Abstract
Background
Ovarian advanced glycation end-products (AGEs) accumulation is associated with ovarian granulosa cells (GCs) dysfunction. Vitamin B6 derivatives positively affected reproduction. The current study was conducted to elucidate the AGEs effects on human luteinized mural GCs steroidogenesis in the presence or absence of pyridoxamine (PM).
Methods and results
Isolated GCs of 50 healthy women were divided into four parts and treated with media alone (Control), PM alone, or human glycated albumin (HGA) with/without PM. Main steroidogenic enzymes and hormones were assessed by qRT-PCR and ELISA. The AGE receptor (RAGE) protein was also determined using Western blotting. The non-toxic concentration of HGA increased the expression of RAGE, StAR, 3β-HSD, and 17β-HSD (P < 0.0001 for all) but decreased the expression of CYP19A1 at mRNA levels. The increased RAGE protein expression was also confirmed by western blot analysis. These effects resulted in declined estradiol (E2), slightly, and a sharp rise in progesterone (P4) and testosterone (T) levels, respectively. PM, on its own, ameliorated the HGA-altered enzyme expression and, thereby, corrected the aberrant levels of E2, P4, and T. These effects are likely mediated by regulating the RAGE gene and protein expression.
Conclusion
This study indicates that hormonal dysfunctions induced by the AGEs-RAGE axis in luteinized GCs are likely rectified by PM treatment. This effect is likely acquired by reduced expression of RAGE. A better understanding of how AGEs and PM interact in ovarian physiology and pathology may lead to more targeted therapy for treating ovarian dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a normal menstrual cycle, ovulation is believed to be triggered by the mid-cycle surge of gonadotropins (both luteinizing hormone [LH] and follicle-stimulating hormone [FSH]). According to the current paradigm, “sustained elevation of estradiol” culminates in the triggering surge of these two gonadotropins and, thereby, ovulation and the end of the follicular phase of the menstrual cycle [1]. However, other research showed that the pre-ovulatory LH surge is not triggered by the sustained elevated estradiol (E2), and it concluded the current role of E2 in ovulation is likely misinterpreted. One reason for this misinterpretation is the supra-physiological E2 level reached in early course ovarian stimulation without eliciting or triggering ovulation [2]. Moreover, well-documented research highlights that progesterone (P4) is the genuine physiological trigger of ovulation during a narrow window at the end of the follicular phase while knowing P4 blocks ovulation when administered out of this narrow window, like in birth-control formulation [3, 4]. Envisaging this role of P4 in eliciting the gonadotropin surge and state of luteinization, therefore, prevention of premature LH-surge by GnRH agonist or antagonist has become part of the routine process COS to improve oocyte quality and prevent premature ovulation [5]. However, whether increased P4 rise during follicular development leads to a higher rate of aneuploidy remains to be answered. It is also well known that premature P4 rise triggered advances in endometrial receptivity and asynchrony between embryo and endometrium, resulting in a freeze-all strategy in such a circumstance with its innate complications [6]. Therefore, a deeper understanding of factors or elements interfering or disturbing the process of steroidogenesis in follicular granulosa cells (GCs) may improve our general understanding of female reproductive physiology and factors affecting oocyte and embryo quality in the process of assisted reproductive techniques (ART).
Advanced glycation end products (AGEs) are a heterogeneous, complex group of compounds formed when reducing sugars react non-enzymatically with amino acids in proteins, lipids, or DNA in vivo and in vitro, especially during the browning process. This phenomenon is referred to as the Maillard reaction [7]. Research has suggested that AGEs are involved in the progression and pathogenesis of several diseases, including diabetes mellitus, cardiovascular disease, Alzheimer’s disease, as well as aging in general [8].
AGEs can cause tissue injury directly or indirectly through proteins crosslinking or binding to multi-ligand transmembrane receptors, known as RAGE (receptor for AGEs) [9]. Activation of RAGE has multiple intracellular downstream effects, including activation of oxidative stress and inflammation signaling pathways. The release of pro-inflammatory cytokines increases the expression of RAGE. This condition results in a vicious cycle of increased pro-inflammatory markers as well as oxidative stress and accounts for the etiology of many diseases, including polycystic ovary syndrome (PCOS) [10,11,12,13].
Previous studies indicated that AGE accumulation and the AGEs/RAGE axis negatively impact ovarian function and aging [14, 15]. In GCs, AGEs alter glucose metabolism [16], steroidogenesis [17], and the production of inflammatory cytokines [18], leading to ovarian complications and infertility-related disorders. Therefore, inhibition of AGEs formation appears to be a promising therapeutic approach for altering the pathogenesis and delaying the progression of reproductive-associated diseases. Several agents have been found to affect tissue and circulating AGEs levels, including aspirin, thiamine, and pyridoxamine [19].
Pyridoxamine dihydrochloride (PM) is a small molecule derived from pyridoxal phosphate with a distinct chemical structure that impedes the formation of AGEs [20]. It is also an essential element of one carbon cycle [21]. A number of pathogenic oxidative reactions that lead to AGEs formation can be inhibited by PM, including toxic carbonyls, reactive oxygen species (ROS), and glycosylated protein conversion to AGEs [20, 22].
A previous report indicated that adding pyridoxine (PN), another member of the vitamin B family, to the maturation medium significantly reduced the activity of cathepsin B, a member of the lysosomal cysteine protease family, in bovine cumulus cells and oocytes. Furthermore, PN improved both the blastocyst and hatched blastocyst rates. According to these results, PN is a promising tool for improving the developmental competence of bovine oocytes and subsequent embryo quality [23]. Metabolomic analysis identifying different metabolites in PCOS revealed that the vitamin B6 metabolism pathway is critically impaired [24]. Therefore, it is not surprising that daily intake of folic acid or B-group vitamins effectively reduces elevated homocysteine levels in PCOS patients undergoing short-term metformin therapy [25]. In another work, there was a significant negative correlation between the free androgen index (FAI) and vitamin B6 intake in women with PCOS [26].
Nevertheless, PM effects on the AGEs-RAGE axis have not been investigated in human luteinized GCs. Based on evidence that suggests PM has a beneficial impact on oocyte developmental competency and inhibits AGEs formation [20, 23], we hypothesized that this water-soluble pyridine compound might support human GCs function against adverse effects induced by AGEs. The present study was conducted to investigate the impact of AGEs on the expression of steroidogenic enzymes in human luteinized GCs in the presence or absence of PM.
