Abstract
Background
Cardiac apoptosis plays a key role in increased morbidity associated with aging-induced-cardiac disorder. Mitochondria play an important role in cardiac apoptosis, and dynamin-related protein 1 (Drp1), as a main mediator of mitochondrial fission, can trigger the mitophagy process to sustain the mitochondrial quality. The present study was done to determine the effect of vitamin D (VitD) treatment on cardiac hypertrophy through mitophagy regulation in aged animals induced by D-galactose (D-GAL).
Methods and results
Male Wistar rats were randomly divided into four groups: control, D-GAL (aging group), D-GAL co-injected with VitD (D-GAL ± VitD), and D-GAL plus ethanol (D-GAL ± Ethanol). Aging was induced by an intraperitoneal (i.p.) administration of D-GAL at 150 mg/kg daily for eight weeks and also VitD (400 IU/kg) or ethanol was injected (i.p.) into aging rats. Then, the levels of cardiac mitophagy and cardiac apoptosis were determined by measuring the expression of tensin homologue (PTEN)-induced putative kinase 1 (PINK1), Drp1, Bcl2-Associated X (Bax), and B-cell lymphoma 2 (Bcl2) genes. Aging in rats was associated with a reduction in mitophagy and also an increase in apoptosis of the heart through down-regulation of Drp1, PINK1, and Bcl2 genes and also up-regulation of Bax. However, VitD improved cardiac hypertrophy through cardiac mitophagy in D-GAL-induced aging rats.
Conclusion
VitD can inhibit cardiac hypertrophy by an increase in mitophagy and a decrease in apoptosis in the aging heart.
Graphical abstract

The illustration of the suggested mechanism underlying of Vitamin D in cardiac hypertrophy induced by aging
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging has been known as an important risk factor for heart failure, which accounts for increased mortality and morbidity in the elderly population worldwide [1]. Cellular senescence can be promoted during the aging process by the accumulation of reactive oxygen species (ROS) in the body. Senescence in the heart is associated with cardiovascular diseases, such as atherosclerosis, valvular heart disease, cardiac hypertrophy, and arrhythmias [2]. Senescent cardiomyocytes are characterized by DNA damage, cardiac apoptosis, increased oxidative stress, and mitochondrial dysfunction in the heart [3]. Among these, cardiac apoptosis is a dominant player in the development of cardiac disorders, and cardiac cell loss due to mitochondrial dysfunction and apoptosis leads to greater vulnerability of the heart to damage in aging hearts [4]. Cardiac apoptosis in aged hearts is mainly mediated by mitochondrial dysfunction [5]. Heart tissue includes many mitochondria occupying 40–60% of the volume of heart cells. These organelles produce approximately 90% of the cardiac energy and play an important role in myocardial function [6]. In this regard, many studies have shown that mitochondrial dysfunction in aged cardiac cells can induce apoptotic cellular damage through the release of cytochrome C into cytoplasm leading to myocardial injury in the aged heart [7].
Furthermore, impaired mitochondria have essential roles in neurodegenerative and cardiovascular diseases, and mitophagy is an important process in mitochondrial quality control, which eliminates dysfunctional mitochondria to maintain cellular homeostasis [8]. However, mitophagy capacity declines with aging [9]; thus, dysfunctional mitochondria predominate in senescent cells exacerbating aging-related disorders [10]. Also, mitophagy is involved in cardiac apoptosis and the modulation of aging-induced damages in the aged hearts [11].
