Abstract
Cancer is one of the leading causes of mortality worldwide; nearly 10 million people died from it in 2020. The high mortality rate results from the lack of effective screening approaches where early detection cannot be achieved, reducing the chance of early intervention to prevent cancer development. Non-invasive and deep-tissue imaging is useful in cancer diagnosis, contributing to a visual presentation of anatomy and physiology in a rapid and safe manner. Its sensitivity and specificity can be enhanced with the application of targeting ligands with the conjugation of imaging probes. Phage display is a powerful technology to identify antibody- or peptide-based ligands with effective binding specificity against their target receptor. Tumour-targeting peptides exhibit promising results in molecular imaging, but the application is limited to animals only. Modern nanotechnology facilitates the combination of peptides with various nanoparticles due to their superior characteristics, rendering novel strategies in designing more potent imaging probes for cancer diagnosis and targeting therapy. In the end, a myriad of peptide candidates that aimed for different cancers diagnosis and imaging in various forms of research were reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is one of the leading causes of mortality in every country of the world [1]. According to World Health Organization, in 2020, approximately 10 million deaths were reported, and this number is rapidly increasing worldwide at an alarming rate [2]. The growing burden of cancer incidence and mortality is normally associated with a lack of sensitive and accurate detection for early diagnosis, leading to the progressive advancement in cancer stage and metastasis, eventually delay in treatment [3]. Cancers are normally detected using conventional invasive (endoscopy and biopsy) and non-invasive imaging techniques (X-ray-based systems, magnetic resonance imaging [MRI], nuclear imaging and ultrasound systems) [4]. However, those non-targeted diagnosis approaches are not sensitive enough to detect tumours in their early development, making physicians unaware of carrying out further investigational steps. Thus, there is an urgent need to develop an optimized and targeted diagnosis method in the oncology field to provide insight into pharmacological responses to therapeutic interventions, arresting cancer development in the early stage.
The widespread use of molecular imaging has contributed to the early detection and effective treatment of cancer where this technique is able to highlight the distinctive features possessed by malignant cells. Such action requires tumour-specific molecules with high affinity to serve as effective targeting vehicles to deliver imaging probes and therapeutic agents to the tumour sites while sparing normal tissues. For this purpose, various tumour-targeting agents have been developed, including antibodies, antibody fragments, peptides, aptamers and small molecules which greatly improve the efficiency of tumour-imaging approaches. Typically, the antibody was the first choice to be employed as molecular imaging agent. Antibodies and their fragments can be engineered to specifically target distinct antigens and cell surface epitopes with high selectivity. FDA approved the first radiolabelled antibody for prostate cancer imaging in 1993, proving the clinical potential of antibodies in cancer diagnostic applications [5]. However, antibodies still exhibit several unfavourable properties which limit their application, including difficulty in infiltrating the whole tumour mass and conjugating to nanoparticles due to their large size, higher batch-to-batch variation, hepatotoxicity due to nonspecific binding to the reticuloendothelial system as well as high production cost [1, 6]. Therefore, smaller-sized ligands or fragments such as peptides become an alternative selective delivery vehicle for diagnostic probes. Peptide-based probes have several critical advantages over antibodies: smaller in size, low immunogenicity, potent in tissue penetration, easy to be synthesized and readily conjugated to chemicals and fluorescent dyes [7,8,9]. For instance, a novel bombesin (BBN) peptide analogue named GB-6 peptide has been used in colorectal cancer imaging, targeting gastrin-releasing peptide receptor (GRPR) that is overexpressed on colorectal cancer cells and showing its potential in early detection of colorectal cancer [10]. Peptide-based targeting ligands can be discovered through several methods, including derivatization from natural ligands or their analogues and rational engineering from the structure of targeted receptors [11]. The most common and easiest way is via screening of phage-display peptide library, known as biopanning [12].
Phage display is a powerful technology that allows the presentation of a large amount of peptide and protein libraries. This technology was first introduced by George Smith in 1985. Numerous peptides, proteins and antibodies were isolated and successfully applied widely in the biomedical field, such as vaccine development, drug discovery, oncology research and epitope mapping [6, 13, 14]. It involves the fusion of the gene encoding the interested polypeptide with gene III or VIII and the displayed proteins are expressed by bacteria hosts in fusion with the coat protein of the phage vector. Therefore, a physical link between the phenotype and genotype of the displayed peptide can be observed [15]. Phage display libraries can be constructed containing up to 1010 different variants simultaneously, which effectively allows affinity screening of the combinatorial peptide library and selection (biopanning) to identify targeted binding-peptide [16]. Recent studies have proved the phage display system to serve as an efficient tool in the rapid identification of novel peptides against different target receptors through high-throughput screening, such as Tanzeum (glucagon-like peptide-1 receptor agonist) being clinically used to control glycemia in patients with type 2 diabetes mellitus [17], peptide ESCP9 targeted oesophageal squamous carcinoma cells with high specificity and were potent to be developed as an imaging detection probes [18] as well as peptide-based inhibitors against β-lactamase enzyme to overcome antibiotic resistance in bacteria [19].
This review will focus on and highlight the diagnostic applications of phage display-based peptides in various cancer types.
Biopanning: selection of phage displayed-based peptides
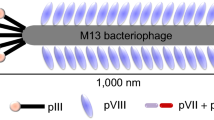
Phage display system is an alternative approach to producing peptide-based imaging molecules via the screening of the phage-display peptide library. The library is made up of billions of bacteriophage clones, with each clone inserted with a distinct foreign DNA, exhibiting a different peptide on the phage surface [20]. Filamentous M13 phage is a well-characterized platform and is commonly employed for phage display as its ability to replicate at higher capacity and accommodate the larger size of foreign DNA [1]. Typically, peptides are incorporated into the N-terminus of minor coat protein III (pIII) or major coat protein VIII (pVIII), which are responsible for peptide presentation. Both libraries are able to display at most five copies and up to a thousand copies of each individual peptide, respectively [20, 21]. The exposure of the inserted peptides on the phage surface is crucial for the subsequent screening process, facilitating the identification of displayed peptides that specifically bind to a given molecular target from a vast number of peptide-displayed clones.
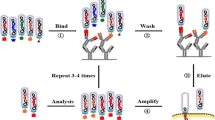
Affinity selection or biopanning is a process of selecting phage clones that selectively bind to the interested target. In general, affinity selection involves five main successive steps (Fig. 1). First and foremost, the preparation of a phage-display peptide library. Secondly, the desired target materials, including coated antigens or immobilized cell surface proteins, are incubated with the naïve library culture in microtiter plates. Next, the unbound or non-specific binders will be removed through the washing process where only the binders with strong affinity are kept. Forth, the bound phages are allowed to be recovered and amplified through bacterial infection after the elution process using acid or high salt solution. The last step is to repeat the aforementioned steps several times, normally three to four times in order to ensure the enrichment of a population of best binders. Sequencing of the individual phage clone genome that encodes the displayed peptide can be carried out to determine the ligands that bind specifically to the target receptors [22].
