Abstract
Background
Disease-resistant cultivars are the best solution to get their maximum yield potential and avoid fungicide application. There is no doubt about the contribution, and use of R genes (resistance genes) in resistance development in plants, while S genes (susceptibility genes) also hold a strong position in pathogenesis by resistance repression, and their loss of function contributes to enhanced resistance. Hence, we attempted to knock out the function of the StERF3 gene in potatoes through CRISPR/Cas9-based genome editing and investigated the CRISPR/Cas9 approach as strategic control against late blight disease in potato plants.
Methods and results
The StERF3 gene was edited in late blight susceptible cv. Lady Rosetta. Full allelic edited plants were identified through DnpI, and N1aIV mediated restriction digestion and then further analyzed through Indel Detection by Amplicon Analysis. Sequence analysis of targeted plants for indel identification showed full allelic editing. The detached leaf assay of full allelic edited plants demonstrated the role of the StERF3 gene in susceptibility to late blight in potatoes. In planta disease assay also showed reduced, slowed, and delayed disease progression in StERF3-loss-of-function mutants compared to wild-type (control) plants. Less fungal biomass was quantified in knockouts through Real-time qPCR that supported less susceptibility of edited plants to late blight. Besides, relatively high expression of pathogens-related genes, StPR1, and StNPR1, were also observed in StERF3-loss-of-function mutants compared to the corresponding control.
Conclusion
The results showed the functional inhibition of StERF3 genes using the CRISPR/Cas9 approach. The functional knockouts (StERF3 gene-edited potato plants) revealed enhanced resistance against Phytophthora infestans, thereby demonstrating the best strategic control for late blight disease in potato plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum L.) is a dicotyledonous, non-grain, nutritionally rich, short-duration, solanaceous food crop. It is grown in tropical and sub-tropical regions with an average production of 17.4 t ha−1 [1]. It is ranked 4th among major staple food; maize, rice, and wheat. It also serves as feedstock for industrial goods. However, potato faces an increasing risk of different stresses that limit their growth, development, and productivity. Biotic factors cause considerable yield losses. Late blight, caused by the oomycete Phytophthora infestans, is a destructive disease of potato plants. It has historical significance as the cause of the Irish Potato Famine during the 1840s [2]. Phytophthora infestans (Mont.) de Bary had a false placement in the kingdom of Fungi because of their fungus-like morphology. Now it is considered a member of the class oomycetes kingdom Chromista. Eighty-nine host species of P. infestans have been reported; out of them, the typical host is potato [3]. It is spread around the world, most commonly through potato seeds. 50–70% yield losses in potato crops have been recorded due to late blight at 16–24 °C and 92–100% relative humidity [4].
Plant-pathogen interaction leads to molecular modulation of stress signals through signaling elements that activate several stress-responsive genes. The products of these genes may have a role in producing regulatory phytohormones (ethylene, salicylic acid, or abscisic acid), which can then trigger the final plant response. Among them, the key defense-related plant hormone is ethylene. This phytohormone also has a core role in plant growth and development [5]. It modulates the expression of defense-related genes through transcription factors called ethylene response factors (ERFs). The ERFs, effectors of ethylene signaling, are specifically bound to the GCC-box of the promoter region of stress-responsive genes and modulate their expression [6]. Among ERF family proteins, several members are reported as transcription activators, i.e., periwinkle, ERF1, ERF2, ERF5, DREB1, DREB2, ORCA2, ORCA3, and CBF1 of Arabidopsis thaliana [7] ERF2, ERF4 and Tsi1 of Nicotiana tabacum [8, 9] JERF3, Pti4 and Pti5 of Solanum lycopersicum [10,11,12]. In contrast, some ERFs have been reported as transcription repressors because of their conserved motif structure ((L/F)DLN(L/F)xP) present at the C terminal of the polypeptide chain known as ERF-associated amphiphilic repression (EAR) motif [7]. These members bind at the GCC-promoter site and repress the expression of the downstream genes [13]. These include ERF3, ERF4, and RAP2.1 of Arabidopsis thaliana, ERF3 of Nicotiana tabacum, ERF3b of Prunus persica, ERF3 and ERF922 of Oryza sativa, ERF3 of Solanum lycopersicum and Solanum tuberosum [7, 14, 18].