Materials and methods
Subjects and controlled ovarian stimulation protocol
The current study was conducted in Shiraz Ghadir Mother and Child Hospital between May 2021 and June 2022 on ovarian mural GCs obtained from 50 women undergoing intracytoplasmic sperm injection (ICSI) treatment.
Inclusion criteria were 18–36 years old and a body mass index between 18.5 and 30 kg/m2. Moreover, this study included infertile couples diagnosed with male factor, tubal factor, as well as egg donors, and women with normal ovarian reserves. Cigarette smokers, alcohol consumers, and women with diabetes, endometriosis, chronic metabolic syndrome, thyroid disorder, obesity, and PCOS were excluded from the present study.
Assessments of the basal serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), anti-müllerian hormone (AMH), and thyroid stimulating hormone (TSH) were performed on the third day of the menstrual cycle. Other blood tests, including fasting blood glucose (FBG), Na+, K+, and hemoglobin concentrations, as well as white blood cells (WBCs) and red blood cells (RBCs) counts, were also performed.
The ovarian stimulation procedure was carried out in all cases using an antagonist protocol. A daily subcutaneous injection of recombinant FSH (150–300 IU, Gonal-F, Merck-Serono, Germany) was initiated on the third day of the current menstrual cycle. Sequential ultrasound scans were conducted to monitor follicular growth. Human recombinant chorionic gonadotropin (hCG) (500 IU, Ovitrelle, Merck-Serono) was administered intramuscularly to induce ovulation when at least three dominant follicles were between 17 and 18 mm in diameter. At this time, the circulating E2 concentration was also determined. Thirty-six hours later, oocyte retrieval was carried out.
Human GCs purification method
Following the harvest of oocytes on the retrieval day, the remaining follicular aspirates were separately collected and transported to the laboratory and centrifuged for 10 min at 4 °C at 1600 rpm. Following removal of the supernatant, the cell pellet was pipetted into cold RBCs lysis buffer (1x) containing ammonium chloride (Sigma, USA), vortexed for approximately 20 s, and then kept at room temperature for about 5 min. Then, the sample was centrifuged at 1600 rpm for 10 min at room temperature to remove the RBCs lysate. The cell pellet was then resuspended in 5 mL phosphate buffer saline (PBS) and centrifuged (1200 rpm, 5 min). The process was repeated two more times.
For the purpose of counting the number of viable cells, GCs pellets were trypsinized (0.25% trypsin/EDTA solution) (Sigma) and resuspended in Dulbecco Modified Eagle medium (DMEM/F12) (Biosera, LM-D1220, Nuaille, France). The percentage of dead cells and cell counts were determined by staining cells with 0.4% Trypan blue (Sigma) and using a hemocytometer, respectively.
Flow cytometry was used to distinguish leukocytes from GCs using mouse anti-human CD45, conjugated with fluorescein isothiocyanate (antiCD45-FITC) (SC-1178 FITC, Santa Cruz), which revealed that the purity of follicular cells exceeded 95%.
Cell culture and study design.
In the first step, MTT (3–4, 5-dimethylthiazol-2-yl-2, 5-diphenyltetrazolium bromide) assay was used to determine percentage cell viability in HGA and PM-treated GCs relative to untreated ones. In order to calculate the percentage of cell viability, the following formula was used:
Cell viability (% of control) = (At - Ab) / (Ac- Ab) × 100
Where At is the absorbance of the test sample, Ac is the absorbance of the Control, and Ab is the absorbance of the blank.
The 400 µg/mL HGA concentrations were chosen based on the previous studies [17]. The PM concentration (50 µM) was determined based MTT test for 50, 100, and 200 µM PM.
Finally, 400 µg/mL HGA and 50 µM PM concentrations were chosen as the concentrations with no toxic effect for the continuation of this study.
The isolated GCs of each participant were divided into four parts and cultured in 6-well plates at a density of 2 × 106 cells/well in DMEM/F12 media (3 mL/well) supplemented with 5% charcoal-stripped fetal bovine serum (FBS), 1% penicillin/streptomycin, and 1% L-glutamine and the corresponding treatments were performed for each group for 48 h under standard culture conditions (37 ℃ and 5% CO2 incubator). Then, the four studied groups were defined as: (1) Control or non-treated (DMSO only), (2) HGA that were treated with 400 µg/mL HGA (dissolved in media), (3) HGA + PM that were treated with 400 µg/mL HGA and 50 µM PM (dissolved in DMSO), and (4) PM that were treated with 50 µM PM.
The final DMSO concentration in the culture medium was 0.5% [27].
Quantitative Real-time PCR (qRT-PCR) technique
To assess the expression of the steroidogenic acute regulatory protein (StAR), 3β-hydroxysteroid dehydrogenase (3β-HSD), 17β‐hydroxysteroid dehydrogenase (17β-HSD), cytochrome P450 aromatase (CYP19A1), and RAGE, total cellular RNA was extracted from GCs using RNXTM–PLUS solution (Sinaclon, Karaj, Iran). The quality of RNA was evaluated using a Nanodrop spectrophotometer (ThermoFisher Scientific, USA). Extracted mRNAs were reversely transcribed into first-strand cDNA by applying a cDNA synthesis kit (Add Bio Co., South Korea) according to the manufacturer’s instructions. qRT-PCR was accomplished using the Applied Biosystems StepOne system with a high ROX SYBR Green PCR Master Mix (Add Bio Co., South Korea). Samples were quantified by the comparative 2-ΔΔCt method with β-actin as an internal control. Experiments were performed in triplicate. The oligonucleotide sequences of primers and reaction conditions used for this study are shown in Table 1.
ELISA for E2, P4, and total T detection in cell culture media
Following 48 h of cell culture, the media were collected to determine the levels of E2, P4, and total testosterone (total T) released by isolated GCs. Assays were conducted using commercially available ELISA kits (DiaMetra, Italy) according to manufacturer recommendations using a microplate reader (Epoch 2TM, BioTek Instruments, USA). The detection limits of E2, P4, and total T were 5 pg/mL, 0.05 ng/mL, and 0.2 ng/mL, respectively. All assays were conducted in duplicate.