Peptides can be identified from the phage-display peptide library through several screening procedures, which are in situ, in vitro and in vivo selections. In situ affinity selection involves screening of a vast variety of receptors that immobilized on a solid surface simultaneously. However, this process is limited by the possibility of conformation alteration of partial denaturation of the target receptors when attached to the plastic surface [23]. In-solution biopanning is established as an alternative against solid-phase biopanning, where the library can be conjugated with the receptors in solution, followed by affinity bead capture which is specific to the target peptide. Based on Fouladi et al. study, magnetic Dynabead was used to capture peptide-bound phage scFv that was specific to Helicobacter pylori urease in the phage-peptide mixture. The specific binders were then separated from the non-bound phage using a magnetic particle concentrator called DynaMag-2 [24]. For the tumour-targeting peptide, in vitro whole-cell panning is commonly used where the peptide identification can be performed on a single cell line or on adherent cells [25]. For example, a CSP3 peptide was discovered via whole-cell subtraction biopanning in Li et al. study, exhibiting the strongest affinity and specificity to SiHa cells (cervical cancer cell line) [26]. It is useful to overcome the shortcomings of using solution-based panning when purified membrane proteins, which are poorly soluble in aqueous media are used as the target receptors. The cell-based panning helps to keep the membrane proteins in their native conformation, which retains their biological functions, proper folding and expression level. Prior knowledge of the cellular receptors is not necessary, making it become an ideal selection technique in tumour-targeting peptides identification [27].
In vivophage display involves affinity selection of phage library via biopanning in living animals, which is effective in isolating organ- or tissue-specific peptides. This powerful selection technique tends to simulate ideal living circumstances, improving the identification of specific ligands against challenging receptors in their native conformation [14]. In vivo screening offers advantages over conventional in vitro and in situ screening methods where the phage-displayed peptide candidates with desired features, including specificity, pharmacokinetics and stability can be selected in the complicated milieu of living animals [28]. Pasqualini and Ruoslahti were the first to demonstrate this technique in 1996, involving the identification of peptides homing to the vascular endothelium of renal and cerebral tissues in vivo [29]. The procedure of this approach is similar to in vitro phage display. Briefly, the peptide phage library is administered intravenously into the living animal. The phages are allowed to circulate for a few hours, promoting the binding of the displayed peptide to the specific targets that are expressed on the organ or tumour surface. Subsequently, the animals will be perfused and sacrificed to wash the unbound phages, followed by the collection of the interested organ to recover the bound phages [28]. Using this approach, various peptides such as CSSTRESAC [30], VAV3 [31], SP5-52 [32] and A54 [33] that target breast cancer cells, glioblastoma, lung tumour blood vessels and liver cancer cells, respectively, were identified in recent studies. One of the drawbacks of this selection system is that the peptide is unable to be translated into humans as different species are employed in the peptide binding [34].
Phage display library possesses several advantages, making this technology established as a preferred platform for isolating specific receptor-targeting ligands. Firstly, large-sized of a combinatorial library can be produced, facilitating rapid identification of target-binding ligands through the screening of a phage library displayed with millions of protein ligands at one time. Unlike the conventional screening technique, screening of the individual molecule which is labour-intensive can be avoided. Besides, it is easy to manipulate and cost-effective as compared to the hybridoma technology where animal cell culture is not being involved. The phage library can be established easily by infecting the bacterial host to amplify and enrich the phage clones that display the desired ligands. Another strength of this system is the ability to discover the interactive regions between the displayed ligands and the receptors without knowing the nature of the interaction.
Labelling peptides for molecular imaging of tumours
Molecular imaging is a type of non-invasive medical imaging that enables visualization, characterization and quantification of biological processes at the cellular and molecular level within living objects [35]. Great advances in the development of targeted imaging contrast agents are one of the critical driving forces of molecular imaging research, assisting in the understanding disease pathologies in the oncology field, especially tumour growth, angiogenesis and metastasis [36]. Initial efforts were made and focused on the radiolabelled small molecules, antibodies and antibody fragments [37]. However, those imaging probes possess unsatisfied results as small molecules exhibit low specificity while antibodies show low target permeability, high immunogenicity and low clearance ability [38]. Although peptide has low binding affinity compared to monoclonal antibodies, it meets certain requirements as a qualified tumour-imaging probe: low molecular weight which facilitate less immunogenicity, better intratumoral diffusion, rapid blood clearance and renal excretion ability, targeting specificity and well-adaptability to harsh conditions during chemical modifications and labelling [39,40,41]. One of the potentials of peptides over antibodies is the flexibility to manage and modify their chemical composition, where the combination with chemical linkers and conjugation with a myriad of imaging agents such as radioactives, fluorescences and nanoparticles can be achieved easily. Such advantage facilitates the development of peptide-based imaging probes, improving the efficiency of imaging tools for the early diagnosis of dangerous cancers.
A peptide-based tumour-imaging probe generally comprises a targeting peptide, a linker and an imaging moiety. Linker is responsible for the linkage between the peptide and moiety, whereas the moiety will assist in the visualization of the probe at the target tumour site in various imaging modalities [36]. To date, conventional tumour imaging techniques include X-ray, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET) and single photon emission computed tomography (SPECT) and ultrasound. Those imaging methods are limited in monitoring microscopic processes in real-time and such shortcomings can be compensated by an optical imaging system, which provides insights into biochemical processes at a cellular level and facilitates diagnosis or response to therapy [42].
Fluorescence-labelled peptide imaging probe
Fluorescence-labelled peptide imaging involves the labelling of peptides with fluorophores. Based on Cho et al. study, a disulfide-bridged cyclic RGD peptide- (iRGD) based monolithic imaging probe was designed for tumour-related angiogenic receptors (αvβ3 integrin and neuropilin-1) targeting and tissue penetration. A fluorescent dye and quencher were conjugated to the N- and C-terminal the of peptides respectively. Owing to the dye/quencher conjugation, the monolithic peptide probe targeted and visualized the tumour regions more specifically with a lower background noise signal, compared to the control probe without quencher conjugation, exhibiting its potential as a promising candidate in tumour imaging for precise diagnosis [43]. Another example of this imaging approach was the utilization of polyethylene glycol (PEG) linked anti-HER2/neu peptide (AHNP-PEG) to detect human epidermal growth factor receptor 2 (HER2) which was overexpressed in gastric cancer cell surface [44]. The peptide binding assays were determined and observed by flow cytometry and fluorescent microscopy in both gastric cancer cells, NCI-N87 (high-level HER2 expression) and MKN45 (low-level HER2 expression). Both assays showed similar results, where fluorescence of the fluorescein isothiocyanate (FITC) labelled peptides (FITC-AHNP-PEG) was significantly displayed and higher in NCI-N87 cells compared to MKN45 cells, showing higher binding affinity to NCI-N87 cells but not to MKN45 cells. This specificity of the peptides towards HER2 was confirmed and it might be potent to be applied for HER2 diagnostic imaging in gastric cancer patients.
