Abstract
3D cell culture approaches are cell culture methods that provide good visualization of interactions between cells while preserving the natural growth pattern. In recent years, several studies have managed to implement magnetic levitation technology on 3D cell culture applications by either combining cells with magnetic nanoparticles (positive magnetophoresis) or applying a magnetic field directly to the cells in a high-intensity medium (negative magnetophoresis). The positive magnetophoresis technique consists of integrating magnetic nanoparticles into the cells, while the negative magnetophoresis technique consists of levitating the cells without labelling them with magnetic nanoparticles. Magnetic levitation methods can be used to manipulate 3D culture, provide more complex habitats and custom control, or display density data as a sensor.The present review aims to show the advantages, limitations, and promises of magnetic 3D cell culture, along with its application methods, tools, and capabilities as a density sensor. In this context, the promising magnetic levitation technique on 3D cell cultures could be fully utilized in further studies with precise control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, 2-dimensional (2D) cell culture approaches have been replaced with 3-dimensional (3D) cell culture methods in various research applications due to the fact that they provide more accurate data simply by offering a more realistic cell growth scenario. As an in vitro model, 3D cell culture allows researchers to conduct studies that are more biologically relevant than 2D cell cultures [1]. Studies using 3D cell culture have shown that changing the extracellular matrix (ECM) around cells from 2D to 3D may significantly affect cell survival, differentiation, proliferation, and mechano-responses [2]. One of those 3D cell culture models is implemented with the integration of magnetic levitation. Although magnetic 3D cell culture is a method that has been studied recently, it is a field that has seen rapid development. Magnetic levitation application to 3D cell cultures could simply be explained as levitating a cell culture during its incubation, which promotes cell-to-cell interactions. Some models not only being a fitting 3D cell culture environment but also provide accurate data on the density of incubating cells in real time, adding another layer of information on top of traditional 3D cell culture methods. Magnetic nanoparticle types that have seen use in the first research done [3] on magnetic levitation based 3D cell culture are still relevant to this day in the positive magnetophoresis method, yet lately emerging negative magnetophoresis methods also show promise of development along with successful results in many studies today. Besides being applicable for highly accurate drug testing studies, the magnetic levitation based 3D cell culture method also offers density-based separation of cells, which could indicate various differentiations in cells during incubation in real-time. Another important parameter for such methods tends to be the equipment and application costs, which often draw the line between a niche method and a widespread method. However, researchers have already made progress on the overall cost problem of the method by having managed to create a system that poses only as little as a 100$ expense and also does not require trained professionals to operate the system [4].
During the literature search, it has been noted that the vast majority of studies cover and study one of two techniques, namely positive magnetophoresis, which has its own advantages over its counterpart but also features its own disadvantages. This article includes fundamental knowledge of magnetic 3D cell culture, its current place in research, and its advantages over similar 3D cell culture methods, considering both positive and negative magnetophoresis methods. Afterward, state-of-the-art magnetic 3D cell culture application methods were explained and compared. It is essential to completely understand both of these methods for conducting successful research on this topic. Therefore, the aim of this article is to present information in order to help many other researchers in their studies, draw attention to this still-growing topic to be improved, and contribute to the database. This review includes English-language articles generally published in 2010–2022, since 2010 was the year when the issue of the implementation of magnetic levitation on 3D cell cultures was first raised. Although the topic is relatively niche, the list of publications was limited to high-quality peer-reviewed journals as much as possible, especially for information that is directly related to the topic. Scopus and ScienceDirect databases were used during this research, which roughly presented 75 articles on the topic. The average citation of these articles, excluding non-academic materials, is 348.
3D cell culture
Cell culture techniques have long been used in biotechnology research for clinical tests in order to aid the development of new diagnostic tools, the discovery of drugs, disease investigation, tissue engineering, and regenerative medicine [5,6,7,8]. The use of cell culture techniques gives a chance to avoid the significant, costly, and ethical difficulties connected with the use of methods on animals in studies [9]. Because of their low cost, simplicity, and repeatability, two dimensional (2D) cell culture models are still commonly used in most experiments [10, 11]. Normal drug development begins with evaluating the medicine in a 2D cell culture technique, proceeding with animal testing, and finally performing the clinical trials phase. Unfortunately, around 90% of novel medications do not prove successful in clinical trials [12], owing mostly to the inability of existing 2D preclinical assays to accurately simulate the diverse and unique human cell structure and, as a result of estimating human biological reaction to molecules [9, 13]. In the 2D cell culture method, cell shape tends to be flat and elongated due to their growing ability only on a two-dimensional surface, whereas in 3D cell culture, natural cell shape will be preserved during growth, which enables cell-to-cell interactions [14,15,16]. Considering the human body is a tremendously complicated organism with many cells, both differing in type and function, interacting with one another to share and propagate the vital information [17], 3D cell culture gives a much better representation of the human body. 3D cell culture has several uses, including cancer and stem cell research, drug development, and cell disease studies [6,7,8].