The knowledge of functional characterization of a gene finds its biological role in disease resistance developing rapidly. Several strategies are used to identify the function of a gene, including gene silencing or knockdown approaches (RNA interference (RNAi) or virus-induced gene silencing (VIGS) and genome editing or knockout approaches (zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) 9 (CRISPR/Cas9).
Tian et al. [14] knockdown the expression of the StERF3 gene through the RNA interference (RNAi) technique resulting in enhanced resistance against P. infestans, causing late blight disease in potato plants. They described that the StERF3 gene is involved in potato plants’ late blight disease prevalence and suggested focusing on the StERF3 gene in plant genome engineering for late blight resistance. However, the RNAi strategy is less likely to be heritable and produce off-targets [15]. It is used only to knock down the expression of the targeted gene as it reduces the gene expression but does not fully inhibit its expression. Often it is used for transient gene silencing in crop plants, most probably to understand the gene function.
Moreover, the RNAi engineered plants need strict biosafety regulations [16] because foreign DNA (transgene) is inserted in the genome. However, genome editing through CRISPR/Cas9 bypasses the regulation because no transgene insertion is required, as required in transgenic plants. Considering this perspective, genome editing through sequence-specific nucleases is gaining importance in obtaining the disease resistance phenotype of plants [17]. Therefore, we employed a CRISPR/Cas9-based genome editing approach to developing StERF3-loss-of-function mutants to evaluate it as strategic control against late blight disease in potato plants. In this contest, an effective and robust CRISPR/Cas9 genome editing tool with its higher efficiency and versatility was used for gene function identification in wheat [18], maize [19], rice [20], soybean [21], tomato [22], and potato [23].
Material and methods
Target sequences selection
The sequence of a target gene (StERF3) was retrieved from the National Center for Biotechnology Information (NCBI) database. The primers were designed using the PrimerQuest™ tool (Table S1) to amplify the target gene sequence in the potato cultivar Lady Rosetta, susceptible to late blight disease. The genomic DNA was extracted using GeneJET Plant Genomic DNA Purification Kit (Thermo Scientific, USA). The PCR reaction was carried out in a thermal cycler (peqSTAR) with a temperature profile that involved initial denaturation (94 °C for 5 min), followed by a loop of 30 cycles; denaturation (95 °C for 40 s), annealing (58 °C for 35 s), extension (72 °C for 60 s), and final extension (72 °C for 7 min). The PCR product was resolved on 0.8% agarose, excised, and eluted from gel using a Gel Purification kit (FavorPrep, Taiwan). The eluted product was direct sequenced (Eurofins Genomics DNA Sequencing Services, USA). The generated sequence was trimmed through the BioEdit tool v. 7.2.6.1. The high-quality sequence was searched for homology using the Blastn tool. Target sequences within the StERF3 gene were selected using an online bio-tool, CHOPCHOP (https://chopchop.rc.fas.harvard.edu), and CRISPOR [http://crisportefor.net] for target design and off-target prediction. This bioinformatics tool provides crRNA or target sequences by detecting proto-spacer adjacent motif (PAM) sequences within subjected DNA sequence and predicts off-target sites for minimizing off-target effects, GC contents, and self-complementarity. The secondary structures of target-sgRNA sequences were analyzed with the mfold web server, RNA Folding Form V2.3.