Mitochondrial fission is necessary to break up inefficient mitochondria during mitophagy [12]. Dynamin-related protein 1 (Drp1) is an important protein modulating mitochondrial fission and is strongly expressed in heart tissue compared to other tissues [13]. Drp1-mediated mitochondrial fission has been indicated to contribute to multiple cardiovascular disorders, including cardiac aging and aging-induced cardiomyopathy [11, 14]. Also, low expression levels of Drp1 and reduced Drp1-dependent mitochondrial fission are involved in aging-induced cardiac hypertrophy, strongly proposing the key role of Drp1-dependent mitochondrial fission in aging-induced cardiac hypertrophy [11]. On the other hand, recent studies have indicated that decreased mitophagy causes myocardial apoptosis, which is mainly induced by Drp1 [11]. Previous studies have reported that the elevation of damaged mitochondria is associated with the reduced expression of tensin homologue (PTEN)-induced putative kinase 1 (PINK1). In addition, Drp1 is involved in the crosstalk with PINK1 for mitophagic control [15]. PINK1-mediated mitophagy is the most extensively studied pathway in autophagic removal of damaged mitochondria [16]. Based on these studies, we hypothesized that abnormal mitophagy could be restored by the protective effect of Vitamin D (VitD) through a new mechanism involving Drp1 and PINK1 in aged hearts.
Previous studies have exhibited the relation between VitD deficiency and increased risk of cardiovascular disorders [17]. In this regard, low levels of VitD are detected in the serum of hypertensive patients with myocardial dysfunction and left ventricular hypertrophy [18]. Also, VitD can be attributed to reduced serum levels of tumor necrosis factor and elevated levels of IL-10 anti-inflammatory cytokine in patients with congestive heart failure [19]. VitD supplementation significantly improves cardiac function in patients with chronic heart failure [20]. VitD is involved in many biological processes, such as oxidative stress, cell adhesion, proliferation, differentiation, and apoptosis [21]. On the other hand, VitD deficiency can influence the immune system and trigger pro-inflammatory signaling pathways in cardiac cells leading to cardiac hypertrophy and fibrosis [22]. Also, VitD deficiency may contribute to adipose tissue dysfunction leading to cardiometabolic disorders by its effects on the expression of adipokines [23]. Overall, VitD treatment affects the function of the cardiovascular system through its receptors in cardiomyocytes and arterial walls; however, the fundamental mechanisms are still unknown. The purpose of the current study was to evaluate the effect of VitD treatment on cardiac hypertrophy through modulating mitophagy and apoptosis in D-galactose (D-GAL)-induced aging rats.
Materials and methods
Vitamin D was obtained from the OSVE pharmaceutical Co. (Tehran, Iran), and D-galactose and Pentobarbital sodium salt were purchased from the Sigma-Aldrich Co. (St. Louis, MO, USA).
Animals
The animals were kept under standard conditions (12 h light/dark cycle, 25 ± 2 °C) and had access to food and water and handled to minimize the stress during the treatment period. All experimental protocols were conducted based on the guidelines of the Animal Experiment Committee (IR.UMSHA.REC.1400.269) considering the Guide for the Care and Use of Animals of the National Institutes of Health (NIH Publication No. 85 − 23, revised 1996).
Treatment schedule of aging rats
After one week of compatibility, male Wistar rats (age: three months; body weight: 290 ± 30 g) were randomly divided into four experimental groups (n = 8):
-
1.
Control group (CONT) without the treatment.
-
2.
D-GAL-induced aged group (D-GAL).
-
3.
Aging group co-injected with D-GAL and ethanol (D-GAL ± Ethanol).
-
4.
Aging group co-injected with D-GAL and VitD (D-GAL ± VitD).
To induce aging in male rats, D-GAL was intraperitoneally (i.p) injected at a dose of 150 mg/kg daily for eight weeks [24]. Also, VitD (400 IU/kg) or ethanol (vehicle, equivalent volume) was co-injected (i.p.) with D-GAL into male rats [25] and VitD ± ethanol was injected (i.p.) into aged animals. Animals receiving VitD and/or ethanol exhibited no significant side effects [25]. Sodium pentobarbital was injected (i.p.) at a dose of 60 mg/kg to anesthetize animals. Then, they were subjected to blood sample collection from the inferior vena cava and isolation of heart tissues for molecular assessment. After weighing and washing with PBS, the tissues were snap-frozen and stored at -80 °C. The cardiac hypertrophy index was defined as the ratio of the heart weight (HW)/ body weight (BW) by the following formula [26]:
Histological examination of the heart
For structural evaluation, the heart tissues were removed from the animals and fixed in 10% neutral buffered formalin. The heart tissues were sectioned (4 μm slices) and stained with hematoxylin and eosin (H&E). The samples were histologically surveyed by a photomicroscope.