Nanoparticle-based peptide imaging probe
In addition to the application of fluorescent molecules in tumour-targeting molecular imaging, advances in nanotechnology generate nanoparticle-based imaging probes that serve as novel candidates to diagnose cancer and detect biomolecules in an effective and safe approach, providing a new platform for peptides. The rapid emergence can be due to their several unique physical features:
-
1.
The nano-sized scale ranging from 1 to 100 nm promotes enhanced permeability and retention (EPR) effects which help to deliver high concentrations of contrast agent to the target tumour site [45].
-
2.
Large surface area to volume ratio allows for greater surface functionality and extent of modification with distinct targeting moieties, enhancing the binding affinity of individual probes [46].
-
3.
The ease of engineering by merging the nanomaterials into a pre-existing product to improve the overall performance, including reduced toxic effects and prolonged blood circulation [47, 48]. There are several well-investigated nanomaterials such as quantum dots (QDs) for optical imaging, superparamagnetic iron oxide nanoparticles for MRI, gold nanoparticles, polymer nanoparticles, liposomes, naposomes and carbon nanotubes.
Magnetic resonance imaging (MRI) is one of the most powerful diagnostic imaging tools that is well-established in clinical practice. Owing to the advances in molecular science, this approach possesses significant progress in the oncology field, responsible for different steps of oncologic management, ranging from detection, characterization, staging process, response evaluation towards therapy and post-therapeutic follow-up [49]. The widespread use can be due to its non-invasive way of producing three-dimensional images with high spatial resolution, facilitating precise tumour evaluation and there is no exposure risk to ionizing radiation [50]. However, limitations such as long postprocessing periods and relatively low sensitivity urge the development of novel molecular probes to optimize detection sensitivity and imaging contrast [36].
Iron oxide nanoparticles (IONPs) are Food and Drug Administration (FDA) clinically approved MRI imaging contrast agents [51]. Although they have been widely employed in the field of molecular imaging, the targeting specificity is affected by the non-specific uptake from the reticuloendothelial system (RES). Besides, owing to their small-sized, IONPs alone will be washed away by the blood circulation system easily, resulting in the inability to accumulate at the tumour site for a longer period and sufficient amount, reducing the imaging signal [52]. As such, targeting peptide conjugation will assist in IONPs tumour cell internalization through receptor-mediated endocytosis, enhancing the imaging contrast of MRI. Boucher et al. had successfully created a Arginine-glycine-aspartic acid (RGD) peptide that labelled with magnetosomes that are made up of bacterial biogenic iron oxide nanoparticles embedded within lipid vesicle to serve as MRI probes for the molecular imaging of glioblastoma [53]. The labelled RGD peptide exhibited specific binding towards its target, ανβ3 integrins that are overexpressed at the U87 tumour cells surface. This effective binding facilitated the internalization of magnetosomes into tumour cells, enhancing MRI contrast between the cancerous and healthy brain tissues. Apart from that, interleukin-6 receptor targeting peptides (I6P7) was covalently modified with superparamagnetic iron oxide (SPIO) nanoprobe, assisting in the T2-weighted MRI [54]. This I6P7 modified nanoprobe was shown to promote transcytosis across the blood-brain-barrier (BBB), bind to low-grade gliomas with higher affinity, low cytotoxicity and accumulate within the tumour region effectively in bEnd.3 endothelial cells and U87 glioma cells. Those investigations suggested the I6P7 peptide-mediated MRI imaging probe was potent to act as a novel strategy to circumvent the difficulty across BBB and diagnose low-grade gliomas. Another novel nanoprobe was developed for pancreatic tumours imaging, comprising spherical iron oxide nanoparticles which were conjugated with fluorescent dye and anchored with PTR86 peptide that targeted somatostatin receptor (SSTR) which were overexpressing on pancreatic tumours [55]. Significant accumulation of PTR86 magnetic nanoprobe in the tumour site was observed in in vivo investigation (Mouse bearing with human pancreatic tumour) compared to the control mouse (not injected), whereas ex vivo tumour tissues fluorescence imaging showed a similar result. The weak fluorescent signal was existed within normal tissue, while bright fluorescent spot was observed in tumours. Both studies proved the potential of this nanosystem in pancreatic tumour detection due to its efficient MR imaging and binding specificity.
Quantum dots (QDs), the fluorescent inorganic semiconductor nano-sized materials, are another commonly studied nanoparticle for preclinical optical imaging systems. Owing to the poor light stability possessed by organic fluorophores, QDs are the alternative option as they exhibit several unique and outstanding optical properties for imaging purposes. This includes the ability to withstand chemical degradation and photobleaching that allows fluorescence imaging for a longer period, high quantum yield and continuous absorption spectra with a high multi-photon absorption coefficient that helps to improve fluorescence imaging resolution [56,57,58]. However, one of the drawbacks is the poor retention within tumour due to their small size, limiting the ability of quantification and deep-tissue imaging [59]. In order to provide specific and reliable images within the target sites, the surface of QDs can be modified to conjugate with a biomolecule such as peptide for targeting imaging. Recently, cysteine-coated ZnCdS QDs were conjugated with RGD peptide and QDs-RGD imaging probe was used to target integrin αvβ3 receptor which is overexpressed on the pancreatic tumour cell surface [60]. Compared to the QDs without RGD peptide conjugation, QDs-RGD exhibited a higher fluorescent signal at the targeted tumour region, suggesting its role serves as a potential specific targeting probe for the fluorescence imaging of pancreatic tumours. In addition, Liu et al. developed a versatile imaging probe constituting luminescent cadmium-free CISe/ZnS quantum dots with the CGKRK tumour-targeting peptide conjugation. This bioimaging agent exhibits significant photostability and markedly tumour-specific homing features which can be seen in in vivo study involving a glioblastoma mouse model. Stronger fluorescent intensity was observed within tumour area, clearly denoting the tumour boundaries and labelling diffusely infiltrating tumour cells from the normal cells [61].
Discovery of phage-displayed peptides for targeting various Cancer types
Modern molecular biology prompts the development of phage display technology, resulting in a wide application for screening tumour affinity peptides which are useful in imaging purposes for early diagnosis and providing a path for therapeutic drug development. In this review, tumour-targeting peptides for distinct cancer diagnostic and imaging purposes generated from phage display library will only be discussed and reviewed.
Breast cancer
Breast cancer is one of the most frequently diagnosed cancers and the leading cause of high mortality in women worldwide. Accurate and early diagnosis of breast cancer becomes challenging as the characterization of different breast tumour subtypes is difficult, which seriously affects the prognosis and treatment management decisions. As such, research on new targets and markers against breast cancer should be prompted to promote breast cancer detection in the early stages. Based on da Fonseca Alves et al. study, recombinant peptides biotin-C3 and biotin-H2 were selected via the screening of phage display library towards MCF-7 cells and employed as biorecognition molecules in the construction of a biosensor. Both synthetic peptides were shown to be capable of differentiating the serum samples of breast cancer patients from benign breast disease patients successfully. Besides, they were also tested to categorize patients with Luminal A and B breast tumours based on the expression of p53 and HER2 respectively [62].
