguez E, Tonn-Eisinger KR, Barrera A, Liu F, Kantor A, Li L, Cigliola V, Hazlett MF, Williams CA, Bartelt LC, Madigan VJ, Bodle JC, Daniels H, Rouse DC, Hilton IB, Asokan A, Ciofani M, Poss KD, Reddy TE, West AE, Gersbach CA (2021) Transgenic mice for in vivo epigenome editing with CRISPR-based systems. Nat Methods 18(8):965–974. https://doi.org/10.1038/s41592-021-01207-2
Şenödeyici E, Şahintürk DK, Akbolat BR, Dİndar A, Sefer S, AnĞay GF, Demİr S (2021) Main genome editing tools: an overview of the literature, future applications and ethical questions. Turk Med Stud J 8(2):50–7
Van Vu T, Thi Hai Doan D, Kim J, Sung YW, Thi Tran M, Song YJ, Das S, Kim JY (2021) CRISPR/Cas-based precision genome editing via microhomology-mediated end joining. Plant Biotechnol J 19(2):230–239. https://doi.org/10.1111/pbi.13490
Miller SM, Wang T, Randolph PB, Arbab M, Shen MW, Huang TP, Matuszek Z, Newby GA, Rees HA, Liu DR (2020) Continuous evolution of SpCas9 variants compatible with non-G PAMs. Nat Biotechnol 38(4):471–481. https://doi.org/10.1038/s41587-020-0412-8
Tan Y, Chu AHY, Bao S, Hoang DA, Kebede FT, Xiong W, Ji M, Shi J, Zheng Z (2019) Rationally engineered Staphylococcus aureus Cas9 nucleases with high genome-wide specificity. Proc Natl Acad Sci USA 116(42):20969–20976. https://doi.org/10.1073/pnas.1906843116
Li P, Zhang L, Li Z, Xu C, Du X, Wu S (2020) Cas12a mediates efficient and precise endogenous gene tagging via MITI: microhomology-dependent targeted integrations. Cell Mol Life Sci 77(19):3875–3884. https://doi.org/10.1007/s00018-019-03396-8
Chen H, Liu X, Li L, Tan Q, Li S, Li L, Li C, Fu J, Lu Y, Wang Y, Sun Y, Luo ZG, Lu Z, Sun Q, Liu Z (2023) CATI: an efficient gene integration method for rodent and primate embryos by MMEJ suppression. Genome Biol 24(1):146. https://doi.org/10.1186/s13059-023-02987-w
Raychowdhury R, Gentili M, Cui A, Schweitzer LD, Li B, Hacohen N (2021) Macrophages from Rosa26-integrated Cas9-expressing C57BL/6J mice have a putative TRIF-mediated defect in the TLR-3/4 signaling. ImmunoHorizons 5(10):818–829. https://doi.org/10.4049/immunohorizons.2100010
Sakurai T, Shindo T (2021) Production of single- and multiple-gene-modified mice via maternal SpCas9-based gene editing. STAR Protocols 2(2):100509. https://doi.org/10.1016/j.xpro.2021.100509
Bouabe H, Okkenhaug K (2013) Gene targeting in mice: a review. Methods Mol Biol (Clifton) 1064:315–336. https://doi.org/10.1007/978-1-62703-601-6_23
Miyasaka Y, Uno Y, Yoshimi K, Kunihiro Y, Yoshimura T, Tanaka T, Ishikubo H, Hiraoka Y, Takemoto N, Tanaka T, Ooguchi Y, Skehel P, Aida T, Takeda J, Mashimo T (2018) CLICK: one-step generation of conditional knockout mice. BMC Genomics 19(1):318. https://doi.org/10.1186/s12864-018-4713-y
Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A (2011) A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474(7351):337–342. https://doi.org/10.1038/nature10163