CRISPR/Cas9 construct preparation for genome editing in potato
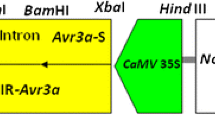
The CRISPR/Cas9 construct targeting the StERF3 gene was generated in the pYLCRISPR/Cas9Pubi-B binary vector [24]. The sgRNA expression cassettes with target sequences were prepared in nested PCR analysis. In the first PCR reaction U-F/UT (−) and gRT (+)/gR-R primers were used (Table S1). In the second PCR reaction, the products of the first PCR (1/10 dilution) were used as templates along with site-specific primers Pps/Pgs containing BsaI sites (detail is given in the supplementary table). Each PCR reaction was performed in 12 μl using Phusion HiFi PCR master mix with HF buffer (Thermo Scientific) for 30 cycles (98 °C for 10 s, 55 °C for 5 s, and 72 °C for 30 s). The products of the second PCR were pooled, electrophoresed, and purified from agarose (high-resolution biotech grade, ACTGene) using a Gel Purification kit (FavorPrep). The Golden Gate cloning (BsaI digestion/ligation reaction) was performed using the NEB Golden Gate assembly Kit (BsaI-HF v2). The reaction was comprised of NEB Golden gate enzyme mix (BsaI-HFv2), T4 DNA ligase buffer (10X), pooled sgRNA expression cassettes (100 ng/μl), CRISPR/Cas9 binary vector pYLCRISPR/Cas9Pubi-B (100 ng/μl), and nuclease-free water. The reaction was incubated in a thermal cycler for thirty cycles (37 °C for 60 s, 16 °C for 60 s) and then 60 °C for 5 min. The Golden Gate assembly reaction’ product was delivered into competent cells of Escherichia coli strain Top10 through the heat shock method. After transformation, cells were cultured on LB (Luria Britani) medium containing 20 mg ml−1 kanamycin for positive colony selection. Plasmid DNA of positive colonies was isolated using GeneJET plasmid Kit (Thermo Scientific) and further analyzed by sequencing and Mlu1 digestion.
Agrobacterium-mediated plant transformation
The CRISPR/Cas9 construct was delivered into electrocompetent cells of Agrobacterium tumefaciens strain AGL1 by electroporation. The cells were then cultured on an LB medium containing 25 mg ml−1 streptomycin and incubated overnight at 28 °C. The overnight grew culture (15 ml) of A. tumefaciens containing CRISPR/Cas9 construct was centrifuged (13,200 rpm for 5 min), decanted the supernatant, and resuspended the pellet in d3H2O (10 ml). The 50 µl acetosyringone at the concentration of 76 mM was added to the Agrobacterium suspension. The calli were co-cultured with the Agrobacterium suspension (A. tumefaciens harboring CRISPR/Cas9 construct with two linked sgRNA expression cassettes with target sequences). The co-culturing was executed at 25 °C for 48 h under dark conditions for the agrobacterium-mediated transformation of the potato.
Selection of transformants
For the selection of transformants, the selected callus induction, regeneration, and root induction media [25] were supplemented with cefotaxime (250 µg ml−1), timentin (100 µg ml−1), and Basta (9 µg ml−1) were designated as selective CIM, selective RM, and Selective ½ MS medium.
Mutation detection
Genomic DNA of selected transformants/putative targeted plants was extracted (GeneJET Plant Genomic DNA Purification Kit, Thermo Scientific, USA), quantified (NANODROP, 8000 Spectrophotometer, Thermo Scientific), and dilutions (50 ng/µl) were made. PCR analysis was carried out using specific primers flanking the target sites (150–250 bp upstream of the target sites) (Forward: 5′GGGAATTCCGTGGAGCTAAA3′; Reverse: 5′GTTTCACGTCAACACGCATAAA3′). Target sequence-1 (Ts1) includes a diagnostic Dnp1 restriction enzyme site spanning the Cas9 cleavage site, 2 bp upstream of the PAM region. However, target sequence-2 (Ts2) includes a diagnostic N1aIV restriction enzyme site spanning the Cas9 cleavage site, 8 bp upstream of the PAM region. Therefore for detecting full allelic edited plants (as potato is tetraploid), purified PCR amplicons of putative targeted plants were subjected to restriction digestions with DnpI and N1aIV endonucleases. The putative targeted plants with full allelic editing were analyzed for Indel Detection by Amplicon Analysis (IDAA). For IDAA, tri-primer PCR analysis of wild-type plants and full allelic edited plants was carried out using target-specific primer pair (flanking the target sites) and universal 6-carboxyfluorescein (6-FAM) 5′-labelled primer (FamF). For analysis, the fluorophore-labeled amplicons were sent to Eurofins genomics services, Europe.
Detached leaf assay
Detached leaf assay was performed on the leaves from the greenhouse-grown plants (age 7 weeks). The experiment was made in triplicate. Inoculation was done by dispensing 50 µl sporangia suspension (2 × 104 sporangia ml−1) [26] of Phytophthora infestans on the leaves’ abaxial side incubated on wet Whatman filter paper, and placed in zipper bags. The samples in sealed bags were kept under 16/8 h light/dark cycle at 18 ± 2 °C in a humidity-controlled Plant Growth Chamber (RGX 400EF). Inoculated leaves were assessed for blight lesion area (%age) on leaves described by Irzhansky and Cohen [27]. The observation was taken after the first day of post-inoculation. The lesion area was also calculated using ImageJ software version 1.53 s.