The first known official 3D cell culture model idea dates back to the 20th century. George Gey originally described the formation of cell clusters in all three dimensions rather than the traditional monolayer formation in the 1950s. Ehrmann and Gey state in a study that they cultured distinct human cell lineages using collagen obtained from the tails of rats that are used as substrate and produced cell agglomerates without requiring a scaffold [18]. Soon after, three-dimensional tissues produced using in vitro methods from human progenitor cells were created by James Rheinwald and Howard Green [19]. In the 1990s, skin organoids from minimum numbers of primary cells from human donors were developed, and the organoids were utilized to heal third-degree burns. Additionally, 3D cornea cultures were used to cure blindness in over 100 people utilizing similar methods around that time [20,21,22].
3D cell culture methods offer a variety of methods that could be summed up as scaffold-based and non-scaffold-based techniques. Hydrogel, polymeric material support, hydrophilic glass fiber, and organoids are examples of scaffold-based approaches. Because of their capacity to replicate the extracellular matrix, hydrogels show unique properties [23, 24]. Hydrogels also facilitate the delivery of soluble substances from the environment to the cells that could be vital for either the cells or the purposes of the study [25]. Hydrogels are very flexible, as they may be utilized to make spheroid cells and could be made in a variety of forms required by the experiment. Due to the scaffold’s capacity to mimic the ECM’s structure, polymeric hard scaffolds are vital tools in the research of cell-to-ECM interactions [26]. According to research, HepG2 liver cells grown in 3D polymeric hard scaffolds had a higher viability and were less susceptible to cytotoxic agents than those produced in 2D [27]. For testing antibodies, detecting cell invasion, and modeling 3D malignancies, hydrophilic glass fibers are considered promising scaffolds since they effectively encourage cell-cell contact and promote the development of 3D cell networks [6]. One such example of drug testing could be given as the study conducted by Lang et al. [28], in which researchers simultaneously administered the dual drug-loaded nanoparticles (Src inhibitor saracatinib (AZD0530) and AKT inhibitor capivasertib (AZD5363)) into the same population of tumor cells. Organoids imitate the microenvironment of certain organs and aggregate into spheroids by generating ECM fibers that link single cells via integrin binding, allowing researchers to study human illnesses using patient-derived pluripotent stem cells [29]. It is possible for researchers to be able to grow tumor models using organoids based on patient-derived tissue cancer cells. This allows scientists to model the patient’s tumor in order to test treatments on a patient-to-patient basis. Furthermore, organoids have shown signs that one day they may be able to aid in an alternative organ transplantation method [6].