Jung CJ, Zhang J, Trenchard E, Lloyd KC, West DB, Rosen B, de Jong PJ (2017) Efficient gene targeting in mouse zygotes mediated by CRISPR/Cas9-protein. Transgenic Res 26(2):263–277. https://doi.org/10.1007/s11248-016-9998-5
Liu ET, Bolcun-Filas E, Grass DS, Lutz C, Murray S, Shultz L, Rosenthal N (2017) Of mice and CRISPR: the post-CRISPR future of the mouse as a model system for the human condition. EMBO Rep 18(2):187–193. https://doi.org/10.15252/embr.201643717
Cao J, Wu L, Zhang SM, Lu M, Cheung WK, Cai W, Gale M, Xu Q, Yan Q (2016) An easy and efficient inducible CRISPR/Cas9 platform with improved specificity for multiple gene targeting. Nucleic Acids Res 44(19):e149. https://doi.org/10.1093/nar/gkw660
Kang K, Huang L, Li Q, Liao X, Dang Q, Yang Y, Luo J, Zeng Y, Li L, Gou D (2019) An improved Tet-on system in microRNA overexpression and CRISPR/Cas9-mediated gene editing. J Animal Sci Biotechnol 10:43. https://doi.org/10.1186/s40104-019-0354-5
Emery DW, Aker M, Stamatoyannopoulos G (2003) Chromatin Insulators and Position Effects. New Comprehensive Biochemistry, Elsevier:381–95. doi: https://doi.org/10.1016/S0167-7306(03)38023-8.
Yoshimi K, Kunihiro Y, Kaneko T, Nagahora H, Voigt B, Mashimo T (2016) ssODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nat Commun 7:10431. https://doi.org/10.1038/ncomms10431
Chen CL, Rodiger J, Chung V, Viswanatha R, Mohr SE, Hu Y, Perrimon N (2020) SNP-CRISPR: a web tool for SNP-specific genome editing. G3 (Bethesda) 10(2):489–94. https://doi.org/10.1534/g3.119.400904
Yang H, Ren S, Yu S, Pan H, Li T, Ge S, Zhang J, Xia N (2020) Methods favoring homology-directed repair choice in response to CRISPR/Cas9 induced-double strand breaks. Int J Mol Sci. https://doi.org/10.3390/ijms21186461
Denes CE, Cole AJ, Aksoy YA, Li G, Neely GG, Hesselson D (2021) Approaches to enhance precise CRISPR/Cas9-mediated genome editing. Int J Mol Sci. https://doi.org/10.3390/ijms22168571
Yao X, Wang X, Hu X, Liu Z, Liu J, Zhou H, Shen X, Wei Y, Huang Z, Ying W, Wang Y, Nie YH, Zhang CC, Li S, Cheng L, Wang Q, Wu Y, Huang P, Sun Q, Shi L, Yang H (2017) Homology-mediated end joining-based targeted integration using CRISPR/Cas9. Cell Res 27(6):801–814. https://doi.org/10.1038/cr.2017.76
Aksoy YA, Nguyen DT, Chow S, Chung RS, Guillemin GJ, Cole NJ, Hesselson D (2019) Chemical reprogramming enhances homology-directed genome editing in zebrafish embryos. Commun Biol 2:198. https://doi.org/10.1038/s42003-019-0444-0
Liu M, Rehman S, Tang X, Gu K, Fan Q, Chen D, Ma W (2018) Methodologies for improving HDR efficiency. Front Genet 9:691. https://doi.org/10.3389/fgene.2018.00691
Ghanta KS, Chen Z, Mir A, Dokshin GA, Krishnamurthy PM, Yoon Y, Gallant J, Xu P, Zhang XO, Ozturk AR, Shin M, Idrizi F, Liu P, Gneid H, Edraki A, Lawson ND, Rivera-Pérez JA, Sontheimer EJ, Watts JK, Mello CC (2021) 5′-modifications improve potency and efficacy of DNA donors for precision genome editing. eLife. https://doi.org/10.7554/eLife.72216