Western Blot technique
The western blotting method was used to examine the RAGE expression level in human GCs. At the end of 48 h experimental period, the GCs were trypsinized and treated with cold-RIPA buffer containing protease inhibitors. Then, cell lysates centrifugation was performed at 7000 rpm for 10 min at 4 °C. After that, supernatant protein concentration was determined by Lowry assay. The proteins were separated by SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, CA, USA). The membranes were blocked with skim milk in Tris-buffered saline supplemented with tween 20 for 120 min. Overnight incubation with primary antibodies [Anti-GAPDH (GTX100118, as housekeeping protein) and anti-RAGE (SC-365,154)] was performed at 4 °C. The membrane was next incubated with HRP-conjugated secondary antibodies [(anti-rabbit IgG, BA1054-2) and (anti-mouse IgG, SC-516,102)] for 2 h at room temperature. Detection of protein bands was done using enhanced chemiluminescence (ECL) kit (Bio-Rad) and X-Ray film (n = 5). Images were analyzed and quantified with ImageJ software (NIH). The experiments were performed in duplicate.
Moreover, the following formula was used to calculate the percentage changes (gene, protein, and hormone assays) in treated samples over control samples:
Percentage change= (Control – Treated)/Control × 100
Statistical analysis
The Shapiro-Wilk test was performed to verify the data normality. Then, one-way ANOVA with post-hoc Tukey test was used to compare the statistical differences between experimental groups with normal data distributions. While in the case that the distribution of data was not normal, a Kruskal-Wallis test was performed. All results were presented as mean ± SEM. Statistical significance was determined at P < 0.05. All statistical analyses were performed using GraphPad Prism software (version 9.0, Inc. La Jolla, California, USA).
Results
Table 2 summarizes the demographic and clinical characteristics of the studied participants. All participants in the present study had acceptable serum TSH and FBG levels, and on the third day of the menstrual cycle, FSH, LH, E2, and AMH levels were also within the normal range, suggesting that women participating in this study were in a normal endocrine and metabolic state.
HGA and PM concentrations
The HGA concentration used in this study was 400 µg/mL based on previous reports (Merhi et al., 2018). MTT assay revealed that 400 µg/mL HGA insignificantly reduced cell viability compared to non-treated GCs (100% vs. 86.06%).
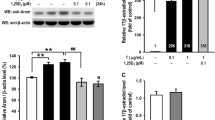
MTT assay was also used to determine the maximum PM concentration with no toxic effect. As shown in Figs. 1, 100 and 200 µM PM significantly reduces GCs viability, while 50 µM was non-toxic to these cells.
Determination of the least toxic dose of PM on human luteinized GCs, based on cell viability assay using MTT. The experiments were performed in duplicate, and data are expressed as mean ± SEM, n = 5. ****P < 0.0001 and **P < 0.01 represent significant differences between studied groups
Abbreviations: PM: pyridoxamine; GCs: granulosa cells; MTT: 3-4, 5-dimethylthiazol-2-yl-2, 5-diphenyltetrazolium bromide
Effect of HGA in the presence or absence of PM on steroidogenic enzymes expression in human luteinized GCs
The other aspect of the current study was investigating the alteration in expression levels of genes involved in steroidogenesis in all studied groups. Considering this aspect, the relative fold changes of StAR, 3β-HSD, 17β-HSD, and CYP19A1 genes were measured by quantitative RT-PCR. The relative expression levels of StAR, 3β-HSD, and 17β-HSD were significantly enhanced, whereas the relative fold change of the CYP19A1 gene was reduced considerably under HGA treatment compared to controls. However, co-treatment GCs with HGA and PM significantly downregulated the expression of StAR, 3β-HSD, and 17β-HSD genes and upregulated the CYP19A1 expression levels compared to the HGA group (Fig. 2).
Effect of HGA (as a precursor for AGEs) (400 µg/mL) with or without PM (50 µM) on StAR (A), 3β-HSD (B), 17β-HSD (C), and CYP19A1 (D) gene expression in human luteinized GCs. Quantitative real-time PCR was done after 48 h incubation with HGA with or without PM. The experiments were performed in duplicate, and data are expressed as mean ± SEM, n = 8. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 represent significant differences between studied groups
Abbreviations: HGA: human glycated albumin; AGEs: advanced glycation end-products; PM: pyridoxamine; StAR: steroidogenic acute regulatory protein; 3β-HSD: 3β-hydroxysteroid dehydrogenase; 17β-HSD: 17β-hydroxysteroid dehydrogenase; CYP19A1: cytochrome P450 aromatase; GCs: granulosa cells
The relative expression levels of StAR and CYP19A1 returned to normal values, while 3β-HSD and 17β-HSD gene expression showed significant differences in the HGA + PM group compared to the control group (Fig. 2).
The percent changes analysis showed that HGA treatment significantly amplified StAR (by 130.16%), 3β-HSD (by 158.28%), and 17β-HSD (by 102.14%) mRNA expression levels, while it diminished CYP19A1 (by 72.11%) mRNA expression (P < 0.0001). The administration of PM along with HGA treatment diminished StAR (by 50.69%), 3β-HSD (by 70.16%), and 17β-HSD (by 21.43%) mRNA levels but enhanced CYP19A1 (by 265.62%) mRNA expression (P < 0.0001). Moreover, PM treatment alone decreased the expression of StAR (by 31.46%), 3β-HSD (by 46.42%), and 17β-HSD (by 29.13%) genes while it increased CYP19A1 (by 19.98%) mRNA expression as compared to controls (Fig. 2).
Effect of HGA in the presence or absence of PM on E2, P4, and total T production by human luteinized GCs
As shown in Fig. 3A, the concentration of E2 in the media was significantly reduced following HGA treatment of the GCs, as compared to the control group (P < 0.0001). Adding PM along with HGA to the GCs culture resulted in an increase in the production of E2 compared to HGA treatment only; however, this difference is not statistically significant. In GCs treated with PM alone, the value of E2 production was approximately equivalent to normal levels.
Effect of HGA (as a precursor for AGEs) with or without PM on E2 (A), P4 (B), and total T (C) release by human luteinized GCs. Cells were treated with HGA (400 µg/mL) with or without PM (50 µM) for 48 h; then, cell culture media was collected for E2, P4, and total T ELISA assay. The experiments were performed in duplicate, and data are expressed as mean ± SEM, n = 20. *P < 0.05, **P < 0.01 and ****P < 0.0001 represent significant differences between studied groups
Abbreviations: HGA: human glycated albumin; AGEs: advanced glycation end-products; PM: pyridoxamine; E2: estradiol; P4: progesterone; total T: total testosterone; GCs: granulosa cells
Furthermore, Fig. 3B shows a slight increase in P4 production in HGA-treated GCs compared to the control group. Administration of PM significantly decreased this undesirable higher P4 secretion in the HGA + PM. In GCs treated with PM alone, the value of P4 production was significantly reduced, but it is still within the normal range of ovulation time.