In planta disease assay and quantification of fungal biomass
Seven-week-old full allelic edited plants and wild-type plants were inoculated with 2 × 104 sporangia ml−1. Disease severity was assessed according to Seifu [28]. For fungal biomass quantification, genomic DNA of infected leaves of knockouts and wild-type plants was isolated using GeneJET genomic DNA Purification Kit (Thermo Scientific, USA), and real-time qPCR analysis was performed using the primer pair PiO8-3-3Fwd/ PiO8-3-3Rev used by Llorente et al. [29] to quantify the Phytophthora infestans biomass in potato. The ubiquitin-conjugating enzyme (Ubc) was used as an endogenous control gene, as Yan and Liou [30] reported it as the best reference gene for real-time qPCR analysis at the stage of the pathogenesis of Phytophthora species.
Expression profiling of pathogenesis-related gene
Real-time quantitative PCR (RT-qPCR) analysis was carried out on the CFX96 Touch Real-Time PCR detection system using Maxima Syber green/ROX/qPCR master mix. Total RNA was extracted from leaves of inoculated wild type (WT, control) and targeted potato plants using GeneJET Plant RNA Purification Kit (Thermo Scientific, USA), and cDNA was synthesized (RevertAid First Strand cDNA Synthesis Kit, Thermo Scientific, USA). Two pathogenesis-related genes, StPR1 and StNPR1, were selected for their comparative expression profiling in wild-type and CRISPR/Cas9 targeted potato plants using primer pairs qRT-StPR1-F/ qRT-StPR1-R [31] and qRT-StNPR1-F/ qRT-StNPR1-R [32]. Their expression level was determined relative to the Ef1-α gene (reference gene) using qPCR primers by Lehtonen et al. [33], and relative expression was calculated by the 2−∆Cq Method.
Results
The StERF3 gene, targeted in this research through a CRISPR/Cas9-based genome editing strategy, is from Class II ERFs of the AP2/ERF protein family [7]. The StERF3 is ERF-associated amphiphilic repression (EAR) type of ERF, which acts as a negative regulator by repressing gene expression [14]. It acts as a repressor by inhibiting the expression of pathogen-induced defense genes that contribute to defense-related pathways and impart disease resistance to plants. The PCR product of the target gene (StERF3) was electrophoresed. The amplicon of size ~ 489 bp was excised and eluted from the gel. The eluted product was direct sequenced. The high-quality (HQ) trimmed sequence showed 96.93% homology with GenBank accession number EF091875.1 using the Blastn tool.
Selection of target sequences
Two target sequences were selected within the StERF3 gene using the CHOPCHOP web tool. The targeting specificity of target sequences was confirmed by subjecting them to a BLAST search against the Solanum tuberosum genome sequence. The mismatch of more than two bases in the PAM-proximal (seed region) of target sequences to the non-target sequence and the difference of more than five bases in the PAM distal region (non-seed region) of targets to the non-target sequences [24, 34] were considered for most specific target selection. The sgRNA secondary structure determines the effectiveness of sgRNA/Cas9 [24, 35]. Hence, the secondary structures of target-sgRNAs were analyzed. Highly efficient targets should have no base pairing with the sgRNA sequence. However, pairing with < 6 base pairs may be tolerated [24]. While selecting a target, the stem-loop formation between more than six base pairs of target and sgRNA sequences should be avoided because stem-loop structure leads to the inhibition of target-sgRNA binding. The analysis of our selected target-sgRNA sequences using RNA Folding Form V2.3 showed no pairing of target-1 with sgRNA (Fig S1) and pairing of three base pairs with sgRNA in the case of target-2 (Fig S2).
The GC content of the target sequence is another strong determinant of editing efficiency in CRISPR/Cas9-based genome editing. The literature has documented that GC contents and editing efficiency are directly proportional; the more GC contents in the target sequence, the higher the editing efficiency will be. Therefore, we selected the target sequences with more than 45% GC contents. However, in clinical research, more GC content in the composition of target sequences result in off-targeting at a high rate [36]. While in plant research, off-targeting is not a critical concern because this risk is not as high as the somatic mutations in tissue culture-based genetic engineering and mutagenesis [37] and can be eliminated by backcrossing the mutant with the parent.