Medert R, Thumberger T, Tavhelidse-Suck T, Hub T, Kellner T, Oguchi Y, Dlugosz S, Zimmermann F, Wittbrodt J, Freichel M (2023) Efficient single copy integration via homology-directed repair (scHDR) by 5’modification of large DNA donor fragments in mice. Nucleic Acids Res 51(3):e14. https://doi.org/10.1093/nar/gkac1150
Ma M, Zhuang F, Hu X, Wang B, Wen XZ, Ji JF, Xi JJ (2017) Efficient generation of mice carrying homozygous double-floxp alleles using the Cas9-Avidin/Biotin-donor DNA system. Cell Res 27(4):578–581. https://doi.org/10.1038/cr.2017.29
Wierson WA, Welker JM, Almeida MP, Mann CM, Webster DA, Torrie ME, Weiss TJ, Kambakam S, Vollbrecht MK, Lan M, McKeighan KC, Levey J, Ming Z, Wehmeier A, Mikelson CS, Haltom JA, Kwan KM, Chien CB, Balciunas D, Ekker SC, Clark KJ, Webber BR, Moriarity BS, Solin SL, Carlson DF, Dobbs DL, McGrail M, Essner J (2020) Efficient targeted integration directed by short homology in zebrafish and mammalian cells. eLife 9. doi: https://doi.org/10.7554/eLife.53968.
Yoshimi K, Oka Y, Miyasaka Y, Kotani Y, Yasumura M, Uno Y, Hattori K, Tanigawa A, Sato M, Oya M, Nakamura K, Matsushita N, Kobayashi K, Mashimo T (2021) Combi-CRISPR: combination of NHEJ and HDR provides efficient and precise plasmid-based knock-ins in mice and rats. Hum Genet 140(2):277–287. https://doi.org/10.1007/s00439-020-02198-4
Gu B, Posfai E, Rossant J (2018) Efficient generation of targeted large insertions by microinjection into two-cell-stage mouse embryos. Nat Biotechnol 36(7):632–637. https://doi.org/10.1038/nbt.4166
Kang ZJ, Liu YF, Xu LZ, Long ZJ, Huang D, Yang Y, Liu B, Feng JX, Pan YJ, Yan JS, Liu Q (2016) The Philadelphia chromosome in leukemogenesis. Chin J Cancer 35:48. https://doi.org/10.1186/s40880-016-0108-0
Zhao Y, Zhao G, Chang Z, Zhu T, Zhao Y, Lu H, Xue C, Saunders TL, Guo Y, Chang L, Chen YE, Zhang J (2023) Generating endogenous Myh11-driven Cre mice for sex-independent gene deletion in smooth muscle cells. JCI insight 8(14). doi: https://doi.org/10.1172/jci.insight.171661.
Nomura J, Takumi T (2012) Animal models of psychiatric disorders that reflect human copy number variation. Neural Plast 2012:589524. https://doi.org/10.1155/2012/589524
Choi PS, Meyerson M (2014) Targeted genomic rearrangements using CRISPR/Cas technology. Nat Commun 5:3728. https://doi.org/10.1038/ncomms4728
Ghezraoui H, Piganeau M, Renouf B, Renaud JB, Sallmyr A, Ruis B, Oh S, Tomkinson AE, Hendrickson EA, Giovannangeli C, Jasin M, Brunet E (2014) Chromosomal translocations in human cells are generated by canonical nonhomologous end-joining. Mol Cell 55(6):829–842. https://doi.org/10.1016/j.molcel.2014.08.002
Lau CH, Suh Y (2017) In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease. F1000Research 6:2153. doi: https://doi.org/10.12688/f1000research.11243.1.