Moreover, Fig. 3C shows the concentration of total T in the media was significantly increased following HGA treatment of the GCs, as compared to the other studied groups. Adding PM along with HGA to the GCs culture and PM treatment alone decreased the production of total T compared to HGA treatment only; so, the amount of total T production was not significantly different from the control values.
Treatment of GCs with HGA significantly reduced E2 release (by 60.60%) (P < 0.0001), enhanced considerably total T production (by 177.70%) (P < 0.01), and slightly raised P4 (by 39.07%) levels compared to controls during 48 h culture time. Co-treatment of HGA and PM leads to hormone balance through increasing E2 level (by 39.93%) and decreasing P4 (by 62.79%) and total T (by 58.46%) production (Fig. 3).
Effect of HGA in the presence or absence of PM on Expression of RAGE mRNA and Protein in human luteinized GCs
The relative expression of RAGE mRNA and protein under treatment of HGA and PM are illustrated in Fig. 4. The highest RAGE mRNA expression and protein level were seen after HGA treatment. However, treating HGA-affected GCs with PM (HGA + PM group) could significantly downregulate RAGE expression. Likewise, the RAGE protein level reduced in the HGA + PM group compared to the HGA group. There were no significant differences in the RAGE protein levels between PM and controls.
Effect of HGA (as a precursor for AGEs) (400 µg/mL) with or without PM (50 µM) on RAGE gene expression (A) and RAGE protein level (B) in human luteinized GCs. The western blotting assay was done after 48 h incubation with HGA with or without PM. The experiments were performed in duplicate, and data are expressed as mean ± SEM, n = 5. **P < 0.01, ***P < 0.001, and ****P < 0.0001 represent significant differences between studied groups
Abbreviations: HGA: human glycated albumin; AGEs: advanced glycation end-products; PM= pyridoxamine; RAGE: receptor for advanced glycation end-products; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; GCs: granulosa cells
Analysis of percentage change showed that HGA addition to the media significantly enhanced RAGE mRNA (by 126.46%) and protein (by 231.61%) levels (P < 0.0001) compared to controls. Co-treatment of HGA and PM significantly blocked the HGA-induced increase in RAGE mRNA (by 61.31%) and protein (by 41.31%) levels (P < 0.0001). These results suggested that PM plays a role in diminishing AGEs effects via RAGE signaling downregulation (Fig. 4).
Discussion
The current study used human luteinized GCs as a model to assess whether AGEs treatment alters steroidogenesis and whether the PM is able to neutralize the harmful effects of AGEs.
Our results showed that HGA significantly upregulates StAR, 3β-HSD, and 17β-HSD mRNA levels, whereas it downregulates CYP19A1 gene expression. Co-treatment of HGA and PM inhibited these expression changes in steroidogenic enzymes. The HGA treatment also altered the release of E2, P4, and total T by GCs in culture media, whereas PM treatment regulated these unwanted changes. Moreover, PM downregulated RAGE mRNA and protein expression, suggesting that the mechanism of PM action involved preventing HGA-induced changes in the expression of steroidogenic enzymes (Fig. 5).
Schematic diagram representing the relationship between PM and HGA (as a precursor for AGEs) in human luteinized GCs. AGEs affect steroidogenesis by upregulating steroidogenic acute regulatory protein (StAR), 3β-hydroxysteroid dehydrogenase (3β-HSD), and 17β-hydroxysteroid dehydrogenase (17β-HSD) (upward red arrows), and downregulating cytochrome P450 aromatase (CYP19A1) (downward red arrows). PM inhibits the expression of the RAGE gene and protein. Moreover, PM restores the effect of AGEs on StAR, 3β-HSD, 17β-HSD, and CYP19A1 (green arrows)
Abbreviations: HGA: human glycated albumin; AGEs: advanced glycation end-products; RAGE: receptor for advanced glycation end-products; PM: pyridoxamine; GCs: granulosa cells
In the current work, we extracted GCs of normal ovulatory individuals in order to avoid the infliction of factors like exposure to high levels of AGEs reported in PCOS [28]. Moreover, in this study, we did not aim to use high concentrations of HGA, which could have a toxic effect on GCs, to mimic the state of follicular atresia. Therefore, 400 µg/mL HGA used in this study was not toxic but was adequate to induce a hormonal imbalance, in which the E2 level is lower, and P4 and T levels are higher than in non-PCOS individuals.
Several studies have shown that AGEs adversely affect ovarian tissues, particularly GCs function [16, 17, 28, 29]. As AGEs concentrations increase in the ovarian microenvironment, female reproductive tissues may become inefficient, leading to PCO-like syndrome [28, 30] and ovarian aging [14, 31]. Due to their long half-life, AGEs could accumulate in the ovary during reproductive life [32] and induce hormonal disturbance. As a result of this accumulation, hypoxia and decreased nutrient uptake by GCs may occur [33], and AGEs-induced NF-κB activation may trigger GCs apoptosis [34].
We have found that in vitro exposure to HGA significantly diminished the release of E2 from GCs, slightly increased P4 secretion, and dramatically raised total T secretion. A negative correlation between E2 and follicular fluid/serum AGEs levels in infertile women has previously been demonstrated [35].
In luteinized GCs, the changes in hormone secretion profiles are probably in line with alterations in the expression of steroidogenic enzymes. Therefore, to improve our understanding of how AGEs lead to hormonal imbalance, we assessed the expression of major enzymes involved in the process of steroidogenesis using the advantage of human luteinized GCs as a model (Fig. 5). Our results showed that HGA significantly upregulates StAR, 3β-HSD, and 17β-HSD mRNA levels, whereas it downregulates CYP19A1 gene expression. As a result of GCs exposure to the HGA, the expression of StAR, 3β-HSD, and 17β-HSD genes, which are involved in P4 and T production, are increased, while CYP19A1 expression, which converts androgens into estrogens, is decreased. This effect is likely due to the acquisition of increased RAGE mRNA and protein expression in GCs induced by HGA, which in turn mediates the toxic effect of HGA [36, 37].