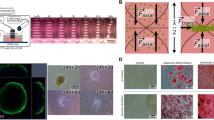
Scaffold-free methods, such as hanging drop microplates, spheroid microplates with ultra-low attachment coating, and magnetic force-based cell patterning, are exceptional in that they allow for unrestricted growth. Hanging drop plates enable the production of spheroid shapes by self-aggregation via gravity [6, 23]. The spheroids are suspended in open bottomless wells that are frequently confined in the plate’s bottom to adjust the ambient humidity of the cells [23, 30]. Because of their vast volume, spheroid microplates with an ultra-low attachment coating are often utilized to research tumor cells as well as establish multicellular cultures. It has also been proven that multicellular spheres produced from two non-small cell lung cancer (NSCLC) cells have substantially distinct development properties than 2D cell cultures [1, 31]. 3D incubated cells demonstrate higher resistance against drugs compared to 2D cells [32]. General models that utilize magnetic force-based cell patterning are bioprinting, ring formation, and levitation (Fig. 1) [9]. In the bioprinting model (Fig. 1b), magnets are placed under the 3D cell culture medium, which increases cell aggregation and matrix formation at the bottom of the well plate. This technique was investigated for secretory epithelial organoids that were created using human dental pulp stem cells (hDPSCs) to assess epithelial regeneration capability following transplantation in ex vivo models [33]. Furthermore, the utilization of human fetal osteoblast cells to bioprint magnetic 3D spheroids in order to study normal bone physiology as well as bone tissue creation and regeneration [34] could be given as an example on heterogeneous 3D cell culture studies concerning nanoparticle-assisted bioprinting. The ring formation model (Fig. 1c) is the most recent approach [9], which is applied similarly to bioprinting by placing a magnet under the 3D cell culture medium but differs in magnet shape. Magnets used for ring formation models are ring-shaped themselves, which alters the shape of the magnetic force they provide and promotes the aggregation of cells in a toroidal shape [9]. In the magnetic levitation model (Fig. 1d), cell levitation and spheroid formation are achieved by placing a magnet above the 3D cell culture medium, which levitates cells that are labeled with magnetic nanoparticles. This method have seen several uses such as magnetic levitation-assisted formation of hDPDC spheroids to test the therapeutic efficacy of these cells in regenerative medicine [35]. In any magnetic nanoparticle-assisted 3D cell culture methods, magnetic nanoparticle-labelled cells tend to form tighter clusters [4], which makes them less likely to be separated by density difference.
Magnetic nanoparticle-based cell patterning techniques, a. Incorporation of magnetic nanoparticles (grey) into cells (yellow) b. Bioprinting c. Ring formation d. Levitation (Imagery modified from open access source [9])
Without utilizing magnetic nanoparticles, the levitation model might also be accomplished by applying a magnetic field to the cells directly in a high-density medium, which causes cells to form spheroid formation by magnetic force produced by magnets [36]. Integration of the magnetic nanoparticles results in a dense cell environment which generates an extracellular matrix. Furthermore, the external magnet may be utilized to alter the 3D culture, enabling more complicated habitats and specific control [37] or to display density data as a sensor [4, 36, 38, 39]. Overall, magnetic levitation can recreate both simple and complex surroundings, rendering it a very versatile approach [6] while providing rapid spheroid development on cells [40,41,42].
Magnetic levitation in 3D cell culture
Integrating magnetic levitation and 3D cell culture studies was started in 2010 by Souza et al. in order to make an addition to 3D cell culture application methods by developing a 3D cell culture in a magnetic environment using hydrogels that contain bacteriophage and magnetic iron oxide nanoparticles [3]. In this study, cells were treated with hydrogels containing magnetic iron oxide and incubated overnight after achieving 80% confluence. The cells were then treated with trypsin and placed on attached plates. Afterward, a magnet was placed on the plate, making cells settle into a spheroid configuration within a few hours as a result of air-liquid exchange toward magnetic levitation; hence, the first implementation of magnetic levitation on 3D cell cultures was achieved [3, 43]. Similarly, a combination of the magnetic levitation technology and scaffolds was studied by other researchers [9, 44], yet the magnetic levitation technique is not limited to only scaffold usage, this technique could be used for vehicle suspension, smart phones, fluorescent imaging, etc. [45, 46].
Levitation-based 3D cell cultures offer many advantages, such as flexibility on medium choice, applicability on 2D cell culture models, applicability on a variety of cell types, overcoming the limitation of traditionally used 96-well plates, requiring less time to form cell spheroids, applicability on scaffold-free studies, and allowing extracellular matrix formation during cell culture configuration [6, 47]. Magnetic levitation techniques on 3D cell cultures can be examined in two groups as positive magnetophoresis and negative magnetophoresis [48, 49]. Several examples of studies on magnetic levitation-based 3D cell culture applications are mentioned in Table 1.
Positive magnetophoresis
The positive magnetophoresis technique utilizes magnetic nanoparticles in order to achieve magnetic levitation on designed systems, which is done by integrating magnetic nanoparticles in cells (Fig. 2a). The technique is described by several authors as incorporating magnetic nanoparticles with cell membranes [50], then levitating the cells during the procedure with the help of magnetic fields to support cell-to-cell aggregation and spheroid formation [5, 23]. The positive magnetophoresis technique on 3D cell cultures allows cells to form homogenous structures with desired levitation [5, 35]. It is also highlighted that positive magnetophoresis is difficult since the labeling procedure using magnetic nanoparticles is labor-intensive, time-consuming, and subject to experimental variability due to differences in cell labeling efficiency [49].