Ikawa M, Tanaka N, Kao WW, Verma IM (2003) Generation of transgenic mice using lentiviral vectors: a novel preclinical assessment of lentiviral vectors for gene therapy. Molecular therapy : the journal of the American Society of Gene Therapy 8(4):666–673. https://doi.org/10.1016/s1525-0016(03)00240-5
Dal Canto MC, Gurney ME (1994) Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am J Pathol 145(6):1271–1279
Johnston JA, Dalton MJ, Gurney ME, Kopito RR (2000) Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 97(23):12571–12576. https://doi.org/10.1073/pnas.220417997
Shelkovnikova TA, Peters OM, Deykin AV, Connor-Robson N, Robinson H, Ustyugov AA, Bachurin SO, Ermolkevich TG, Goldman IL, Sadchikova ER, Kovrazhkina EA, Skvortsova VI, Ling SC, Da Cruz S, Parone PA, Buchman VL, Ninkina NN (2013) Fused in sarcoma (FUS) protein lacking nuclear localization signal (NLS) and major RNA binding motifs triggers proteinopathy and severe motor phenotype in transgenic mice. J Biol Chem 288(35):25266–25274. https://doi.org/10.1074/jbc.M113.492017
Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ (1989) The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science (New York, NY) 244(4912):1578–1580. https://doi.org/10.1126/science.2662404
Egorova TV, Zotova ED, Reshetov DA, Polikarpova AV, Vassilieva SG, Vlodavets DV, Gavrilov AA, Ulianov SV, Buchman VL, Deykin AV (2019) CRISPR/Cas9-generated mouse model of Duchenne muscular dystrophy recapitulating a newly identified large 430 kb deletion in the human DMD gene. Dis Models Mech. https://doi.org/10.1242/dmm.037655
Chemello F, Wang Z, Li H, McAnally JR, Liu N, Bassel-Duby R, Olson EN (2020) Degenerative and regenerative pathways underlying Duchenne muscular dystrophy revealed by single-nucleus RNA sequencing. Proc Natl Acad Sci USA 117(47):29691–29701. https://doi.org/10.1073/pnas.2018391117
Chemello F, Chai AC, Li H, Rodriguez-Caycedo C, Sanchez-Ortiz E, Atmanli A, Mireault AA, Liu N, Bassel-Duby R, Olson EN (2021) Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci Adv. https://doi.org/10.1126/sciadv.abg4910
Pendse AA, Arbones-Mainar JM, Johnson LA, Altenburg MK, Maeda N (2009) Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J Lipid Res 50:S178-82. https://doi.org/10.1194/jlr.R800070-JLR200
Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N (1992) Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA 89(10):4471–4475. https://doi.org/10.1073/pnas.89.10.4471
Golforoush P, Yellon DM, Davidson SM (2020) Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic Res Cardiol 115(6):73. https://doi.org/10.1007/s00395-020-00829-5
Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der WJ, van der Hoorn J, Princen HM, Kooistra T (2007) Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol 27(8):1706–21. https://doi.org/10.1161/atvbaha.107.142570
Jackson HM, Onos KD, Pepper KW, Graham LC, Akeson EC, Byers C, Reinholdt LG, Frankel WN, Howell GR (2015) DBA/2J genetic background exacerbates spontaneous lethal seizures but lessens amyloid deposition in a mouse model of Alzheimer’s disease. PLoS ONE 10(5):e0125897. https://doi.org/10.1371/journal.pone.0125897
Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP (1996) Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84(3):491–495. https://doi.org/10.1016/s0092-8674(00)81294-5
Wang B, Chandrasekera PC, Pippin JJ (2014) Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 10(2):131–145. https://doi.org/10.2174/1573399810666140508121012