The above findings are consistent with previous studies stating mice exposed to high-AGE diets prenatally have altered expression of steroidogenic enzymes, including CYP19A1 [38]. AGEs/RAGE activation upregulates the expression of StAR, CYP17A1, 3β-HSD, and 17β-HSD, which may lead to increased P4 and T production and hyper-androgenic state [15]. Moreover, other research showed that P4 is a physiological trigger of ovulation during a narrow window at the end of the follicular phase, while knowing P4 disturbs ovulation when administered out of this narrow window [3, 4]. Indeed, increased P4 level in the pre-ovulatory phase has been shown to impair embryonic development, interrupt embryo-endometrial synchrony, and may account for reduced ART outcomes [6, 39].
On the other hand, interference of HGA with LH and FSH signaling in human granulosa KGN cells was also confirmed. Furthermore, the direct effect of AGEs on LH disturbs its pathway and contributes to the pathophysiology of anovulation [40]. It suggested that exogenous AGEs could potentially alter follicular development.
Similarly, the results of a recent study indicated that treatment of human cumulus GCs with HGA leads to an increased expression of StAR, CYP11A1, 3β-HSD, CYP17A1, and LHR genes. While HGA-treated cumulus GCs released higher E2 content in culture media, their CYP19A1 expression did not change [17]. The latter finding is contrary to the results of the present study. This contradiction is related to the fact that in the mentioned study, cumulus GCs were used, whereas, in our research, mural GCs were used. Overall, the level of E2 production in cumulus GCs is higher than in mural GCs, while the P4 release is not different. These differences are likely related to differences in the metabolomes of cumulus and mural GCs [41, 42].
It was interesting to note that PM, on its own, slightly but significantly downregulated the relative expression of StAR, 3β-HSD, and 17β-HSD and upregulates the relative expression of CYP19A1, which had an outstanding effect on E2 increase and P4 and total T decreases. This effect is related to the ability of PM to hamper the formation of AGEs from glycated proteins by scavenging the pathogenic reactive carbonyl compounds, the intermediates of AGEs formation [43]. This property may account for the anti-oxidant capacity of PM as the AGEs/RAGE interaction induces ROS production [20]. An alternative possibility is that a shortage of vitamin B6 can lead to increased homocysteine in the follicular fluid, resulting in the production of ROS and the formation of AGEs which might be related to poor oocyte quality [44].
Moreover, it is clear that vitamin Bs acts through a specific mechanism, modulation of transcriptional activation, to regulate the physiological actions of multiple members of the steroid hormone receptor superfamily [45]. Analysis of premenopausal women who took a multivitamin or nutrition supplements showed an inverse association between intakes of vitamins B2, B6, and B12 and the risk of ovulatory infertility [46, 47]. Additionally, combined treatment of P4 and vitamin B resulted in lower levels of prolactin, increased FSH and E2, and normalized the menstrual cycle of patients with amenorrhea caused by antipsychotic drugs [48].
Moreover, another study showed that compared to healthy women, women with PCOS had lower plasma concentrations of vitamin B6 [49], and their vitamin B6 metabolism pathway was critically impaired [24]. In this regard, it has been shown that daily intake of B-group vitamins effectively reduced elevated homocysteine levels in PCOS patients undergoing short-term metformin therapy [25]. Another role of vitamin B6 is the production of hydrogen sulfide (H2S), which can induce nuclear factor erythroid–related factor 2 (Nrf2) production as a result of excess ROS production in “oxidative” or “reductive” stress conditions. Increased Nrf2 production led to the production of anti-oxidant enzymes and higher GSH levels [50, 51]. Therefore, the positive effect of B6 could be accounted for by its effect on H2S/Nrf2 axis.
Moreover, supplementation of PN during in vitro maturation improved the developmental competence and quality of the blastocysts [23, 52]. In addition, other work showed that PN inhibits cathepsin B activity (a member of the lysosomal cysteine proteases family) in bovine cumulus-oocyte complexes (COCs) and oocytes [23]. This evidence confirms that PM has beneficial effects on the female reproductive system.
Co-treatment of HGA and PM completely ameliorated the effect of HGA on the relative expression of StAR and CYP19A1 and slightly but significantly decreased and increased the relative expression of 3β-HSD and 17β-HSD, respectively. These effects resulted in a slight but significant improvement in E2 and a significant reduction in P4 and total T. These effects are likely acquired through reduced expression RAGE.
Due to the protective effects of vitamin B6 on GCs function, especially steroidogenesis, it can be considered as a reason for its beneficial effects in past clinical studies and the importance of its adjustment in the preconception period.
Maternal vitamin status contributes to the probability of conception, clinical spontaneous abortion, subclinical early pregnancy loss, and other maternal and fetal health aspects. Pyridoxal 5´-phosphate (PLP) is the main form of vitamin B6 in humans and functions as a coenzyme for more than 160 different enzymatic reactions and plays an important role in maternal health and fetal development [53].
Folate and vitamins B12 and B6 are required for DNA synthesis and cell growth and are involved in homocysteine metabolism. A clinical study suggested that low maternal RBC folate and high homocysteine values in mid-pregnancy are associated with subsequent reduced fetal growth [54]. Similarly, in another study, impaired vitamin B6 levels and folate status were associated with low birth weight [55]. The risk of preterm birth was 50% higher among Chinese women who were vitamin B6 deficient [56]. The results of a prospective cohort of young Chinese women indicated that poor preconception vitamin B6 status was associated with an increased risk of early pregnancy loss and reduced probabilities of conception and clinical pregnancy [57]. Similar studies also showed that lower plasma concentrations of vitamin B6 were associated with recurrent spontaneous and placental abruption or infarction [58, 59].
A recent clinical study discovered that a plasma PLP concentration in the range of 50–100 nmol/L seems to ensure an optimal vitamin B6 status for never-pregnant women, whereas a plasma PLP > 30 nmol/L in pregnancy week 28 ensures an adequate vitamin B6 status during pregnancy and lactation [60]. These observations suggest that maternal vitamin B6 status may influence reproductive events throughout the entire course of pregnancy, from the time of conception through delivery.