Quantitative real-time PCR (qRT-PCR) analysis
Drp1 is a key gene regulating mitochondrial fission in mammalian cells, which is accompanied by mitophagy. It is the upstream activator of PINK1. To survey mitophagy in the aged heart, we evaluated the expression of Drp1 and PINK1 by real-time qRT-PCR analysis. First, fresh tissue spices (30 mg) were obtained from the isolated hearts and then homogenized in RNX- plus (Sinaclon, Iran). The isolation of total RNA was performed according to the manufacturer’s protocols. Then, the purity and concentration of total RNA were assessed through the NanoDrop apparatus (Thermo Fisher Scientific, USA). The corresponding cDNA was synthesized through a cDNA synthesis kit (Parstous, Iran) according to the manufacturer’s protocols. Then, qRT-PCR was performed on diluted cDNA with SYBR green Master Mix (Parstous, Iran) through a real-time PCR apparatus (Light Cycler 96, Germany). The expression of genes was calculated by the 2−ΔΔCT formula. The sequences of primers used in the current study are listed in Table 1. The relative mRNA levels of PINK1, Drp1, Bcl2-Associated X (Bax), and B-cell lymphoma 2 (Bcl2) were normalized with internal control (beta-actin).
Statistical analysis
Results were presented as means ± standard error (SE). The analysis of variance (ANOVA) and post hoc Tukey’s test were used to compare the mean values between the groups. A p-value < 0.05 was considered statistically significant.
Results
Cardiac hypertrophy
The D-GAL rats treated with ethanol indicated significantly lower body weight than the D-GAL group (p < 0.001). Chronic ethanol exposure through i.p. injection significantly decreases body weight in aged male rats. The total volume of distribution of the ethanol and lean body mass are negatively correlated with age. A lower volume of distribution, in association with a decrease in lean body mass, most likely describes an increase in blood ethanol concentration and a decrease in body weight as a risk factor for long-term alcohol use in aging [27]. In addition, alcohol enhances catecholamine secretion and vasodilatation. Thus, thermogenesis and resting energy expenditure are increased [28]. Cardiac hypertrophy was significantly enhanced in the aged group compared to the control group (p < 0.05), which indicates the apparent cardiac hypertrophy in aged hearts. However, VitD treatment remarkably decreased cardiac hypertrophy in aging rats (VitD ± D-GAL group) compared to the D-GAL given animals (D-GAL- induced aging group) (P < 0.05, Table 2).
Effect of treatments on the histological changes in the hearts
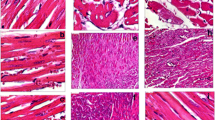
Figure 1 illustrates the histological photographs of heart tissues in experimental animals. Histological assessment of cardiac tissues obtained from normal control rats (A) indicated clear integrity of the myocardial membrane. In addition, normal untreated animals (A) exhibited normal cardiac fibers. Heart tissues from aging rats induced by D-GAL (B) and aging rats treated with ethanol (C) indicated widespread myocardial structure disorder and increased intercellular space compared to control rats (A), indicating the increased size of the cardiomyocytes. Treatment with VitD depicted decreased intercellular space and increased integrity of the myocardial membrane. However, the intercellular space in aging rats treated with VitD (D) still remained greater than that of the control rats (A).