Brophy CM, Tilson JE, Braverman IM, Tilson MD (1988) Age of onset, pattern of distribution, and histology of aneurysm development in a genetically predisposed mouse model. J Vasc Surg 8(1):45–48
Blunt T, Finnie NJ, Taccioli GE, Smith GC, Demengeot J, Gottlieb TM, Mizuta R, Varghese AJ, Alt FW, Jeggo PA, Jackson SP (1995) Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell 80(5):813–823. https://doi.org/10.1016/0092-8674(95)90360-7
Blunt T, Gell D, Fox M, Taccioli GE, Lehmann AR, Jackson SP, Jeggo PA (1996) Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc Natl Acad Sci USA 93(19):10285–10290. https://doi.org/10.1073/pnas.93.19.10285
Pantelouris EM (1968) Absence of thymus in a mouse mutant. Nature 217(5126):370–371. https://doi.org/10.1038/217370a0
Žuklys S, Handel A, Zhanybekova S, Govani F, Keller M, Maio S, Mayer CE, Teh HY, Hafen K, Gallone G, Barthlott T, Ponting CP, Holländer GA (2016) Foxn1 regulates key target genes essential for T cell development in postnatal thymic epithelial cells. Nat Immunol 17(10):1206–1215. https://doi.org/10.1038/ni.3537
Rahman MA, Thomas R (2017) The SKG model of spondyloarthritis. Best Pract Res Clin Rheumatol 31(6):895–909. https://doi.org/10.1016/j.berh.2018.07.004
Peccoud J, Dellabona P, Allen P, Benoist C, Mathis D (1990) Delineation of antigen contact residues on an MHC class II molecule. EMBO J 9(13):4215–4223. https://doi.org/10.1002/j.1460-2075.1990.tb07869.x
Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D (1996) Organ-specific disease provoked by systemic autoimmunity. Cell 87(5):811–822. https://doi.org/10.1016/s0092-8674(00)81989-3
Arbeit JM, Münger K, Howley PM, Hanahan D (1993) Neuroepithelial carcinomas in mice transgenic with human papillomavirus type 16 E6/E7 ORFs. Am J Pathol 142(4):1187–1197
Hay AM, Howie HL, Gorham JD, D’Alessandro A, Spitalnik SL, Hudson KE, Zimring JC (2021) Mouse background genetics in biomedical research: the devil’s in the details. Transfusion 61(10):3017–3025. https://doi.org/10.1111/trf.16628
Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW (2002) Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet 30(1):81–85. https://doi.org/10.1038/ng794
Erway LC, Willott JF, Archer JR, Harrison DE (1993) Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res 65(1–2):125–32. https://doi.org/10.1016/0378-5955(93)90207-h
Kovács AD, Pearce DA (2015) Finding the most appropriate mouse model of juvenile CLN3 (Batten) disease for therapeutic studies: the importance of genetic background and gender. Dis Model Mech 8(4):351–361. https://doi.org/10.1242/dmm.018804
Coley WD, Bogdanik L, Vila MC, Yu Q, Van Der Meulen JH, Rayavarapu S, Novak JS, Nearing M, Quinn JL, Saunders A, Dolan C, Andrews W, Lammert C, Austin A, Partridge TA, Cox GA, Lutz C, Nagaraju K (2016) Effect of genetic background on the dystrophic phenotype in mdx mice. Hum Mol Genet 25(1):130–145. https://doi.org/10.1093/hmg/ddv460
Eshraghi M, McFall E, Gibeault S, Kothary R (2016) Effect of genetic background on the phenotype of the Smn2B/- mouse model of spinal muscular atrophy. Hum Mol Genet 25(20):4494–4506. https://doi.org/10.1093/hmg/ddw278
Kim BW, Ryu J, Jeong YE, Kim J, Martin LJ (2020) Human motor neurons With SOD1-G93A mutation generated from CRISPR/Cas9 gene-edited iPSCs develop pathological features of amyotrophic lateral sclerosis. Front Cell Neurosci 14:604171. https://doi.org/10.3389/fncel.2020.604171