There are limitations in this current work. Three types of follicular cells, including cumulus GCs, mural GCs, and theca cells, participate in ovary steroidogenesis [47]. Our study focused on mural GCs that display different characteristics from theca cells. Simultaneously analyzing these three types of human cells responsible for steroidogenesis in laboratory conditions is not easily possible.
In conclusion, due to the long in vivo half-life of AGEs, they become potential signaling molecules that can alter GCs function by accumulating in the ovary. It adversely affects oocyte competence and embryo development via alterations in the follicular microenvironment. Therefore, understanding the mechanisms behind ovarian dysfunction is especially important for women with elevated levels of AGEs, such as women with obesity or PCOS or those with unhealthy diets. As a result, AGEs inhibitors may be considered as new approaches for preserving regular reproductive function. PM (50 µM) reduces mRNA and protein levels of RAGE and protects against steroidogenesis alterations caused by HGA in the present study. Based on our results, the non-toxic properties of PM make it an important candidate as a natural drug for treating AGEs-induced ovarian dysfunction, such as infertile women with PCOS or diabetes or individuals with unhealthy lifestyles and disturbed menstrual cycles aiming for pregnancy.
Furthermore, based on current study findings and other clinical aspects, there is a growing need to counsel pregnant women on the right diets to ensure adequate vitamin B6 is consumed. However, more studies are needed to clarify the molecular mechanism of PM actions in the ovary. To the best of our knowledge, there is no study about PM impacts on human female steroidogenesis affected by AGEs.
Data Availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Andersen CY, Ezcurra D (2014) Human steroidogenesis: implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod Biol Endocrinol 12:128. https://doi.org/10.1186/1477-7827-12-128
Hurst BS, Merriam KS, Elliot M, Matthews ML, Marshburn PB, Usadi RS, Hurst BS (2015) A sustained elevated estradiol is not the trigger for the pre-ovulatory luteinizing hormone surge. Women’s Health Gynecol 3:10–18
Dozortsev DI, Pellicer A, Diamond MP (2020) Premature progesterone rise as a trigger of polycystic ovarian syndrome. Fertil Steril 114:943–944. https://doi.org/10.1016/j.fertnstert.2020.07.007
Dozortsev DI, Diamond MP (2020) Luteinizing hormone-independent rise of progesterone as the physiological trigger of the ovulatory gonadotropins surge in the human. Fertil Steril 114:191–199. https://doi.org/10.1016/j.fertnstert.2020.06.016
Venetis CA, Storr A, Chua SJ, Mol BW, Longobardi S, Yin X, D’Hooghe T (2023) What is the optimal GnRH antagonist protocol for ovarian stimulation during ART treatment? A systematic review and network meta-analysis. Hum Reprod Update. https://doi.org/10.1093/humupd/dmac040
Lawrenz B, Melado L, Fatemi H (2018) Premature progesterone rise in ART-cycles. Reprod Biol 18:1–4
Shen C-Y, Lu C-H, Wu C-H, Li K-J, Kuo Y-M, Hsieh S-C, Yu C-L (2020) The development of Maillard reaction, and Advanced Glycation End product (AGE)-Receptor for AGE (RAGE) signaling inhibitors as Novel therapeutic strategies for patients with AGE-Related Diseases. Molecules 25:5591
Prasad C, Davis KE, Imrhan V, Juma S, Vijayagopal P (2019) Advanced Glycation End Products and Risks for Chronic Diseases: intervening through Lifestyle Modification. Am J Lifestyle Med 13:384–404. https://doi.org/10.1177/1559827617708991
Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M (2022) Advanced Glycation End-Products (AGEs): formation, Chemistry, classification, receptors, and Diseases related to AGEs. Cells 11. https://doi.org/10.3390/cells11081312
Bongarzone S, Savickas V, Luzi F, Gee AD (2017) Targeting the receptor for Advanced Glycation End-products (RAGE): a Medicinal Chemistry Perspective. J Med Chem 60:7213–7232. https://doi.org/10.1021/acs.jmedchem.7b00058
Masjedi F, Keshtgar S, Zal F, Talaei-Khozani T, Sameti S, Fallahi S, Kazeroni M (2020) Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic anti-oxidant defense in human granulosa cells of normal and polycystic ovaries. J Steroid Biochem Mol Biol 197:105521. https://doi.org/10.1016/j.jsbmb.2019.105521
Mehdinejadiani S, Amidi F, Mehdizadeh M, Barati M, Safdarian L, Aflatoonian R, Alyasin A, Aghahosseini M, Pazhohan A, Hayat P, Mohammadzadeh Kazorgah F, Sobhani A (2018) The effects of letrozole and clomiphene citrate on ligands expression of Wnt3, Wnt7a, and Wnt8b in proliferative endometrium of women with polycystic ovarian syndrome. Gynecol Endocrinol 34:775–780. https://doi.org/10.1080/09513590.2018.1446934
Merhi Z, Kandaraki EA, Diamanti-Kandarakis E (2019) Implications and future perspectives of AGEs in PCOS Pathophysiology. Trends Endocrinol Metab 30:150–162. https://doi.org/10.1016/j.tem.2019.01.005
Stensen MH, Tanbo T, Storeng R, Fedorcsak P (2014) Advanced glycation end products and their receptor contribute to ovarian ageing. Hum Reprod 29:125–134. https://doi.org/10.1093/humrep/det419
Garg D, Merhi Z (2016) Relationship between Advanced Glycation End Products and Steroidogenesis in PCOS. Reprod Biol Endocrinol 14:71. https://doi.org/10.1186/s12958-016-0205-6
Diamanti-Kandarakis E, Chatzigeorgiou A, Papageorgiou E, Koundouras D, Koutsilieris M (2016) Advanced glycation end-products and insulin signaling in granulosa cells. Exp Biol Med 241:1438–1445
Merhi Z, Buyuk E, Cipolla M (2018) Advanced glycation end products alter steroidogenic gene expression by granulosa cells: an effect partially reversible by vitamin D. Mol Hum Reprod 24:318–326