Development of CRISPR/Cas9 construct and plant transformation
The binary vector, pYLCRISPR/Cas9Pubi-B with vector backbone pCAMBIA1300 containing maize ubiquitin promoter, was used for generating the CRISPR/Cas9 construct for targeting the StERF3 gene. The sgRNA intermediate vector was prepared by synthesizing the OsU6a-sgRNA sequence [24] in the pUC57 backbone. In the sgRNA expression cassette, target sequences were introduced in an overlapping polymerase chain reaction (PCR). In the first PCR reaction of nested PCR, promoter and sgRNAs were amplified using chimeric primers U-F/UT (−) and gRT (+)/gR-R, respectively, in which target sequences were engineered in gRT (+) and UT (−) primers. In the second PCR reaction of nested PCR site-specific primer pairs, Pps/Pgs containing BsaI-cutting sites were used to amplify the sgRNA expression cassette with target sequences. The BsaI sites were engineered in site-specific primers for Golden Gate ligation. The BsaI is of type IIs restriction endonucleases with a novel cleavage attribute of generating non-palindromic distinct, sticky ends to circumvent self-ligation and ligation non-compatible ends [38]. We prepared the pYLCRISPR/Cas9Pubi-BstERF3 construct using the Golden Gate cloning strategy, carrying two sgRNA expression cassettes driven by the OsU6a promoter for gene targeting in potatoes. The Golden Gate cloning is an efficient method to join multiple DNA fragments in designed order. The delivery of the CRISPR/Cas9 construct to electrocompetent cells of Agrobacterium tumefaciens strain AGL1 was mediated by electroporation. The strain has C58 RecA chromosomal background containing Ti plasmid, pTiBo542DT-DNA having strepR (Streptomycin resistance) gene, as screening tag imparting streptomycin resistance to the strain.
The Explant of potato cultivar. Lady Rosetta was cultured on a callus induction medium, CIM (MS Salt with vitamin 4.33 g/L; Sucrose 30 g/L, 2, 4-D 4.75 mg/L; Myoinositol 0.1 g/L; Glycine 2 mg/L; Gellan Gum Powder 2.66 g/L) [25]. The co-culturing of 2 weeks old calli was made with 1 ml of Agrobacterium suspension at 25 °C under dark for 2 days. The calli were washed with d3H2O and cultured on selective CIM that was CIM supplemented with cefotaxime (250 µg ml−1), timentin (100 µg ml−1), and Basta (9 µg ml−1) for 2 weeks, by adopting the potato transformation protocol described by Veillet et al. [39], and Kieu et al. [26]; however, with some modifications. After 2 weeks, the 28 days old calli cultured on selective CIM were transferred to regeneration media, RM, (MS Salt with vitamin 4.33 g/L; Sucrose 30 g/L, BAP 5 mg/L; Myoinositol 0.1 g/L; Glycine 2 mg/L; Gellan Gum Powder 2.66 g/L) [25] supplemented with cefotaxime (250 µg ml−1), timentin (100 µg ml−1), and Basta (9 µg ml−1) (selective RM). The emerged shoots on selective RM were transferred to a selective rooting medium. The shoots that initiated rooting and turned to plantlet were multiplied by putting them in a multiplication medium (MS Salt with vitamin 4.33 g/L; Sucrose 30 g/L, Myoinositol 0.1 g/L; Glycine 2 mg/L; Gellan Gum Powder 2.66 g/L). We got six plantlets under continued selection pressure and were transferred to a multiplication medium before molecular characterization.