In positive magnetophoresis applications, typically two magnetic nanoparticle types have been developed on a commercial state and are commonly used, which are iron oxide nanoparticles [51, 52], and a combination of gold and iron oxide nanoparticles [33, 53]. In a study conducted by Caleffi et al. in 2021 it was stated that gold nanoparticles were allowed by the United States Food and Drug Administration (FDA). Additionally, Caleffi et al. [5] demonstrated that iron oxide nanoparticles have no negative effects on mammalian cells. Some other studies have utilized different nanoparticles that are arguably in experimental states, such as glycopolymer-coated gold nanoparticles as a pH-responsive drug carrier system [54], carbon-coated cobalt nanoparticles in order to incubate breast and colorectal cancer cells [55], and iron (II,III) oxide (Fe3O4) magnetic nanoparticles to achieve magnetic levitation [50, 56]. In these studies, it is stated that iron (II, III) oxide and carbon-coated cobalt nanoparticles did not cause any cytotoxicity [50, 55], whereas drug carrier glycopolymer-coated gold nanoparticles caused more toxicity in cancer cells compared to healthy cells, and the reason is the linkage to the drug used rather than nanoparticles [54].
Negative magnetophoresis
The negative magnetophoresis technique in 3D cell culture consists of levitating cells without labeling them with magnetic nanoparticles [57]. Biochemical and genetic variables, such as differential gene expression and energy consumption, can cause density disparities within cell populations [58, 59], which is simply how single cell separation is achieved by negative magnetophoresis (Fig. 2b). While being exposed to a magnetic field, non-labeled cells tend to move to the center, where the magnetic field is least effective [60], then cells become stationary at the place where the equilibrium between magnetic and buoyancy occurs [61]. The equilibrium point of each type of cell differs due to their density, which creates a unique height profile for each cell in the capillary column. In the system, cells with more density tend to be closer to the bottom magnet, which is the reason for separation, and the difference in height between cells allows for the detection of the density of the cells [4, 36, 38, 62]. Traditional non-label magnetic levitation systems (Fig. 3a) include a capillary tube, two magnets placed on its vertical sides facing each other, and two 45° tilted mirrors that allow observation of the capillary tunnel [4, 38, 62].
a Simple description of the frontal view of the traditional magnetic levitation tool used in the negative magnetophoresis method (Black arrows representing pathway of microscope sight and light) b Simplified illustration of upward magnetic waves (blue dotted lines) effecting the culture in ring magnet levitation setup
One example could be a study done by Anil-Inevi et al. [49] by creating a ring magnet levitation system in the presence of paramagnetic gadolinium (Gd3+) agents for biofabrication of MDA-MB-231 (breast cancer cell line) and D1 ORL UVA (bone marrow stem cell line) cells by manipulating living cells on an axial-circular angle (Fig. 3b). During their research, Anil-Inevi et al. observed that changes in cell density over time lead to changes in the altitude of cell cultures in magnetic levitation systems. Possible sensory applications could be given as the detecting density of blood cells, which could potentially indicate illness [63], or drug treatments that would cause a change in the density of yeast or bacteria [61]. In addition to the non-scaffold approach, Gd3+ is an often used material in a negative magnetophoresis cell culture medium to create a magnetic susceptibility difference [46, 49, 62]. Gd3+ could possibly be accompanied by Ficoll in order to increase media density, which makes magnetic levitation achievable for specific cell cultures [49]. However, Gd3+ has also been noted to cause toxicity in operations where cells metabolize calcium (Ca2+) by being a competitive inhibitor, which researchers overcame by adding a gadobutrol agent with Gd3+ to attain high kinetic stability while still being able to use its features [49].