Takahashi N, Harada M, Azhary JM, Kunitomi C, Nose E, Terao H, Koike H, Wada-Hiraike O, Hirata T, Hirota Y (2019) Accumulation of advanced glycation end products in follicles is associated with poor oocyte developmental competence. Mol Hum Reprod 25:684–694
Chen JL, Francis J (2012) Pyridoxamine, advanced glycation inhibition, and diabetic nephropathy. J Am Soc Nephrol 23:6–8. https://doi.org/10.1681/asn.2011111097
Ramis R, Ortega-Castro J, Caballero C, Casasnovas R, Cerrillo A, Vilanova B, Adrover M, Frau J (2019) How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of Its Primary Anti-oxidant Activity. Anti-oxidants (Basel) 8. https://doi.org/10.3390/antiox8090344
Lyon P, Strippoli V, Fang B, Cimmino L (2020) B vitamins and One-Carbon Metabolism: implications in Human Health and Disease. Nutrients 12. https://doi.org/10.3390/nu12092867
Glaeser JD, Ju D, Tawackoli W, Yang JH, Salehi K, Stefanovic T, Kanim LEA, Avalos P, Kaneda G, Stephan S, Metzger MF, Bae HW, Sheyn D (2020) Advanced glycation end product inhibitor pyridoxamine attenuates IVD degeneration in type 2 Diabetic rats. Int J Mol Sci 21. https://doi.org/10.3390/ijms21249709
Aboelenain M, Balboula AZ, Kawahara M, El-Monem Montaser A, Zaabel SM, Kim SW, Nagano M, Takahashi M (2017) Pyridoxine supplementation during oocyte maturation improves the development and quality of bovine preimplantation embryos. Theriogenology 91:127–133. https://doi.org/10.1016/j.theriogenology.2016.12.022
Chen X, Lu T, Wang X, Sun X, Zhang J, Zhou K, Ji X, Sun R, Wang X, Chen M, Ling X (2020) Metabolic alterations associated with polycystic ovary syndrome: a UPLC Q-Exactive based metabolomic study. Clin Chim Acta 502:280–286. https://doi.org/10.1016/j.cca.2019.11.016
Kilicdag EB, Bagis T, Tarim E, Aslan E, Erkanli S, Simsek E, Haydardedeoglu B, Kuscu E (2005) Administration of B-group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: a randomized trial. Hum Reprod 20:1521–1528. https://doi.org/10.1093/humrep/deh825
Hestiantoro A, Astuti BPK, Joyo EO, Febri RR, Silvana V, Muharam R (2022) Vitamin B(3) (niacin), B(6), C, and iron intake are associated with the free androgen index, especially in normoandrogenic polycystic ovary syndrome. J Turk Ger Gynecol Assoc 23:130–136. https://doi.org/10.4274/jtgga.galenos.2022.2022-2-1
Chen X, Thibeault S (2013) Effect of DMSO concentration, cell density and needle gauge on the viability of cryopreserved cells in three dimensional hyaluronan hydrogel. Annu Int Conf IEEE Eng Med Biol Soc 2013:6228–6231. https://doi.org/10.1109/embc.2013.6610976
Tatone C, Di Emidio G, Placidi M, Rossi G, Ruggieri S, Taccaliti C, D’Alfonso A, Amicarelli F, Guido M (2021) AGEs-related dysfunctions in PCOS: evidence from animal and clinical research. J Endocrinol 251:R1–r9. https://doi.org/10.1530/joe-21-0143
Mouanness M, Merhi Z (2022) Impact of Dietary Advanced Glycation End Products on Female Reproduction: Review of Potential Mechanistic Pathways. Nutrients [serial on the Internet]. ; 14(5)
Garg D, Merhi Z (2015) Advanced glycation end products: link between diet and ovulatory dysfunction in PCOS? Nutrients 7:10129–10144
Pertynska-Marczewska M, Diamanti-Kandarakis E (2017) Aging ovary and the role for advanced glycation end products. Menopause 24:345–351
Wang X, Wang L, Xiang W (2023) Mechanisms of ovarian aging in women: a review. J Ovarian Res 16:67. https://doi.org/10.1186/s13048-023-01151-z
Merhi Z, Irani M, Doswell AD, Ambroggio J (2014) Follicular fluid soluble receptor for advanced glycation end-products (sRAGE): a potential indicator of ovarian reserve. J Clin Endocrinol Metab 99:E226–E233
Zhu J-l, Cai Y-q, Long S-l, Chen Z, Mo Z-c (2020) The role of advanced glycation end products in human infertility. Life Sci 255:117830. https://doi.org/10.1016/j.lfs.2020.117830
Ravichandran G, Lakshmanan DK, Raju K, Elangovan A, Nambirajan G, Devanesan AA, Thilagar S (2019) Food advanced glycation end products as potential endocrine disruptors: an emerging threat to contemporary and future generation. Environ Int 123:486–500. https://doi.org/10.1016/j.envint.2018.12.032
Niu G, Guo J, Tian Y, Zhao K, Li J, Xiao Q (2018) α–lipoic acid can greatly alleviate the toxic effect of AGES on SH–SY5Y cells. Int J Mol Med 41:2855–2864
Prantner D, Nallar S, Vogel SN (2020) The role of RAGE in host pathology and crosstalk between RAGE and TLR4 in innate immune signal transduction pathways. FASEB J 34:15659–15674. https://doi.org/10.1096/fj.202002136R
Merhi Z, Du XQ, Charron MJ (2020) Perinatal exposure to high dietary advanced glycation end products affects the reproductive system in female offspring in mice. Mol Hum Reprod 26:615–623
Huang B, Ren X, Wu L, Zhu L, Xu B, Li Y, Ai J, Jin L (2016) Elevated progesterone levels on the day of oocyte maturation may affect top quality embryo IVF cycles. PLoS ONE 11:e0145895
Kandaraki EA, Chatzigeorgiou A, Papageorgiou E, Piperi C, Adamopoulos C, Papavassiliou AG, Koutsilieris M, Diamanti-Kandarakis E (2018) Advanced glycation end products interfere in luteinizing hormone and follicle stimulating hormone signaling in human granulosa KGN cells. Exp Biol Med (Maywood) 243:29–33. https://doi.org/10.1177/1535370217731288
Dompe C, Kulus M, Stefańska K, Kranc W, Chermuła B, Bryl R, Pieńkowski W, Nawrocki MJ, Petitte JN, Stelmach B, Mozdziak P, Jeseta M, Pawelczyk L, Jaśkowski JM, Piotrowska-Kempisty H, Spaczyński RZ, Nowicki M, Kempisty B (2021) Human granulosa Cells-Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. https://doi.org/10.3390/cells10061396. Cells 10