Hematoxylin and eosin (H&E) staining indicating the cardiac tissue architecture in control (A); aged rats induced by D-GAL (B); aged rats induced by a combination of D-GAL and ethanol (C); aged rats induced by a combination of D-GAL and Vit D (D). The images of myocardial architecture were magnified at 100X. Arrowheads indicate cardiomyocytes and the stars show empty spaces between the heart cells
The expression of mitophagic and apoptotic genes
The expression of PINK1, Drp1, and Bcl2 genes has been reported in mitophagy and apoptosis processes. In our study, their expression significantly decreased in the heart tissue of aged animals. In contrast, the expression of Bax in the heart tissue significantly increased in the aged rats compared to the control group. On the other hand, VitD treatment led to increased expression of PINK1, Drp1, and Bcl2 genes in the hearts of aging animals. Consistently, decreased Bax expression, an apoptotic marker gene, was observed in the aged heart after VitD treatment compared to the aged group. These results suggest that VitD exerts its inhibitory effects on aging-related cardiac hypertrophy through enhanced mitophagy and a reduction in apoptosis in the heart of aged rats (Figs. 2, 3 and 4).
Cardiac expression of PINK1 gene in real-time PCR. The findings have been presented as mean ± SEM in different groups. Eight rats were included in each group (n = 8). Also, ### p < 0.001 compared with control group, *** p < 0.001 compared to the D-GAL group. CONT: control, D-GAL: D-galactose -induced aged rats, D-GAL ± Ethanol: D-galactose and Ethanol co-treated rats, D-GAL ± VitD: D-galactose and VitD co-injected group
Cardiac expression of Drp1 gene in real-time PCR. The findings have been presented as mean ± SEM in different groups. Eight rats were included in each group (n = 8). Also, ### p < 0.001 compared with control group, *** p < 0.001 compared to the D-GAL group. CONT: control, D-GAL: D-galactose -induced aged rats, D-GAL ± Ethanol: D-galactose and Ethanol co-treated rats, D-GAL ± VitD: D-galactose and VitD co-injected group
Cardiac expression of Bax(A) and Bcl-2(B) genes in real-time PCR. The findings have been presented as mean ± SEM in different groups. Eight rats were included in each group (n = 8). Also, ## p < 0.01, ### p < 0.001 compared with control group, * p < 0.05 compared to the D-GAL group. CONT: control, D-GAL: D-galactose -induced aged rats, D-GAL ± Ethanol: D-galactose and Ethanol co-treated rats, D-GAL ± VitD: D-galactose and VitD co-injected group
Discussion
Aging is an important risk factor for the development and progression of cardiovascular disorders. It has been reported that aging is associated with reduced cardiac supplies and impaired cardiac function [29]. In the current study, cardiac hypertrophy occurred in D-GAL-induced aging rats compared to normal rats. Histological evaluations showed that D-GAL injection increased the size of myocytes. Researchers have demonstrated myofibrillar loss and derangement in D-GAL-treated myocardium. Besides, our study showed that cardiac apoptosis was enhanced in cardiac aging [30]. Apoptosis results in myocardial dysfunction and cardiac hypertrophy, and also, mitochondrial pathway-related apoptosis plays a key role in deficient cardiac function in the aging heart. Animal studies have revealed that the structure and function of the mitochondria are disrupted in cardiac aging [30]. Mitochondrial quality is regulated by mitochondrial dynamics, mitochondrial biogenesis, and mitophagy. Mitophagy eradicates the impaired mitochondria through the lysosomal digestive pathway to sustain mitochondrial homeostasis and reduction of the repositioning in damaged mitochondria [31]. On the other hand, the heart endures an increased burden of mitophagy to ensure normal cardiac function under physiological conditions. Elevated mitochondrial autophagy also causes increased longevity in Caenorhabditis elegans [32]. It has been known that the inhibition of mitophagy leads to enhanced apoptosis in cardiac aging [11] and the current investigation indicated that mitophagy was notably reduced in cardiac aging. Mitochondrial cleavage happens before mitophagy, and impaired mitochondria will merely be decayed through mitophagy [12]. Mitochondrial fission-induced mitophagy is involved in liver injury [33].