Heiman-Patterson TD, Sher RB, Blankenhorn EA, Alexander G, Deitch JS, Kunst CB, Maragakis N, Cox G (2011) Effect of genetic background on phenotype variability in transgenic mouse models of amyotrophic lateral sclerosis: a window of opportunity in the search for genetic modifiers. Amyotrophic Lateral Sclerosis 12(2):79–86. https://doi.org/10.3109/17482968.2010.550626
Getz GS, Reardon CA (2009) Apoprotein E as a lipid transport and signaling protein in the blood, liver, and artery wall. J Lipid Res 50:S156-61. https://doi.org/10.1194/jlr.R800058-JLR200
Leigh SE, Foster AH, Whittall RA, Hubbart CS, Humphries SE (2008) Update and analysis of the University College London low density lipoprotein receptor familial hypercholesterolemia database. Ann Hum Genet 72(Pt 4):485–498. https://doi.org/10.1111/j.1469-1809.2008.00436.x
Méndez-Lara KA, Farré N, Santos D, Rivas-Urbina A, Metso J, Sánchez-Quesada JL, Llorente-Cortes V, Errico TL, Lerma E, Jauhiainen M, Martín-Campos JM, Alonso N, Escolà-Gil JC, Blanco-Vaca F, Julve J (2019) Human ApoA-I overexpression enhances macrophage-specific reverse cholesterol transport but fails to prevent inherited diabesity in mice. Int J Mol Sci. https://doi.org/10.3390/ijms20030655
Vecoli C, Cao J, Neglia D, Inoue K, Sodhi K, Vanella L, Gabrielson KK, Bedja D, Paolocci N, L’Abbate A, Abraham NG (2011) Apolipoprotein A-I mimetic peptide L-4F prevents myocardial and coronary dysfunction in diabetic mice. J Cell Biochem 112(9):2616–2626. https://doi.org/10.1002/jcb.23188
Rubin EM, Ishida BY, Clift SM, Krauss RM (1991) Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci USA 88(2):434–438. https://doi.org/10.1073/pnas.88.2.434
Schinnerling K, Rosas C, Soto L, Thomas R, Aguillón JC (2019) Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Front Immunol 10:203. https://doi.org/10.3389/fimmu.2019.00203
Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, El-Naggar AK, Lozano G (2004) Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119(6):861–872. https://doi.org/10.1016/j.cell.2004.11.006
Hanahan D (1985) Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 315(6015):115–122. https://doi.org/10.1038/315115a0
Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, Matsumoto H, Takano H, Akiyama T, Toyoshima K, Kanamaru R, Kanegae Y, Saito I, Nakamura Y, Shiba K, Noda T (1997) Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science (New York) 278(5335):120–123. https://doi.org/10.1126/science.278.5335.120
Marinkovic D, Marinkovic T, Mahr B, Hess J, Wirth T (2004) Reversible lymphomagenesis in conditionally c-MYC expressing mice. Int J Cancer 110(3):336–342. https://doi.org/10.1002/ijc.20099
O’Doherty A, Ruf S, Mulligan C, Hildreth V, Errington ML, Cooke S, Sesay A, Modino S, Vanes L, Hernandez D, Linehan JM, Sharpe PT, Brandner S, Bliss TV, Henderson DJ, Nizetic D, Tybulewicz VL, Fisher EM (2005) An aneuploid mouse strain carrying human chromosome 21 with down syndrome phenotypes. Science (New York) 309(5743):2033–2037. https://doi.org/10.1126/science.1114535
Sheppard O, Wiseman FK, Ruparelia A, Tybulewicz VL, Fisher EM (2012) Mouse models of aneuploidy. Sci World J 2012:214078. https://doi.org/10.1100/2012/214078
Wolvetang EJ, Wilson TJ, Sanij E, Busciglio J, Hatzistavrou T, Seth A, Hertzog PJ, Kola I (2003) ETS2 overexpression in transgenic models and in Down syndrome predisposes to apoptosis via the p53 pathway. Hum Mol Genet 12(3):247–255. https://doi.org/10.1093/hmg/ddg015