Several studies have been conducted on negative magnetophoresis, some of which even contributed to the traditional non-label magnetic 3D cell culture technique. Traditional system requires costly optical microscopes [61] and trained personnel to operate them. It should also be noted that different brands or self-built systems will inevitably have minor differences in mirror placement and capillary tube size. Thus, having varying distances between the object and microscope makes the procedure harder to standardize [4]. Some studies have offered alternative magnetic levitation-based devices that are portable, low-cost, and allow measurements with mobile phone cameras. Although the brand difference was still an issue, without suitable lighting and extra optical setup giving an efficient magnification scheme, these investigations could hardly distinguish features the size of a white blood cell (20 μm) [4, 63, 64]. Alternatively, another compact design utilizing magnetic levitation was studied, one which had low-cost and was capable of making rapid analysis. However, the design required users to manually focus on microparticles, thus being incompatible with automation and again requiring trained personnel [65]. In an effort to eliminate shortcomings of previous magnetic levitation systems, Delikoyun et al., created a system that aimed to combine magnetic levitation technology and a lensless holographic microscopy imaging system that researchers named HologLev [4]. HologLev does not only eliminate the need for a trained professional to be able to use it but also is very cheap (100$ for the cost of all the parts, according to researchers), portable, and able to be used either with or without a fluorescence microscope to provide high-resolution images (< 2 μm). It is also possible to use the HologLev system with an embedded microcomputer that could make it cell phone attachable or integrate the HologLev system with fluid pumping mechanisms for high sample density analysis besides 3D cell culture experiments [4]. HologLev has been tested by researchers on 2D and 3D drug response experiments by incubating MDA-MB-231 (human breast cancer) cells, then adding varying concentrations of Docetaxel (a chemotherapy drug) to the cell medium. It has been noted that besides the system giving more accurate results in the 3D experiment, a comparison with traditional methods shows that the overall accuracy of the results was equal. During the experiment, the density of dying cells was increased by the loss of cell osmolarity, which made dead cells denser than others. That difference in density also caused a separation [4], thus proving HologLev to be an accurate real-time measurement device for dead cell count. The separation of live cells from dead cells is a critical step in cell-based toxicity tests for medicines, environmental pollutants, and other chemical substances [66]. Moreover, changes in cell density may reflect certain conditions in cells, like cell differentiation or disease [4].
Challenges of Magnetophoresis
Magnetic levitation is a recently developed method, which means it still has obstacles to overcome by researchers in order to fully utilize both positive and negative magnetophoresis. For sensory applications, negative magnetophoresis was the preferred method of many researchers, as the integration of magnetic nanoparticles would change the characteristic density of cells unequally. As a result, density measurement by levitation height is not possible with positive magnetophoresis at the current state. Another reason that made certain researchers focus on negative magnetophoresis was the fact that magnetic nanoparticles would not be removed from the cell structure, causing cytotoxicity and DNA damage [40, 67, 68]. However, Sarigil et al. [48] noted that using negative magnetophoresis caused problems in the adipogenic cell levitation study as cells attached to the surface of the capillary structure that provides the magnetic field, which is a problem that could be overcome by reducing Gd3+ concentration or forming a co-culture with other cells. Another possible problem that could occur in negative magnetophoresis was reported as weak cell-to-cell interactions that were present in adipocytes. Tightly packed cells merge better between cellular units, whereas loosely packed cells (e.g., cancer cells) merge slowly or not at all [49].On the other hand, in the positive magnetophoresis technique, the biocompatibility of magnetic nanoparticles is an essential parameter for nanoparticle integration in the 3D cell culture method. The material used should not develop toxic effects on cells, alter the proliferation or metabolism performance of the cells, promote pro-inflammatory responses, or create oxidative stress [5, 33, 42]. In one case, researchers reported that either cell morphology, viability, or proliferation were not affected by magnetic nanoparticles, and more than 90% of the cells were viable after 3 days of culture [33]. In another case, magnetic nanoparticles did not cause any intracellular oxidative stress or any inflammatory effects, so it was decided that the overall cytotoxicity of magnetic nanoparticles was negligible since cell metabolism was not significantly affected during both incubation and when exposed to a magnetic field [69, 70]. However, it should also be noted that different cells could show different types and levels of responses against magnetic nanoparticles; therefore, nanoparticles that are intended to be used could be tested on biocompatibility levels specific to the cell before starting an experiment. Malhotra et al. reported that commonly used iron oxide nanoparticles could show toxic effects such as inflammation, ulceration, decreases in growth rate, declines in viability, and triggering of neurobehavioral alterations in plants and cell lines (human leukemia monocytic (THP-1), HeLa, human lung carcinoma (A549), human embryonic kidney (HeK293), etc.) as well as in animal models (adult zebrafish, mice, chick embryos, etc.). However, it has been noted that gold coated iron oxide nanoparticles show lesser and ignorable levels of toxicity depending on cell types [71], which proves gold-coated nanoparticles are a step in the right direction. Also, the coating of nanoparticles with different materials such as chitosan, carbon, polyethylene glycol (PEG), polyvinyl alcohol (PVA), and silver significantly enhanced the biocompatibility of nanoparticles [71]. Both positive and negative magnetophoresis techniques pose different merits and demerits when compared with each other; therefore, their evaluation of viability differs from study to study. On the other hand, a study conducted by Meng et al., in 2011 for observing the effects of abnormal gravity conditions on human mesenchymal cells has shown that human mesenchymal cells cultured in a magnetic levitation environment even under µg conditions have shown apoptosis in 56.95% of cells [72]. This study emphasizes that not every cell is applicable to a magnetic 3D cell culture system, yet many other studies done in the following years, utilizing magnetic 3D cell culture systems, only prove that the capabilities of the systems require more research to be fully discovered.