Apart from the breast cancer subtypes characterization, phage-displayed peptides also demonstrate their potential in homing of brain metastatic breast cancer tumour. Fu et al. successfully isolated a novel peptide named BRBP1 (MYPWTEPSYLSN) through in vitro phage display screening against 231-BR cells (human brain-seeking breast carcinoma cells). For the targeting potential in vivo evaluation, BRBP1 was injected into 231-BR tumour-bearing mice intravenously and the immunofluorescent staining results showed the preferentially homing of the BRBP1 peptide to the tumours while the uptake by normal tissues and organs was limited. The most interesting finding was the internalization ability of BRBP1 where this fluorescent-labelled 231-BR tumour-targeting peptide exhibited its distribution into the cytoplasm and nucleus during 2 h-post incubation. As such, apart from its potential in targeting brain metastatic breast cancer tumour specifically which is useful in early diagnosis, it is also potent to serve as anticancer drug carrier [63].
Another breast cancer-targeting peptide, CK3 (CLKADKAKC) was isolated by Feng et al. through the phage display technique, demonstrating its cryptic C-end rule motif-mediated binding specificity towards neuropilin-1 (NRP-1) receptor. NRP-1 receptor is a potential biomarker of breast cancer as it is considered as a multifunction protein that is responsible for angiogenesis and tumour invasion [64]. Hence, it can be served as an attractive diagnostic and therapeutic agent for NRP-1 overexpressed breast cancer. After the binding affinity of CK3 towards NRP-1 was confirmed, the bio-distribution investigation of CK3 was established through the injection of 99mTc-labeled CK3 into mice bearing MDA-MB-231 breast tumours. According to single-photon emission computed tomography (SPET) imaging and near-infrared fluorescence imaging (NIRF), the accumulation of labelled peptides in the tumours and in less extent in kidneys for excretion was observed, indicating its potential for clinical applications such as SPET and NIRF imaging of breast cancer and opening new pathways for breast cancer diagnosis and therapeutic response assessment [65].
Colorectal cancer
Colorectal cancer is one of the causes leading to high mortality rate in both sexes worldwide [66]. This can be due to advanced-stage diagnosis in most patients regularly, which is normally resulted from the lack of sensitivity of conventional colonoscopy diagnostic methods and the absence of early clinical targeting molecules [67, 68]. Thus, the identification of specific targeting probes is crucial for early diagnosis of colorectal cancer and the following treatment decision.
A cyclic peptide named TCP-1 (c[CTPSPFSHC]OH) was characterized by Liu et al. for its ability in targeting imaging of colon cancer. It was originally synthesized through screening of an orthotopic mouse model of colorectal cancer using phage display selection technology [69, 70]. The radiolabelled TCP-1 ((99 m)Tc-TCP-1) was subjected to in vitro binding assay in human HCT116 colon cancer cells, showing its binding selectivity for colorectal cancer cells. An evaluation was also made through intravenously injection of (99 m)Tc-TCP-1 into SCID mice with human HCT116 and PC3 prostate cancer xenografts, followed by SPECT imaging, which further confirmed the binding specificity of TCP-1 to colorectal cancer but not to other solid tumours. In addition, TCP-1 was revealed its accumulation and localization not only within metabolically active tumour regions but also tumour-associated vasculature, as defined by direct positron imaging of (18)F-fluorodeoxyglucose ((18)F-FDG) and fluorescence microscopy respectively in SCID mice with HCT116 xenografts in dorsal skinfold window chambers (DSWC) model. Hence, those findings suggested the potential of TCP-1 to be a promising imaging probe for malignant colorectal lesions detection [71].
Another novel peptide named RKOpep (CPKSNNGVC) was selected through in vitro biopanning with RKO cells (colorectal canprobe for malignant colorectal lesions decer cells) due to its highest binding ability. The binding specificity and selectivity of RKOpep towards RKO cells and other human colorectal cancer cells (HCT 116, Caco-2 and HCT-15) but not to normal non-cancerous cells (CCD-841-CoN) was further confirmed through fluorescence microscopy and cytometry analysis. A similar result was shown when RKOpep was tested with clinical human colorectal cancer tissues. Through the structural bioinformatics approach, the potential target of RKOpep named monocarboxylate transporter 1 (MCT1) was identified. Validation of the aforementioned RKOpep target was made via gene knockdown approaches on RKO cells as a significant reduction in the fluorescence signal of FAM-RKOpep was observed after MCT1 silencing. The binding selectivity of the peptide towards MCT1 was also proved through immunocytochemistry, showing the co-localization of the FAM-RKOpep and anti-MCT1 antibody. As such, those findings exhibited the potential of RKOpep as a valuable colorectal cancer diagnostic tool and novel treatment alternative [67].
From the study conducted by Hou et al., through the screening of phage-displayed peptide library with human colon cancer cell line (COLO320HSR) and a normal human intestinal epithelial cell line (NCM460), a novel peptide termed CBP-DWS (DWSSWVYRDPQT) were isolated. The binding affinity and specificity of CBP-DWS was verified through FITC-based immunofluorescence involving a panel of human colorectal cancer cell lines (COLO320HSR, NCM460, HCT116, HT29, SW480, and LoVo cells) and biopsy specimens. Markedly fluorescence signal was shown within the cancerous cells while sparing the normal colon tissues. Further study on this peptide was performed by conducting bioinformatics analysis to explore its molecular target. Glypican-3 was discovered as a potential target of CBP-DWS among various receptor targets in the databases and researchers have proved the overexpression of glypican-3 receptor in HCT116 and Lovo cell lines [72]. The binding specificity of CBP-DWS towards glypican-3 was proved through the competition inhibition between anti-glypican-3 antibody and excess amount of CBP-DWS peptide. Hence, based on the excellent binding selectivity of CBP-DWS, it may be developed as a novel imaging probe for the detection and targeted therapy of colorectal cancer [73].
Lung cancer
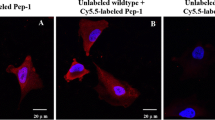
An in vivo phage-displayed peptide screening was performed through the injection of M13 phage-displayed 2.9 × 109 random peptides library into lung adenocarcinoma-bearing xenograft mouse model, discovering a new lung adenocarcinoma-specific peptide named Pep-1 (CAKATCPAC). A series of investigations were carried out to analyse its binding specificity and targeted imaging ability, including in vitro competitive assay to A549 lung cancer cell line, in vivo NIR fluorescence whole-body imaging of Cy5.5-labeled lung tumour-bearing mice, and immunohistochemistry application. Accumulation of Pep-1 at the tumour regions only as time passed and ability in targeting lung tumours specifically in mice bearing various tumour xenografts was observed, indicating its outstanding binding selectivity and the possibility to be developed as a diagnostic probe of non-invasive imaging system for human lung adenocarcinoma [74].