Proper mitochondrial longitude is essential for normal mitochondrial function [30]. Also, mitochondrial cleavage and fusion regulate the mitochondrial morphology, which is changed during apoptosis [34]. Mitochondrial cleavage is modulated through Drp1, which also is involved in apoptosis and mitophagy in ischemic conditions of the heart [35]. Drp1-related mitophagy improves mitochondrial dysfunction and obesity-induced cardiac hypertrophy [36]. However, triptolide-induced hepatotoxicity is associated with decreased Drp1 expression [37], highlighting the role of Drp1-induced mitochondrial fission in mitophagy and mitochondrial homeostasis [38]. Parkin-irrelevant mitophagy sustains the integrity of the brain and heart in mammalians by Drp1 expression [39]. In addition, reduced expression of Drp1 in aged hearts leads to various cardiovascular diseases, diabetes, and cardiac hypertrophy [40]. Drp1 inhibition can mediate the suppression of mitophagy, mitochondrial dysfunction, oxidative stress, and finally, the induction of apoptotic pathways [41]. Also, PINK1 modulates mitophagy and mitochondrial quality in cardiac cells, and decreased expression of PINK1 causes the accumulation of impaired mitochondria in the cells [42]. The expression of Drp1 not only is involved in mitochondrial fission but also significantly increases PINK1 expression in the cardiomyocytes stimulating mitophagic mitochondrial composition in cardiac hypertrophy and cardiomyopathy [43]. Our findings indicated that PINK1 as a mitophagy-related critical gene was notably decreased in parallel with the decreased expression of Drp1 in the hearts of aging rats. On the other hand, mitophagy could cause pro-apoptotic protein release from mitochondria to cytosol, resulting in apoptosis and finally, cell death [44]. Emerging evidence also indicates that mitophagy affects multiple interactions with apoptosis [45]. Drp1-mediated mitochondrial fission is considered a potential upstream regulator for subsequent mitophagy [46].
Our findings propose that aging leads to mitophagy reduction and apoptosis by suppressing the PINK1. In addition, these results indicate that Drp1 might act as an upstream molecule and regulate the PINK1 as a downstream regulator, and also mitophagic and mitochondrial apoptotic responses. In addition, it has been demonstrated that cardiac hypertrophy is closely associated with apoptosis, which has a close relationship with mitophagy [46]. Our findings indicated increased myocardial apoptosis induced by D-GAL injection in aged hearts, whereas VitD treatment dramatically improved the apoptotic activities, evidenced by the reduced expression of pro-apoptotic gene Bax and the increased expression of anti-apoptotic gene Bcl-2. Therefore, VitD treatment could inhibit myocardial apoptosis by increasing Drp1-mediated mitophagy, thereby delaying the development of D-GAL-induced cardiac hypertrophy.
VitD is involved in critical physiologic processes, including oxidative stress, mitochondrial activity, mitochondrial respiratory chain, and apoptosis, which explain its significant effects on different systems, such as the skeletomuscular, immune system, and cardiovascular systems [47]. VitD has protective effects on cardiac ischemia-reperfusion, cardiac infarction, Doxorubicin-induced cardiotoxicity, and cardiac injury in breast cancer through mitophagy and cardiac apoptosis induction in animal models [48,49,50]. VitD exerts anti-fibrotic and anti-hypertrophic effects on myocardial tissue via changing cardiovascular-related risk factors or/and directly affects cardiomyocytes [51]. VitD receptor (VDR) has been identified in ventricular cardiomyocytes and fibroblasts mediating rapid non-genomic and genomic effects of VitD on the cardiac cells [52]. VitD, as a steroid hormone, has two types of receptors, including membrane-bound and cytosolic receptors, involving non-genomic and genomic pathways, respectively [51]. Membrane-bound receptors after VitD trapping increase cytosolic calcium levels by elevated calcium release from intercellular organelles. However, VitD binding to cytosolic receptors causes the formation of nucleolar heterodimeric complexes, which specifically recognize the promotor of responding genes in the nucleus [51]. VitD effects on the expression of downstream genes result in favorable events, ranging from the reduction of thrombogenesis and inhibition of foam cell differentiation to endothelial repair and vascular relaxation [53]. VDR deletion is associated with cardiac hypertrophy in knockout mice [54]. The findings of our study exhibited that VitD led to mitophagy via promoting the expression of PINK1 and Drp1 genes, as markers of mitophagy activity, and also inhibited apoptosis by up-regulation of Bcl-2 anti-apoptotic gene in D-GAL-induced aging rats. Also, VitD supplementation enhances the function and density of mitochondria in a rat model [55]. In general, it can be suggested that VitD modulates mitochondrial function, oxidative stress, and inflammatory signaling to improve cardiac function in aging models.