Conclusion
The magnetic 3D cell culture method is a recently developed method that offers the incubation of cells in a 3 dimensional environment by utilizing external magnetic forces to achieve cell levitation. Incubation of cells in this manner leads more accurate results on testing drugs and treatments since it allows the cell culture to represent an organism more accurately. Studies that have been conducted on magnetic 3D cell culture so far not only demonstrate the availability of this method but also bring out key factors to look out for in future studies. It has been noted that compared to positive magnetophoresis, the number of negative magnetophoresis based research was significantly limited, possibly due to the negative magnetophoresis being a more recent method. Even as branches of the same method, positive and negative magnetophoresis present crucial differences that allow them to cover a widespread area of applications together. In the case of positive magnetophoresis studies, the possible effects of the chosen magnetic nanoparticles on the cells that are involved in the study should be known in order to identify possible errors. At this point, coating materials seem to be a promising approach to enhance the biocompatibility of nanoparticles. Likewise, the possible difference in density between cells in a negative magnetophoresis based 3D cell culture should be known since it could lead to looser cell formation, which would affect cell-to-cell interactions and provide inaccuracy in results. Additionally, magnetic 3D cell culture shows promise even outside of tissue engineering. To our knowledge, there is no negative magnetophoresis based research aiming solely to isolate a species of microorganisms from a co-culture formed in their natural habitat, which could potentially open up new topics of research. In any case, further research on the topic would not only improve the already existing method but also provide material and application alternatives, possibly making the magnetic levitation method cheaper and easier to use, to turn 3D cell culture and any other possible applications into widespread methods.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Ravi M, Paramesh V, Kaviya SR, Anuradha E, Paul FD, Solomon (2015) J Cell Physiol 230:16
Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, Chen Z (2017) Physiology 32:266
Souza GR, Molina JR, Raphael RM, Ozawa MG, Daniel J, Levin CS, Bronk LF, Ananta JS, Mandelin J, Georgescu M, Bankson JA, Gelovani JG, Killian TC, Arap W (2010) HSS Public Access 5:291
Delikoyun K, Yaman S, Yilmaz E, Sarigil O, Anil-Inevi M, Telli K, Yalcin-Ozuysal O, Ozcivici E, Tekin HC (2021) ACS Sens 6:2191
Caleffi JT, Aal MCE, de Gallindo H, Caxali GH, Crulhas BP, Ribeiro AO, Souza GR, Delella FK (2021) Life Sci 286:1
Jensen C, Teng Y (2020) Front Mol Biosci 7:1
de Antonino D, Soares MM, de Júnior J, de Alvarenga PB, de Mohallem R, Rocha CD, Vieira LA, de Souza AG, Beletti ME, Alves BG, Jacomini JO, Goulart LR, Alves KA (2019) Reprod Biomed Online 38:300
Costa EC, Moreira AF, de Melo-Diogo D, Gaspar VM, Carvalho MP, Correia IJ (2016) Biotechnol Adv 34:1427
Marques IA, Fernandes C, Tavares NT, Salom A (2022) Int J Mol Sci 23:1
Nunes AS, Barros AS, Costa EC, Moreira AF, Correia IJ (2019) Biotechnol Bioeng 116:206