Large cell carcinoma (LCC) is categorized into the group of non-small cell lung carcinoma (NSCLC), sharing genetic similarity with adenocarcinoma and squamous cell carcinoma (SCC) [75]. Thus, it is difficult to differentiate LCC from the others as it does not express specific biomarkers and is not glandular or squamous [76]. Chi et al. identified three targeting peptides (HSP1, HSP2 and HSP4) that exhibit broad subtype specificity for both SCLC and NSCLC. Those peptides were selected through H460 LCC cells in vitro biopanning and showed binding specificity to SCLC, NSCLC cell lines and clinical specimens but not to normal pneumonic tissues. HSP1 was shown to exhibit the highest tumour-binding affinity and the longest tumour retention period among three peptides during the investigations of in vivo optical imaging and MR imaging with peptide-labelled supermagnetic iron oxide nanoparticles (SPIONs), implying its potential application in multimodal molecular imaging systems for lung cancer diagnosis. Besides, receptor-mediated endocytosis upon binding of peptide towards target cancer cells was revealed, where this action was tested for intracellular drug and imaging probe delivery for those three peptides and HSP4 had the highest efficiency as the delivery vehicle. Overall, the selected peptides were potent to serve as a promising agent for combinatorial drug delivery and non-invasive diagnostic imaging for both SCLC and NSCLC [77].
MT2-MMP is one of the subfamily members of the membrane-type matrix metalloproteinases (MT-MMPs) and is found to be overexpressed in various cancer types [78]. It mainly plays an important role in the extracellular matrix (ECM) degradation and pro-MMP2 activation, contributing to the invasion and metastasis of tumour cells through vasculogenesis [79, 80]. Besides, MT2-MMP was revealed as an antiapoptotic factor in human lung adenocarcinoma [81]. As such, it is an interesting biomarker to be explored for lung cancer diagnosis and targeted therapy. Ren et al. had successfully isolated a MT2-MMP targeting peptide termed MT2-AF5p (HHRLHSAPPPQA) through the screening of phage-displayed 12-mer peptide library, having a better binding affinity and selectivity towards H1299, human non-small cell lung carcinoma cell line. It was then conjugated with fluorescent mesoporous silica nanoparticles (FMSN-NH2) and intravenously injected into an H1299 tumour-bearing mice model. Through the ex vivo fluorescence imaging of fluorescent-labelled MT2-AF5p, the histological staining examination revealed its accumulation within tumour site with specificity, whereas no obvious histological changes were observed in other organs. Thus, MT2-AF5p is possible to be developed as a clinically used targeting probe for lung cancer diagnosis [82].
Prostate cancer
The development of prostate cancer can be owing to the elevated expression of oncofetal fibronectin (onfFN) extracellular matrix (ECM) protein such as extradomain-B fibronectin (EDB-FN) which contributes to angiogenesis, metastasis and generation of invasive mesenchymal cells found in high-grade prostate tumours [83, 84]. Hence, the development of novel imaging and diagnostic probes that target EDB-FN will be useful in high-risk prostate cancer detection. ZD2 (CTVRTSADC), an EDB-FN-specific cyclic nonapeptide was identified by Han et al. via a peptide-based phage display technique. Cy5-labeled ZD2 was synthesized to evaluate its binding specificity and imaging ability towards prostate cancer via fluorescence imaging. Effective binding of Cy5-ZD2 to overexpressed EDB-FN receptors that were presented on TGF-β-induced post EMT-PC3 cancer cells was observed. Besides, the accumulation of the Cy5-ZD2 probes within GFP-labelled PC3 tumour site compared to the absence of control CREAK-Cy5 fluorescent signal was also revealed in mice bearing PC3-GFP flank tumour xenograft models. ZD2 was also proved for its ability to differentiate Gleason score-based prostate cancer aggressiveness as stronger binding happened towards tumour specimens with a Gleason score of 9 (GS9) as compared to lower a Gleason score (GS7), with no binding capability in EDB-FN absence-benign prostatic hyperplasia (BPH). Overall, ZD2 was valuable to be explored as a useful targeting imaging biomolecule which is feasible to various imaging tools for effective non-invasive diagnosis of high-risk prostate cancers [85].
Prostate cancer patients with aggressive tumours have higher mortality compared to those with localized tumour owing to the lack of effective biomarkers to target and differentiate between the tumour types during the early phase. CD44v6, the mutated receptors resulting from the alternative splicing of CD44 under pathological conditions, was shown to be highly expressed in aggressive prostate cancer tissues, mainly contributing to increased tumour progression and metastasis [86]. A CD44v6-specific peptide termed PFT (PFTVSVPFVWNFTAD) was identified through biopanning of a phage-displayed peptide library. PFT was shown to selectively bind to aggressive human prostate cancer cell lines such as PC3M andMDA-PCa-2b which are CD44v6 overexpression, compared to its absence of binding towards the PC3 cell line which was considered less aggressive. Furthermore, the binding affinity of PFT towards patient tissues using human prostate cancer tissue microarrays (TMA) through immunohistofluorescence studies was investigated. Based on the results, fluorescent signals were detected in CD44v6+ samples with distinct stages and grades, indicating the precise binding of PFT towards its target. Besides, higher PFT staining positivity was found to be associated with late stage (T3-T4), higher grade (III-V) and metastatic (M1) samples. As such, PFT can facilitate the early diagnosis of aggressive prostate cancer type which assists in the rapid administration of accurate therapy options [87].
Conclusion
Small molecules such as peptides are gaining attraction to be explored for their various functionalities in different biological science fields. Antibody is still playing a critical role in both diagnostics and therapeutic agents manufacturing owing to its high affinity and specificity, however, its larger size results in a slow clearance rate, high risk of immunogenicity, poor tumour penetration and high liver uptake, limiting its clinical application. Smaller-sized peptides are an alternative choice to be developed as novel diagnostic and imaging agents as they possess several advantages over antibodies, including lower manufacturing cost, higher stability, minimal immunogenicity with little or non-observable toxicity and ease of modification for bio-conjugation. Typically, phage display technology is used to isolate interested peptides through the screening of a vast amount of targeted samples simultaneously. The widespread use of phage display technique to identify target-binding peptides from a library comprising millions of distinct proteins is the advantage of eliminating the need for prior knowledge of the cellular receptor target. However, receptor identification should be given priority and this process is more challenging than the discovery of novel targeting ligands. This can be due to receptor identification being informative, providing any changes or modifications in the cell surface profile which is useful in cancer biomarker discovery. The mechanism of the receptor or receptor-ligand interaction leading to cancer biological processes such as angiogenesis, metastasis, immune evasion and progression of tumour can be explored, opening a broad and new vision in the oncology field.