Conclusion
Cardiac apoptosis is increased in aging. In addition, we demonstrated that reduced mitophagy is likely an important mediator involved in the enhanced apoptosis of the aged heart. Our findings propose that mitophagy is regulated by Drp1 and PINK1 proteins in cardiac aging. Also, VitD treatment improves aging-induced cardiac hypertrophy by enhancing mitophagy and up-regulation of anti-apoptotic genes. Our results suggest that VitD could be regarded as a potential candidate for the prevention and treatment of cardiac aging.
Data Availability
The data used and analyzed in this study are available from the Corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- Bax :
-
Bcl-2-associated X protein
- Bcl-2 :
-
B-cell lymphoma 2
- BW:
-
Body Weight
- CH:
-
Cardiac hypertrophy
- D-GAL:
-
D-galactose
- Drp1 :
-
Dynamin-related protein 1
- HW:
-
Heart Weight
- i.p.:
-
Intraperitoneally
- PINK1 :
-
tensin homologue (PTEN)-induced putative kinase 1
- QRT-PCR:
-
Quantitative Real-Time PCR
- ROS:
-
Reactive oxygen species
- SE:
-
Standard error
- VitD:
-
Vitamin D
- VDR:
-
VitD receptor
References
Tepp K et al (2016) Bioenergetics of the aging heart and skeletal muscles: modern concepts and controversies. Ageing Res Rev 28:1–14
Chadda KR et al (2018) Ageing, the autonomic nervous system and arrhythmia: from brain to heart. Ageing Res Rev 48:40–50
Thai PN et al (2019) Mitochondrial quality control in aging and heart failure: influence of ketone bodies and mitofusin-stabilizing peptides. Front Physiol 10:382
Wang K et al (2013) Thioredoxin reductase was nitrated in the aging heart after myocardial ischemia/reperfusion. Rejuvenation Res 16(5):377–385
Kwak H-B (2013) Effects of aging and exercise training on apoptosis in the heart. J Exerc Rehabil 9(2):212
Zhang R et al (2020) Mitophagy in cardiovascular homeostasis. Mech Ageing De 188:111245
No M-H et al (2020) Aging promotes mitochondria-mediated apoptosis in rat hearts. Life 10(9):178
Luo H et al (2020) A healthy heart and a healthy brain: looking at mitophagy. Front Cell Dev Biol 8:294
Ma L et al (2017) Restoring pharmacologic preconditioning in the aging heart: role of mitophagy/autophagy. J Gerontol: Series A 72(4):489–498
Chen G, Kroemer G, Kepp O (2020) Mitophagy: an emerging role in aging and age-associated diseases. Front Cell Dev Biol 8:200
Wei X et al (2021) Decreased dynamin-related protein 1-related mitophagy induces myocardial apoptosis in the aging heart. Acta Biochim Biophys Sin 53(10):1354–1366
Tong M, Zablocki D, Sadoshima J (2020) The role of Drp1 in mitophagy and cell death in the heart. J Mol Cell Cardiol 142:138–145
Tong M and J, et al (2016) Mitochondrial autophagy in car-diomyopathy. Curr Opin Genet Dev 38:8–15
Ren XC et al (2017) Resveratrol ameliorates mitochondrial elongation via Drp1/Parkin/PINK1 signaling in senescent-like cardiomyocytes. Oxid Med Cell Longev
Tsubouchi K et al (2018) PINK1-PARK2-mediated mitophagy in COPD and IPF pathogeneses. Inflamm Regen 38
Ashrafi G et al (2013) The Pathways of Mitophagy for Quality Control and Clearance of Mitochondria. Cell Death Differ 20(1):31–42
Nardin M et al (2016) Vitamin D status, diabetes mellitus and coronary artery disease in patients undergoing coronary angiography. Atherosclerosis 250 114 – 21