Gao EM, Turathum B, Wang L, Zhang D, Liu YB, Tang RX, Chian RC (2022) The Differential Metabolomes in Cumulus and Mural Granulosa cells from human pre-ovulatory follicles. Reprod Sci 29:1343–1356. https://doi.org/10.1007/s43032-021-00691-3
Turgut F, Bolton WK (2010) Potential New Therapeutic Agents for Diabetic kidney disease. Am J Kidney Dis 55:928–940. https://doi.org/10.1053/j.ajkd.2009.11.021
Steegers-Theunissen RP, Steegers EA, Thomas CM, Hollanders HM, Peereboom-Stegeman JH, Trijbels FJ, Eskes TK (1993) Study on the presence of homocysteine in ovarian follicular fluid. Fertil Steril 60:1006–1010. https://doi.org/10.1016/s0015-0282(16)56401-2
Allgood VE, Cidlowski JA (1992) Vitamin B6 modulates transcriptional activation by multiple members of the steroid hormone receptor superfamily. J Biol Chem 267:3819–3824
Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC (2008) Use of multivitamins, intake of B vitamins, and risk of ovulatory infertility. Fertil Steril 89:668–676. https://doi.org/10.1016/j.fertnstert.2007.03.089
Govahi A, Amjadi F, Nasr-Esfahani MH, Raoufi E, Mehdizadeh M (2022) Accompaniment of Time-Lapse Parameters and Cumulus Cell RNA-Sequencing in embryo evaluation. Reprod Sci 29:395–409. https://doi.org/10.1007/s43032-021-00748-3
Zhao Y, Hu S, Zhai W, Wang M, Ran L (2022) Clinical study of Progesterone combined with vitamin B6 in the treatment of Amenorrhea Endocrine Disorders caused by antipsychotics. Comput Math Methods Med 2022:2436322. https://doi.org/10.1155/2022/2436322
Szczuko M, Hawryłkowicz V, Kikut J, Drozd A (2020) The implications of vitamin content in the plasma in reference to the parameters of carbohydrate metabolism and hormone and lipid profiles in PCOS. J Steroid Biochem Mol Biol 198:105570. https://doi.org/10.1016/j.jsbmb.2019.105570
Scammahorn JJ, Nguyen ITN, Bos EM, Van Goor H, Joles JA (2021) Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Anti-oxidants (Basel) 10. https://doi.org/10.3390/antiox10030373
Yang J, Minkler P, Grove D, Wang R, Willard B, Dweik R, Hine C (2019) Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun Biol 2:194. https://doi.org/10.1038/s42003-019-0431-5
Golestanfar A, Niasari-Naslaji A, Jafarpour F, Rouhollahi S, Rezaei N, Menezo Y, Dattilo M, Nasr-Esfahani MH (2022) Metabolic enhancement of the one carbon metabolism (OCM) in bovine oocytes IVM increases the blastocyst rate: evidences for a OCM checkpoint. Sci Rep 12:20629. https://doi.org/10.1038/s41598-022-25083-8
Ueland PM, Ulvik A, Rios-Avila L, Midttun Ø, Gregory JF (2015) Direct and functional biomarkers of vitamin B6 status. Annu Rev Nutr 35:33–70. https://doi.org/10.1146/annurev-nutr-071714-034330
Furness D, Fenech M, Dekker G, Khong TY, Roberts C, Hague W (2013) Folate, vitamin B12, vitamin B6 and homocysteine: impact on pregnancy outcome. Matern Child Nutr 9:155–166. https://doi.org/10.1111/j.1740-8709.2011.00364.x
Deepa R, Mandal S, Van Schayck OCP, Babu GR (2023) Vitamin B6 levels and impaired Folate Status but not vitamin B12 Associated with Low Birth Weight: results from the MAASTHI Birth Cohort in South India. Nutrients 15. https://doi.org/10.3390/nu15071793
Ronnenberg AG, Goldman MB, Chen D, Aitken IW, Willett WC, Selhub J, Xu X (2002) Preconception homocysteine and B vitamin status and birth outcomes in chinese women. Am J Clin Nutr 76:1385–1391. https://doi.org/10.1093/ajcn/76.6.1385
Ronnenberg AG, Venners SA, Xu X, Chen C, Wang L, Guang W, Huang A, Wang X (2007) Preconception B-vitamin and homocysteine status, conception, and early pregnancy loss. Am J Epidemiol 166:304–312. https://doi.org/10.1093/aje/kwm078
Goddijn-Wessel TA, Wouters MG, van de Molen EF, Spuijbroek MD, Steegers-Theunissen RP, Blom HJ, Boers GH, Eskes TK (1996) Hyperhomocysteinemia: a risk factor for placental abruption or infarction. Eur J Obstet Gynecol Reprod Biol 66:23–29. https://doi.org/10.1016/0301-2115(96)02383-4
Wouters MG, Boers GH, Blom HJ, Trijbels FJ, Thomas CM, Borm GF, Steegers-Theunissen RP, Eskes TK (1993) Hyperhomocysteinemia: a risk factor in women with unexplained recurrent early pregnancy loss. Fertil Steril 60:820–825
Bjørke-Monsen AL, Varsi K, Sakkestad ST, Ulvik A, Ueland PM (2023) Assessment of vitamin B6 status in never-pregnant, pregnant and postpartum women and their infants. Eur J Nutr 62:867–878. https://doi.org/10.1007/s00394-022-03033-4
Acknowledgements
The authors wish to thank Ms. Sheryl Thomas-Nikpoor, Language Editor, Springer Publications, for her valuable comments in editing this manuscript.
Funding
The study was funded by the Vice Chancellor of Research Affairs, Shiraz University of Medical Sciences. This manuscript is extracted from the Ph.D. thesis of Maryam Mirani (Grant number: 23078).
Author information
Authors and Affiliations
Contributions
SMT and MHN participated in the study conception and design, data interpretation, and manuscript revision. MM, FM, ZD, and MD contributed to performing experimental methods and data acquiring and analyzing. MM and FM wrote the manuscript. SB advised some experimental protocols and was involved in the critical revision of the manuscript. All authors reviewed and approved the final manuscript for submission.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local Medical Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1400.458) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mirani, M., Bahmanpour, S., Masjedi, F. et al. Pyridoxamine protects human granulosa cells against advanced glycation end-products-induced steroidogenesis disturbances. Mol Biol Rep 50, 8537–8549 (2023). https://doi.org/10.1007/s11033-023-08723-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08723-8