Tumour-targeting peptide is one of the important components of non-invasive imaging systems. Targeting peptide is widely applied in various imaging modalities which aid in enhancing the sensitivity in cancer diagnosis and contrast imaging ability which associate with nanoparticle conjugation. Optical imaging with high spatial resolution is a valuable tool for various cancer imaging as the risk of harmful radiation exposure can be reduced, but is limited to its low specificity. To date, fluorescent probes or nanoparticles conjugated-antibody is generally used in molecular imaging owing to their excellent binding affinity and selectivity. However, peptides will be preferentially used as the tumour-targeting ligand to guide the imaging probe to target tumour site specifically due to its ability of tissue penetration where it cannot be achieved by antibody. Although the research on peptides in the field of molecular imaging is increasing, little tumour-targeting peptides are being clinically used in human whereas the application in animals is more stable and acceptable. Several barriers that are required to be considered including the problems of immunogenicity and clearance, as well as the understanding towards the usage of peptides especially towards human is insufficient and is still in the exploring process. Overall, efforts such as modification strategies to enhance their potentials should be carried out as peptide is a promising targeting agent of molecular imaging for various cancer diagnosis.
References
Liu R, Li X, Xiao W, Lam KS (2017) Tumor-targeting peptides from combinatorial libraries. Adv Drug Deliv Rev 110–111:13–37. https://doi.org/10.1016/J.ADDR.2016.05.009
World Health Organization (2022) Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 24 Oct 2022
Tobore TO (2020) On the need for the development of a cancer early detection, diagnostic, prognosis, and treatment response system. Futur Sci OA 6. https://doi.org/10.2144/FSOA-2019-0028
Karpuz M, Silindir-Gunay M, Ozer AY (2018) Current and future approaches for effective Cancer imaging and treatment. Cancer Biother Radiopharm 33:39–51. https://doi.org/10.1089/CBR.2017.2378
Olafsen T, Wu AM (2010) Novel antibody vectors for imaging. Semin Nucl Med 40:167. https://doi.org/10.1053/J.SEMNUCLMED.2009.12.005
Deutscher SL (2010) Phage display in molecular imaging and diagnosis of cancer. Chem Rev 110:3196–3211. https://doi.org/10.1021/CR900317F
Wang AZ, Gu F, Zhang L et al (2008) Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin Biol Ther 8:1063–1070. https://doi.org/10.1517/14712598.8.8.1063
Li Z, Cho C (2010) Development of peptides as potential drugs for cancer therapy. Curr Pharm Des 16:1180–1189. https://doi.org/10.2174/138161210790945913
Newman MR, Benoit DSW (2018) In vivo translation of peptide-targeted drug Delivery Systems discovered by Phage Display. Bioconjug Chem 29:2161–2169. https://doi.org/10.1021/ACS.BIOCONJCHEM.8B00285/ASSET/IMAGES/MEDIUM/BC-2018-00285Y_0003.GIF
Liu P, Tu Y, Tao J et al (2020) GRPR-targeted SPECT imaging using a novel bombesin-based peptide for colorectal cancer detection. Biomater Sci 8:6764–6772. https://doi.org/10.1039/D0BM01432J
Nagpal R, Behare P, Rana R et al (2011) Bioactive peptides derived from milk proteins and their health beneficial potentials: an update. Food Funct 2:18–27. https://doi.org/10.1039/C0FO00016G
Gray BP, Brown KC (2014) Combinatorial peptide libraries: mining for cell-binding peptides. Chem Rev 114:1020. https://doi.org/10.1021/CR400166N
Smith GP (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228:1315–1317. https://doi.org/10.1126/SCIENCE.4001944
Li C, Li J, Xu Y et al (2021) Application of phage-displayed peptides in Tumor Imaging diagnosis and targeting therapy. Int J Pept Res Ther 27:587. https://doi.org/10.1007/S10989-020-10108-5
Bazan J, Całkosiñski I, Gamian A (2012) Phage display—A powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum Vaccin Immunother 8:1817. https://doi.org/10.4161/HV.21703
Arap MA (2005) Phage display technology: applications and innovations. Genet Mol Biol 28:1–9. https://doi.org/10.1590/S1415-47572005000100001
Fala L (2015) Tanzeum (Albiglutide): a Once-Weekly GLP-1 receptor agonist subcutaneous injection approved for the treatment of patients with type 2 diabetes. Am Heal Drug Benefits 8:126
Ma C, Li C, Jiang D et al (2015) Screening of a specific peptide binding to esophageal squamous carcinoma cells from phage displayed peptide library. Mol Cell Probes 29:182–189. https://doi.org/10.1016/J.MCP.2015.04.001
Muteeb G, Rehman M, Ali S et al (2017) Phage display technique: a Novel Medicinal Approach to overcome an tibiotic resistance by using peptide-based inhibitors against β-Lactamases. Curr Drug Metab 18:90–95. https://doi.org/10.2174/1389200217666160727100434
Scott JK, Smith GP (1990) Searching for peptide ligands with an epitope library. Science 249:386–390. https://doi.org/10.1126/SCIENCE.1696028
Greenwood J, Hunter GJ, Perham RN (1991) Regulation of filamentous bacteriophage length by modification of electrostatic interactions between coat protein and DNA. J Mol Biol 217:223–227. https://doi.org/10.1016/0022-2836(91)90534-D
Stellwagen SD, Sarkes DA, Adams BL et al (2019) The next generation of biopanning: next gen sequencing improves analysis of bacterial display libraries. BMC Biotechnol 19. https://doi.org/10.1186/s12896-019-0577-8
Alfaleh MA, Alsaab HO, Mahmoud AB et al (2020) Phage Display Derived Monoclonal antibodies: from bench to Bedside. Front Immunol. https://doi.org/10.3389/FIMMU.2020.01986/BIBTEX. 11:1986
Fouladi M, Sarhadi S, Tohidkia M et al (2019) Selection of a fully human single domain antibody specific to Helicobacter pylori urease. Appl Microbiol Biotechnol 103:3407–3420. https://doi.org/10.1007/S00253-019-09674-6
Saw PE, Song EW (2019) Phage display screening of therapeutic peptide for cancer targeting and therapy. Protein Cell 10:787. https://doi.org/10.1007/S13238-019-0639-7
Li C, Gao N, Xue Q et al (2017) Screening and identification of a specific peptide binding to cervical cancer cells from a phage-displayed peptide library. Biotechnol Lett 39:1463–1469. https://doi.org/10.1007/S10529-017-2381-7