Seker T et al (2015) Lower serum 25-hydroxyvitamin D level is associated with impaired myocardial performance and left ventricle hypertrophy in newly diagnosed hypertensive patients. Anatol J Cardiol 15(9):744
Hazique M et al (2022) A study of vitamin D and its correlation with severity and complication of congestive heart failure: a systematic review. Cureus 6 14(9):28873
Witte KK et al (2016) Effects of vitamin D on cardiac function in patients with chronic HF: the VINDICATE study. J Am Coll Cardiol 67(22):2593–2603
Norman P et al (2014) Vitamin D and cardiovascular disease. Circ Re 17 114(2) 379 – 93
Greco D et al (2018) Vitamin D replacement ameliorates serum lipoprotein functions, adipokine profile and subclinical atherosclerosis in pre-menopausal women. Nutr Metab Cardiovasc Dis 28(8):822–882
Stokić E et al (2015) Vitamin D and dysfunctional adipose tissue in obesity. Angiology 66(7):613–618
Dehghani A et al (2019) Resveratrol and 1, 25-dihydroxyvitamin D co-administration protects the heart against D-galactose-induced aging in rats: evaluation of serum and cardiac levels of klotho. Aging Clin Exp Res 31 1195 – 205
Jeremy M, Gurusubramanian G, Roy VK (2019) Vitamin D3 regulates apoptosis and proliferation in the testis of D-galactose-induced aged rat model. Sci Rep 9(1):14103
Liu J et al (2020) Taurine protects against cardiac dysfunction induced by pressure overload through SIRT1–p53 activation. Chem Biol Interact 317:108972
Matthews D, Mittleman G (2017) Age-dependent effects of chronic intermittent ethanol treatment: gross motor behavior and body weight in aged, adult and adolescent rats. Neurosci Lett 14:657:146–150
Husain K et al (2014) Alcohol-induced hypertension: mechanism and prevention. World J Cardio 6(5):245–252
Liu D et al (2019) Heat shock factor 1-mediated transcription activation of Omi/HtrA2 induces myocardial mitochondrial apoptosis in the aging heart. Aging 11(20):8982
Pereira RM et al (2018) Protective molecular mechanisms of clusterin against apoptosis in cardiomyocytes. Heart Fail Rev 23:123–129
Qiu Z et al (2019) The role of myocardial mitochondrial quality control in heart failure. Front Pharmacol 10:1404
Ryu D et al (2016) Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med 22(8):879–888
Hasnat M et al (2019) Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: a novel mechanism in triptolide-induced hepatotoxicity. Cell Biol Toxicol 35:267–280
Wang DB et al (2013) Declines in Drp1 and parkin expression underlie DNA damage-induced changes in mitochondrial length and neuronal death. J Neurosci Res 33(41):357–365
Li Y, Liu X (2018) Novel insights into the role of mitochondrial fusion and fission in cardiomyocyte apoptosis induced by ischemia/reperfusion. J Cell Physiol 233(8):5589–5597
Tong Met al et al (2023) Distinct roles of DRP1 in conventional and alternative mitophagy in obesity cardiomyopathy. Circ Res 133(1):6–21
Shirakabe A et al (2015) Drp1-dependent mitochondrial autophagy plays a protective role in response to pressure overload Induced mitochondrial dysfunction and heart failure. Circulation 132:17574
Zhang Y et al (2020) Drp1-dependent mitochondrial fission contributes to cr (VI)-induced mitophagy and hepatotoxicity. Ecotoxicol Environ Saf 203:110928
Buhlman L et al (2014) Functional interplay between parkin and Drp1 in mitochondrial fission and clearance. Biochim Biophys Acta Mol Cell Res 1843(9):2012–2026