Masemann D, Ludwig S, Boergeling Y (2020) Advances in transgenic mouse models to study infections by human pathogenic viruses. Int J Mol Scie. https://doi.org/10.3390/ijms21239289
McCray PB Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, Meyerholz DK, Kirby P, Look DC, Perlman S (2007) Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 81(2):813–821. https://doi.org/10.1128/jvi.02012-06
Tseng CT, Huang C, Newman P, Wang N, Narayanan K, Watts DM, Makino S, Packard MM, Zaki SR, Chan TS, Peters CJ (2007) Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human angiotensin-converting enzyme 2 virus receptor. J Virol 81(3):1162–1173. https://doi.org/10.1128/jvi.01702-06
Yang XH, Deng W, Tong Z, Liu YX, Zhang LF, Zhu H, Gao H, Huang L, Liu YL, Ma CM, Xu YF, Ding MX, Deng HK, Qin C (2007) Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med 57(5):450–459
Fréminet A, Leclerc L (1980) Effect of fasting on liver and muscle glycogen in rats and guinea pigs. J de Physiol 76(8):877–880
Bruter AV, Korshunova DS, Kubekina MV, Sergiev PV, Kalinina AA, Ilchuk LA, Silaeva YY, Korshunov EN, Soldatov VO, Deykin AV (2021) Novel transgenic mice with Cre-dependent co-expression of GFP and human ACE2: a safe tool for study of COVID-19 pathogenesis. Transgenic Res 30(3):289–301. https://doi.org/10.1007/s11248-021-00249-8
Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82(15):7264–7275. https://doi.org/10.1128/jvi.00737-08
Oladunni FS, Park JG, Pino PA, Gonzalez O, Akhter A, Allué-Guardia A, Olmo-Fontánez A, Gautam S, Garcia-Vilanova A, Ye C, Chiem K, Headley C, Dwivedi V, Parodi LM, Alfson KJ, Staples HM, Schami A, Garcia JI, Whigham A, Platt RN 2nd, Gazi M, Martinez J, Chuba C, Earley S, Rodriguez OH, Mdaki SD, Kavelish KN, Escalona R, Hallam CRA, Christie C, Patterson JL, Anderson TJC, Carrion R Jr, Dick EJ Jr, Hall-Ursone S, Schlesinger LS, Alvarez X, Kaushal D, Giavedoni LD, Turner J, Martinez-Sobrido L, Torrelles JB (2020) Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun 11(1):6122. https://doi.org/10.1038/s41467-020-19891-7
Rathnasinghe R, Strohmeier S, Amanat F, Gillespie VL, Krammer F, García-Sastre A, Coughlan L, Schotsaert M, Uccellini MB (2020) Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg Microbes Infect 9(1):2433–2445. https://doi.org/10.1080/22221751.2020.1838955
Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, Qu Y, Li F, Lv Q, Wang W, Xue J, Gong S, Liu M, Wang G, Wang S, Song Z, Zhao L, Liu P, Zhao L, Ye F, Wang H, Zhou W, Zhu N, Zhen W, Yu H, Zhang X, Guo L, Chen L, Wang C, Wang Y, Wang X, Xiao Y, Sun Q, Liu H, Zhu F, Ma C, Yan L, Yang M, Han J, Xu W, Tan W, Peng X, Jin Q, Wu G, Qin C (2020) The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583(7818):830–833. https://doi.org/10.1038/s41586-020-2312-y
Zheng J, Wong LR, Li K, Verma AK, Ortiz ME, Wohlford-Lenane C, Leidinger MR, Knudson CM, Meyerholz DK, McCray PB Jr, Perlman S (2021) COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 589(7843):603–607. https://doi.org/10.1038/s41586-020-2943-z
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 181(2):271–80.e8. https://doi.org/10.1016/j.cell.2020.02.052
Ohka S, Igarashi H, Nagata N, Sakai M, Koike S, Nochi T, Kiyono H, Nomoto A (2007) Establishment of a poliovirus oral infection system in human poliovirus receptor-expressing transgenic mice that are deficient in alpha/beta interferon receptor. J Virol 81(15):7902–7912. https://doi.org/10.1128/jvi.02675-06