Dantas-Barbosa C, Brigido M, de Maranhao M AQ (2012) Antibody phage display libraries: contributions to oncology. Int J Mol Sci 13:5420–5440. https://doi.org/10.3390/IJMS13055420
André AS, Moutinho I, Dias JNR, Aires-da-Silva F (2022) In vivo Phage Display: a promising selection strategy for the improvement of antibody targeting and drug delivery properties. Front Microbiol 13:3704. https://doi.org/10.3389/FMICB.2022.962124/BIBTEX
Pasqualini R, Ruoslahti E (1996) Organ targeting in vivo using phage display peptide libraries. Nature 380:364–366. https://doi.org/10.1038/380364A0
Staquicini FI, Hajitou A, Driessen WHP et al (2021) Targeting a cell surface vitamin D receptor on tumor-associated macrophages in triple-negative breast cancer. Elife 10. https://doi.org/10.7554/ELIFE.65145
Liu JK, Lubelski D, Schonberg DL et al (2014) Phage display discovery of novel molecular targets in glioblastoma-initiating cells. Cell Death Differ 21:1325–1339. https://doi.org/10.1038/CDD.2014.65
Lee TY, Lin CT, Kuo SY et al (2007) Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res 67:10959–10965. https://doi.org/10.1158/0008-5472.CAN-07-2233
Du B, Han H, Wang Z et al (2010) Targeted drug delivery to hepatocarcinoma in vivo by phage-displayed specific binding peptide. Mol Cancer Res 8:135–144. https://doi.org/10.1158/1541-7786.MCR-09-0339
Wu CH, Liu IJ, Lu RM, Wu HC (2016) Advancement and applications of peptide phage display technology in biomedical science. J Biomed Sci 2016 231 23:1–14. https://doi.org/10.1186/S12929-016-0223-X
Weissleder R, Ross BD, Rehemtulla A et al (2011) Molecular imaging: principles and practice. J Nucl Med 52:1003–1003. https://doi.org/10.2967/JNUMED.111.092270
Khondee S, Piyawattanametha W, Khondee S, Piyawattanametha W (2019) Targeting peptides derived from Phage Display for Clinical Imaging. https://doi.org/10.5772/INTECHOPEN.84281. Bacteriophages - Perspect Futur
Wu AM, Olafsen T (2008) Antibodies for molecular imaging of cancer. Cancer J 14:191–197. https://doi.org/10.1097/PPO.0B013E31817B07AE
Rowe SP, Pomper MG (2022) Molecular imaging in oncology: current impact and future directions. CA Cancer J Clin 72:333–352. https://doi.org/10.3322/CAAC.21713
Okarvi SM (2008) Peptide-based radiopharmaceuticals and cytotoxic conjugates: potential tools against cancer. Cancer Treat Rev 34:13–26. https://doi.org/10.1016/J.CTRV.2007.07.017
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128. https://doi.org/10.1126/SCIENCE.AAA1348
Le Joncour V, Laakkonen P (2018) Seek & destroy, use of targeting peptides for cancer detection and drug delivery. Bioorg Med Chem 26:2797–2806. https://doi.org/10.1016/J.BMC.2017.08.052
Lee S, Xie J, Chen X (2010) Peptide-based probes for targeted Molecular Imaging. Biochemistry 49:1364. https://doi.org/10.1021/BI901135X
Cho HJ, Lee SJ, Park SJ et al (2016) Activatable iRGD-based peptide monolith: Targeting, internalization, and fluorescence activation for precise tumor imaging. J Control Release 237:177–184. https://doi.org/10.1016/J.JCONREL.2016.06.032
Guan SS, Wu CT, Chiu CY et al (2018) Polyethylene glycol-conjugated HER2-targeted peptides as a nuclear imaging probe for HER2-overexpressed gastric cancer detection in vivo. J Transl Med 16:168. https://doi.org/10.1186/S12967-018-1550-3
Oh I, Min HS, Li L et al (2013) Cancer cell-specific photoactivity of pheophorbide a-glycol chitosan nanoparticles for photodynamic therapy in tumor-bearing mice. Biomaterials 34:6454–6463. https://doi.org/10.1016/J.BIOMATERIALS.2013.05.017
Minchin RF, Martin DJ (2010) Minireview: nanoparticles for Molecular Imaging—An overview. Endocrinology 151:474–481. https://doi.org/10.1210/EN.2009-1012
Montet X, Montet-Abou K, Reynolds F et al (2006) Nanoparticle imaging of integrins on tumor cells. Neoplasia 8:214–222. https://doi.org/10.1593/NEO.05769
Wang JL, Du XJ, Yang JX et al (2018) The effect of surface poly(ethylene glycol) length on in vivo drug delivery behaviors of polymeric nanoparticles. Biomaterials 182:104–113. https://doi.org/10.1016/J.BIOMATERIALS.2018.08.022
Higgins LJ, Pomper MG (2011) The evolution of imaging in cancer: current state and future challenges. Semin Oncol 38:3–15. https://doi.org/10.1053/J.SEMINONCOL.2010.11.010
Guimaraes MD, Schuch A, Hochhegger B et al (2014) Functional magnetic resonance imaging in oncology: state of the art. Radiol Bras 47:101. https://doi.org/10.1590/S0100-39842014000200013
Accardo A, Tesauro D, Morelli G (2013) Peptide-based targeting strategies for simultaneous imaging and therapy with nanovectors. Polym J 2013 455 45:481–493. https://doi.org/10.1038/pj.2012.215
Corot C, Robert P, Idée JM, Port M (2006) Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 58:1471–1504. https://doi.org/10.1016/J.ADDR.2006.09.013
Boucher M, Geffroy F, Prévéral S et al (2017) Genetically tailored magnetosomes used as MRI probe for molecular imaging of brain tumor. Biomaterials 121:167–178. https://doi.org/10.1016/J.BIOMATERIALS.2016.12.013
Tan J, Sun W, Lu L et al (2019) I6P7 peptide modified superparamagnetic iron oxide nanoparticles for magnetic resonance imaging detection of low-grade brain gliomas. J Mater Chem B 7:6139–6147. https://doi.org/10.1039/C9TB01563A
Ahmadi Y, Kostenich G, Oron-Herman M et al (2017) In vivo magnetic resonance imaging of pancreatic tumors using iron oxide nanoworms targeted with PTR86 peptide. Colloids Surf B Biointerfaces 158:423–430. https://doi.org/10.1016/J.COLSURFB.2017.06.051
Cheng Z, Yan X, Sun X et al (2016) Tumor Molecular Imaging with Nanoparticles. Engineering 2:132–140. https://doi.org/10.1016/J.ENG.2016.01.027
Kim J, Lee N, Hyeon T (2017) Recent development of nanoparticles for molecular imaging. Philos Trans R Soc A Math Phys Eng Sci 375. https://doi.org/10.1098/RSTA.2017.0022
Siddique S, Chow JCL (2020) Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomater (Basel Switzerland) 10:1–41. https://doi.org/10.3390/NANO10091700
Cai W, Hsu AR, Li ZB, Chen X (2007) Are quantum dots ready for in vivo imaging in human subjects? Nanoscale Res Lett 2:265–281. https://doi.org/10.1007/S11671-007-9061-9