Morales PE et al (2020) Emerging role of mitophagy in cardiovascular physiology and pathology. Mol Aspects Med 71:100822
Lin X-H et al (2020) Suppressing DRP1-mediated mitochondrial fission and mitophagy increases mitochondrial apoptosis of hepatocellular carcinoma cells in the setting of hypoxia. Oncogenesis 9(7):67
Bakula D and M (2020) Scheibye-Knudsen, MitophAging: mitophagy in aging and disease. Front Cell Dev Biol 8:239
Song M et al (2015) Interdependence of parkin-mediated mitophagy and mitochondrial fission in adult mouse hearts. Circ Res 117(4):346–351
Wan Q et al (2018) Mir-499-5p attenuates mitochondrial fission and cell apoptosis via P21 in Doxorubicin Cardiotoxicity. Front Genet 9:734
Ham SJ et al (2020) Decision between Mitophagy and apoptosis by parkin via VDAC1 ubiquitination. Proc Natl Acad Sci 117(8):4281–4291
Ren X et al (2017) Resveratrol ameliorates mitochondrial elongation via Drp1/Parkin/PINK1 signaling in senescent-like cardiomyocytes. Oxid Med Cell Longev 2017:4175353
Wan M et al (2022) YQFM alleviated cardiac hypertrophy by apoptosis inhibition and autophagy regulation via PI3K/AKT/mTOR pathway. J Ethnopharmacol 1(285):114835
Reddy AM et al (2022) Pivotal role of vitamin D in mitochondrial health, cardiac function, and human reproduction. EXCLI j 21:967
Lee T-L et al (2020) Vitamin D attenuates ischemia/reperfusion-induced cardiac injury by reducing mitochondrial fission and mitophagy. Front Pharmacol 11:604700
Milazzo V et al (2017) Vitamin D and acute myocardial infarction. World J Cardiol 9(1):14
Lee KJ et al (2021) Cytoprotective effect of vitamin d on doxorubicin-induced cardiac toxicity in triple negative breast cancer. Int J Mol Sci 22(14):7439
Latic N, Erben RG (2020) Vitamin D and cardiovascular disease, with emphasis on hypertension, atherosclerosis, and heart failure. Int J Mol Sci 21(18):6483
Ferder Met al et al (2013) The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol 304(11):1027–1039
Pilz S et al (2016) Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol 13(7):404–417
Chen S et al (2011) Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 124(17):1838–1847
Acknowledgements
The authors would like to thank and appreciate the Hamadan University of Medical Sciences for funding the present research.
Funding
This study was funded by the Vice-Chancellor for Research and Technology, Hamadan University of Medical Sciences (No. 140005124073).
Author information
Authors and Affiliations
Contributions
SSh and KHR-A contributed to data collection and interpretation and wrote the manuscript. AK performed the experiments and provided reagents.IS conceived and designed the experiments.SH provided reagents and materials and analyzed the data. SSA and PH contributed to data collection and analysis.FR-A designed the study, contributed to data collection and interpretation, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical approval was granted by the Ethics Committee of Hamadan University of Medical Sciences (Ethics code: IR.UMSHA.REC.1400.269).
Consent for publication
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahidi, S., Ramezani-Aliakbari, K., Komaki, A. et al. Effect of vitamin D on cardiac hypertrophy in D-galactose-induced aging model through cardiac mitophagy. Mol Biol Rep 50, 10147–10155 (2023). https://doi.org/10.1007/s11033-023-08875-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08875-7