Kuehn MR, Bradley A, Robertson EJ, Evans MJ (1987) A potential animal model for Lesch-Nyhan syndrome through introduction of HPRT mutations into mice. Nature 326(6110):295–298. https://doi.org/10.1038/326295a0
Kubekina MV, Kalmykov VA, Kusov PA, Silaeva YY, Deikin AV (2019) Lesch-Nyhan syndrome: from patient to mouse model. J Anim Sci 97:46–47. https://doi.org/10.1093/jas/skz258.092
Bell S, Kolobova I, Crapper L, Ernst C (2016) Lesch-Nyhan syndrome: models, theories, and therapies. Mol Syndromol 7(6):302–311. https://doi.org/10.1159/000449296
Keebaugh AC, Mitchell HA, Gaval-Cruz M, Freeman KG, Edwards GL, Weinshenker D, Thomas JW (2011) PRTFDC1 is a genetic modifier of HPRT-deficiency in the mouse. PLoS ONE 6(7):e22381. https://doi.org/10.1371/journal.pone.0022381
Keebaugh AC, Sullivan RT, Thomas JW (2007) Gene duplication and inactivation in the HPRT gene family. Genomics 89(1):134–142. https://doi.org/10.1016/j.ygeno.2006.07.003
Oblak AL, Forner S, Territo PR, Sasner M, Carter GW, Howell GR, Sukoff-Rizzo SJ, Logsdon BA, Mangravite LM, Mortazavi A, Baglietto-Vargas D, Green KN, MacGregor GR, Wood MA, Tenner AJ, LaFerla FM, Lamb BT (2020) Model organism development and evaluation for late-onset Alzheimer’s disease: MODEL-AD. Alzheimer’s Dementia (New York) 6(1):e12110. https://doi.org/10.1002/trc2.12110
Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK, St Clair DK (2006) Beta-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer’s disease. Am J Pathol 168(5):1608–1618. https://doi.org/10.2353/ajpath.2006.051223
Foidl BM, Humpel C (2020) Can mouse models mimic sporadic Alzheimer’s disease? Neural Regen Res 15(3):401–406. https://doi.org/10.4103/1673-5374.266046
Carmona S, Zahs K, Wu E, Dakin K, Bras J, Guerreiro R (2018) The role of TREM2 in Alzheimer’s disease and other neurodegenerative disorders. The Lancet Neurol 17(8):721–730. https://doi.org/10.1016/s1474-4422(18)30232-1
Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C (2008) Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res 193(2):174–182. https://doi.org/10.1016/j.bbr.2008.05.008
Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C (2005) Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res 159(1):1–14. https://doi.org/10.1016/j.bbr.2004.09.019
Franco R, Cedazo-Minguez A (2014) Successful therapies for Alzheimer’s disease: why so many in animal models and none in humans? Front Pharmacol 5:146. https://doi.org/10.3389/fphar.2014.00146
Morris GP, Clark IA, Vissel B (2014) Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol Commun 2:135. https://doi.org/10.1186/s40478-014-0135-5
Funding
The work was supported by the Russian Science Foundation project no. 22-15-00227 and by grant 075-15-2021-668 (29.07.2021) from the UNU Transgenbank. Chapter “Concerns” section was financed through the Russian Science Foundation and all other parts were performed by the grant support of the UNU Transgenbank.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review conception. The first draft of the manuscript was written by AVB, images were prepared by EAV and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies involving human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bruter, A.V., Varlamova, E.A., Okulova, Y.D. et al. Genetically modified mice as a tool for the study of human diseases. Mol Biol Rep 51, 135 (2024). https://doi.org/10.1007/s11033-023-09066-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09066-0