Brancato V, Oliveira JM, Correlo VM, Reis RL, Kundu SC (2020) Biomaterials 232:119744
Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Assay Drug Dev Technol 12:207
Lv D, Hu Z, Lu LIN, Lu H, Xu X (2017) Oncol Lett 14:6999
Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, Unterleuthner D, Unger C, Kramer N, Hengstschlager M, Kenner L, Pfeiffer D, Krupitza G, Dolznig H (2017) Co Biol 130:203
Lee KH, Kim TH (2021) Biosensors 11,
Fey SJ, Wrzesinski K (2013) in Valproic Acid Pharmacol Mech Action Clin Implic pp. 141–165
Gupta N, Liu JR, Patel B, Solomon DE, Vaidya B, Gupta V (2016) Bioeng Transl Med 1:63
Ehrmann RL, Gey GO (1956) J Natl Cancer Inst 16:1375
Rheinwatd JG, Green H (1975) Cell 6:331
Lindberg K, Brown ME, Chaves HV, Kenyon KR, Rheinwald JG (1993) Invest Ophthalmol Vis Sci 34:2672
Pellegrini G, Traverso CE, Franzi AT, Mario Z, Cancedda R (1997) and M. De Luca, Lancet 349, 990
Sakalem ME, De Sibio MT, da Costa FA, de Oliveira M (2021) Biotechnol. J. 16,
Langhans SA (2018) Front Pharmacol 9:1
Antoni D, Burckel H, Josset E, Noel G (2015) Int J Mol Sci 16:5517
van Duinen V, Trietsch SJ, Joore J, Vulto P, Hankemeier T (2015) Curr Opin Biotechnol 35:118
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS (2011) Int. J. Polym. Sci. (2011)
Lei KF, Wu MH, Hsu CW, Chen YD (2014) Biosens Bioelectron 51:16
Lang L, Shay C, Zhao X, Xiong Y, Wang X, Teng Y (2019) J Hematol Oncol 12:1
Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O (2016) Cell Stem Cell 18:25
Zuppinger C (2019) Front Cardiovasc Med 6:1
Godugu C, Patel AR, Desai U, Andey T, Sams A, Singh M (2013) PLoS ONE 8,
Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S, Nakatsura T, Minami H (2015) Oncol Rep 33:1837
Adine C, Ng KK, Rungarunlert S, Souza GR, Ferreira JN (2018) Biomaterials 180:52
Gaitán-Salvatella I, López-Villegas EO, González-Alva P, Susate-Olmos F, Álvarez-Pérez MA (2021) Front Mol Biosci 8:1
Chan YH, Lee YC, Hung CY, Yang PJ, Lai PC, Feng SW (2021) Stem Cell Rev Reports 17:1810
Sarigil O, Anil-Inevi M, Yilmaz E, Mese G, Tekin HC, Ozcivici E (2019) Analyst 144:2942
Haisler WL, Timm DM, Gage JA, Tseng H, Killian TC, Souza GR (2013) Nat Protoc 8:1940
Baday M, Ercal O, Sahan AZ, Sahan A, Ercal B, Inan H, Demirci U (2019) Adv Healthc Mater 8:1
Turker E, Yildiz ÜH, Arslan Yildiz A (2019) Int J Biol Macromol 139:1054
Khawar IA, Ghosh T, Park JK, Kuh HJ (2021) J Pharm Investig 51:541
Whatley BR, Li X, Zhang N, Wen X (2014) J Biomed Mater Res - Part A 102:1537
Tseng H, Gage JA, Shen T, Haisler WL, Neeley SK, Shiao S, Chen J, Desai PK, Liao A, Hebel C, Raphael RM, Becker JL, Souza GR (2015) Sci Rep 5:1
Ayvaz I, Sunay D, Sariyar E, Erdal E, Karagonlar ZF (2021) J Gastrointest Cancer 52:1294
Penland N, Choi E, Perla M, Park J, Kim DH (2019) Mater Today Proc 27:0
Jaganathan H, Gage J, Leonard F, Srinivasan S, Souza GR, Dave B, Godin B (2014) Sci Rep 4:1
Dabbagh SR, Alseed MM, Saadat M, Sitti M, Tasoglu S (2022) Adv NanoBiomed Res 2:2100103
Talukdar S, Kundu SC (2012) Adv Funct Mater 22:4778
Sarigil O, Anil-Inevi M, Firatligil-Yildirir B, Unal YC, Yalcin-Ozuysal O, Mese G, Tekin HC, Ozcivici E (2021) Biotechnol Bioeng 118:1127
Anil-Inevi M, Delikoyun K, Mese G, Tekin HC, Ozcivici E (2021) Biotechnol Bioeng 118:4771