Shi X, Shi C, Ye W et al (2020) Targeted fluorescence imaging and Biological Effects of peptide conjugated Quantum dots on pancreatic Cancer cells. J Nanosci Nanotechnol 20:1351–1357. https://doi.org/10.1166/JNN.2020.16949
Liu X, Braun GB, Zhong H et al (2016) Tumor-targeted Multimodal Optical Imaging with Versatile Cadmium-Free Quantum Dots. Adv Funct Mater 26:267–276. https://doi.org/10.1002/ADFM.201503453/FULL
da Fonseca Alves R, Martins IC, Franco DL et al (2022) A novel peptide-based electrochemical biosensor for breast cancer characterization over a poly 3-(3-aminophenyl) propionic acid matrix. Biosens Bioelectron 205:114081. https://doi.org/10.1016/J.BIOS.2022.114081
Fu B, Zhang Y, Long W et al (2014) Identification and characterization of a novel phage display-derived peptide with affinity for human brain metastatic breast cancer. Biotechnol Lett 36:2291–2301. https://doi.org/10.1007/S10529-014-1608-0
Prud’homme GJ, Glinka Y (2012) Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 3:921–939. https://doi.org/10.18632/ONCOTARGET.626
Feng GK, Liu R, Bin, Zhang MQ et al (2014) SPECT and near-infrared fluorescence imaging of breast cancer with a neuropilin-1-targeting peptide. J Control Release 192:236–242. https://doi.org/10.1016/J.JCONREL.2014.07.039
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30. https://doi.org/10.3322/CAAC.21332
Ferreira D, Silva AP, Nobrega FL et al (2019) Rational identification of a colorectal Cancer targeting peptide through Phage Display. Sci Rep 9. https://doi.org/10.1038/S41598-019-40562-1
Kwak MH, Yi G, Yang SM et al (2020) A Dodecapeptide selected by Phage Display as a potential theranostic probe for Colon cancers. Transl Oncol 13:100798. https://doi.org/10.1016/J.TRANON.2020.100798
Li ZJ, Wu WKK, Ng SSM et al (2010) A novel peptide specifically targeting the vasculature of orthotopic colorectal cancer for imaging detection and drug delivery. J Control Release 148:292–302. https://doi.org/10.1016/J.JCONREL.2010.09.015
Li ZJ, Cho CH (2012) Peptides as targeting probes against tumor vasculature for diagnosis and drug delivery. J Transl Med 10 Suppl 11–9. https://doi.org/10.1186/1479-5876-10-S1-S1/TABLES/1
Liu Z, Gray BD, Barber C et al (2016) Characterization of TCP-1 probes for molecular imaging of colon cancer. J Control Release 239:223–230. https://doi.org/10.1016/J.JCONREL.2016.08.033
Zhu D, Qin Y, Wang J et al (2016) Novel glypican-3-Binding peptide for in vivo Hepatocellular Carcinoma fluorescent imaging. Bioconjug Chem 27:831–839. https://doi.org/10.1021/ACS.BIOCONJCHEM.6B00030
Hou L, Zhu D, Liang Y et al (2018) Identification of a specific peptide binding to colon cancer cells from a phage-displayed peptide library. Br J Cancer 118:79–87. https://doi.org/10.1038/BJC.2017.366
Lee KJ, Lee JH, Chung HK et al (2016) Application of peptide displaying phage as a novel diagnostic probe for human lung adenocarcinoma. Amino Acids 48:1079–1086. https://doi.org/10.1007/S00726-015-2153-4
Bunn PA, Franklin W, Doebele RC (2013) The evolution of Tumor classification: a role forGenomics? Cancer Cell 24:693. https://doi.org/10.1016/J.CCR.2013.11.019
Chen Z, Fillmore CM, Hammerman PS et al (2014) Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14:535–546. https://doi.org/10.1038/NRC3775
Chi YH, Hsiao JK, Lin MH et al (2017) Lung Cancer-Targeting peptides with multi-subtype indication for Combinational Drug Delivery and Molecular Imaging. Theranostics 7:1612. https://doi.org/10.7150/THNO.17573
Sato H, Takino T, Okada Y et al (1994) A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370:61–65. https://doi.org/10.1038/370061A0
Tanaka M, Sato H, Takino T et al (1997) Isolation of a mouse MT2-MMP gene from a lung cDNA library and identification of its product. FEBS Lett 402:219–222. https://doi.org/10.1016/S0014-5793(96)01537-2
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/J.CELL.2011.02.013
Abraham R, Schäfer J, Rothe M et al (2005) Identification of MMP-15 as an anti-apoptotic factor in cancer cells. J Biol Chem 280:34123–34132. https://doi.org/10.1074/JBC.M508155200
Ren L, Ma Z, Li Q et al (2019) Identifying a membrane-type 2 Matrix metalloproteinase-targeting peptide for human Lung Cancer Detection and Targeting Chemotherapy with Functionalized Mesoporous silica. ACS Appl bio Mater 2:397–405. https://doi.org/10.1021/ACSABM.8B00633
Freire-de-Lima L, Gelfenbeyn K, Ding Y et al (2011) Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process. Proc Natl Acad Sci U S A 108:17690–17695. https://doi.org/10.1073/PNAS.1115191108/SUPPL_FILE/PNAS.201115191SI.PDF
Barron DA, Rowley DR, Barron A, Rowley DR (2012) The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer 19:R187–R204. https://doi.org/10.1530/ERC-12-0085
Han Z, Zhou Z, Shi X et al (2015) EDB fibronectin specific peptide for prostate cancer targeting. Bioconjug Chem 26:830–838. https://doi.org/10.1021/ACS.BIOCONJCHEM.5B00178/ASSET/IMAGES/MEDIUM/BC-2015-00178P_0009.GIF
Ni J, Cozzi PJ, Hao JL et al (2014) CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate 74:602–617. https://doi.org/10.1002/PROS.22775
Peng Y, Prater AR, Deutscher SL (2017) Targeting aggressive prostate cancer-associated CD44v6 using phage display selected peptides. Oncotarget 8:86747. https://doi.org/10.18632/ONCOTARGET.21421
Acknowledgements
The authors express gratitude to the Institute for Research in Molecular Medicine (INFORMM), Universiti Sains Malaysia for their support. We would also like to thank Gan Shin Yi for her kind guidance.
Funding
This work was supported by the Malaysian Ministry for Education Higher Institution Centre of Excellence (HICoE) Grant (311/CIPPM/44001005).
Author information
Authors and Affiliations
Contributions
PHS and LNS had the idea for the article, guided the study, performed the literature search and analysis, and wrote the paper. TGJ, LCH and NWK critically revised the final draft. All authors critically reviewed progressive drafts of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pung, H.S., Tye, G.J., Leow, C.H. et al. Generation of peptides using phage display technology for cancer diagnosis and molecular imaging. Mol Biol Rep 50, 4653–4664 (2023). https://doi.org/10.1007/s11033-023-08380-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08380-x