Bonfim L, de Queiroz Souza Passos P, de Oliveira K, Gonçalves LC, Courrol FR, de Oliveira, Silva, Vieira DP (2019) Appl Nanosci 9:1707
Beola L, Asín L, Fratila RM, Herrero V, De La Fuente JM, Grazú V, Gutiérrez L (2018) ACS Appl Mater Interfaces 10:44301
Labusca L, Herea DD, Minuti AE, Stavila C, Danceanu C, Grigoras M, Ababei G, Chiriac H, Lupu N (2021) J Biomed Mater Res - Part B Appl Biomater 109:630
Ferreira JN, Hasan R, Urkasemsin G, Ng KK, Adine C, Muthumariappan S, Souza GR (2019) J Tissue Eng Regen Med 13:495
Yilmaz G, Guler E, Geyik C, Demir B, Ozkan M, Odaci Demirkol D, Ozcelik S, Timur S (2018) and C. Remzi Becer, Mol. Syst. Des. Eng. 3, 150
Bumpers HL, Janagama DG, Manne U, Basson MD, Katkoori V (2015) J Surg Res 194:319
Jeong YG, Lee JS, Shim JK, Hur W (2016) Cytotechnology 68:2323
Sarabi MR, Yetisen AK, Tasoglu S (2022) Trends Biotechnol 40:915
Bryan AK, Hecht VC, Shen W, Payer K, Grover WH, Manalis SR (2014) Lab Chip 14:569
Grover WH, Bryan AK, Diez-Silva M, Suresh S, Higgins JM, Manalis SR (2011) Proc. Natl. Acad. Sci. U. S. A. 108, 10992
Ge S, Nemiroski A, Mirica KA, Mace CR, Hennek JW, Kumar AA, Whitesides GM (2020) Angew Chemie - Int Ed 59:17810
Durmus NG, Tekin HC, Guven S, Sridhar K, Yildiz AA, Calibasi G, Ghiran I, Davis RW, Steinmetz LM, Demirci U (2015) Proc. Natl. Acad. Sci. U. S. A. 112, E3661
Turker E, Demirçak N, Arslan-Yildiz A (2018) Biomater Sci 6:1745
Knowlton S, Yu CH, Jain N, Ghiran IC (2015) PLoS ONE 13:1
Ozefe F, Arslan Yildiz A (2020) Analyst 145:5816
Sobieranski AC, Inci F, Tekin HC, Yuksekkaya M, Comunello E, Cobra D, Von Wangenheim A, Demirci U (2015) Light Sci Appl 4,
Fritzsche M, Mandenius CF (2010) Anal Bioanal Chem 398:181
Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K (2018) Sci Rep 8:1
Abakumov MA, Semkina AS, Skorikov AS, Vishnevskiy DA, Ivanova AV, Mironova E, Davydova GA, Majouga AG, Chekhonin VP (2018) J Biochem Mol Toxicol 32:1
Ali EA, Bordacahar B, Mestas JL, Batteux F, Lafon C, Camus M, Prat F (2018) PLoS ONE 13:1
Chung J, Sriram G, Keefer CL (2020) Biotechnol Lett 42:2083
Malhotra N, Lee JS, Liman RAD, Ruallo JMS, Villaflore OB, Ger TR (2020) and C Der Hsiao Molecules 25:1
Meng R, Xu HY, Di SM, Shi DY, Qian AR, Wang JF, Shang P (2011) Acta Biochim Biophys Sin (Shanghai) 43:133
Kotze LA, Beltran CGG, Lang D, Loxton AG, Cooper S, Meiring M, Koegelenberg CFN, Allwood BW, Malherbe ST, Hiemstra AM, Glanzmann B, Kinnear C, Walzl G (2021) and N. du Plessis, MSphere 6, 1
Urbanczyk M, Zbinden A, Layland SL, Duffy G, Schenke-Layland K (2020) Tissue Eng - Part A 26:387
Perez JE, Nagle I, Wilhelm C (2021) Biofabrication 13,
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. U.T.: Conceptualization, Writing – original draft, B.A.G.: Conceptualization, Writing – original draft; E.I.: Conceptualization, Supervision, Writing ─ review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing financial interest.
Ethics approval
No ethical approval is required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tepe, U., Aslanbay Guler, B. & Imamoglu, E. Applications and sensory utilizations of magnetic levitation in 3D cell culture for tissue Engineering. Mol Biol Rep 50, 7017–7025 (2023). https://doi.org/10.1007/s11033-023-08585-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08585-0