Mutation detection in putative targeted plants
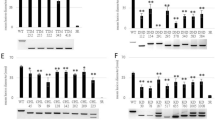
Due to the indel-mediated destruction of the DnpI and N1aIVsites, we got a DNA fragment at ~ 400 bp in two putative targeted plants (PTP2 and PTP3), as shown in Fig. 1a. However, restriction digestion with DnpI yields two DNA fragments of ~ 180 bp and ~ 216 bp in wild-type plants (control) and four putative targeted plants (no editing) (PTP1, PTP4, PTP5, and PTP6). Similarly, indel-mediated destruction of the N1aIV site gave two DNA fragments of ~ 128 bp and ~ 268 bp in wild-type plants (control) and four putative targeted plants (no editing) (PTP1, PTP4, PTP5, and PTP6). Of six putative targeted plants, two showed editing (33%), and four displayed no allele editing (67%). Sequence analysis of edited plants for indel identification through IDAA assay revealed that both PTP2 and PTP3 were full allelic edited. In target sequence 1, the targeted plant, PTP2, had 5 bp nucleotide substitution in allele 1, 5 bp deletion in allele 2, 3 bp deletion & 2 bp nucleotide substitution in allele 3, and 1 bp nucleotide substitution & 3 bp deletion in allele 4. However, target 2 of this plant had 4 bp & 6 bp deletion in allele 1 and allele 3, respectively, and 1 bp & 2 bp addition in allele 2, and allele 4, respectively (Fig. 1b). The targeted plant PTP3 had 1 bp addition in allele 1, 1 bp addition in allele 2, 4 bp deletion in alleles 3 and 4, in target sequence 1. However, the target sequence 2 of this plant had 2 bp addition in alleles 1 and 2 and 5 bp & 6 bp deletion in alleles 3 and 4, respectively (Fig. 1c).
Mutation detection in CRISPR/Cas9 targeted StERF3 gene in potato plants. a Restriction digestion assay: Mutation detection in StERF3 targeted plants through restriction digestion of diagnostic restriction enzyme sites b Sequence analysis of edited plant, PTP2, for indel identification through IDAA assay c Sequence analysis of edited plant, PTP3, for indel identification through IDAA assay
Pathological analyses
The detached leaf assay of full allelic edited and control plants was performed to evaluate the role of the StERF3 gene in susceptibility to late blight in potatoes. The detached leaves of targeted plants and control (Wild type) were inoculated with P. infestans. Small dark green to brownish-black water-soaked lesions appeared on the leaf of control plants on the third day of post inoculation (3dpi), which expanded rapidly and covered more than ~ 60% of the leaf area after 7 days. However, in the case of detached leaves of knockouts (PTP2 and PTP3), infinitesimal symptoms were observed at 5dpi, which progressed slowly compared to a corresponding control, and after 7 days, ~ 15% leaf area was infected. The lesion area calculated by ImageJ software was 1233mm2 (WT), 315mm2 (PTP2), and 314 mm2 (PTP3) (Fig. 2). Validating the results of detached leaf assay, In planta, disease assay was performed parallelly, in which seven-week-old full allelic edited plants and wild type (WT) plants or control were inoculated with sporangial suspension (2 × 104 sporangia ml−1). Disease symptoms started appearing on wild-type plants after 10 days post-inoculation (10dpi). We analyzed the WT plants for disease rating at 12dpi and observed 5% disease severity according to the scale described by Seifu [28]. Disease on StERF3-loss-of-function mutant plants has appeared on 23dpi, and at 28dpi, disease severity was 2%. However, in wild-type plants at 28dpi, 40% disease severity was observed. These results showed that the disease severity and progression in knockouts were reduced, slowed, and delayed. We took the observations to 42dpi, which revealed lower disease progression in knockouts than wild types. At 42dpi, 75% infection was observed in WT plants, and 30% disease severity was observed on edited plants. These results showed increased tolerance of edited potato plants to late blight.
Detached leaf assay of StERF3-loss-of-function mutants (PTP2 and PTP3): Inoculated targeted plants and control (Wild type) with P. infestans showed small brownish-black lesions that covered more than ~ 60% of leaf area after 7 days of post inoculation (7dpi) in control; however, infinitesimal symptoms were observed on the leaves of knockouts at 7dpi. It showed increased tolerance of edited potato plants to late blight
Fungal genomic DNA was extracted from infected leaves taken at 23dpi from StERF3-loss-of-function mutants and WT plants. Fungal biomass was quantified at 23dpi. Real-time qPCR analysis showed less target amplicon level or copy number of the target gene in edited plants than in wild-type (Fig. 3a). Less copy number of target gene showed less fungal biomass and supported reduced susceptibility of mutants to late blight. However, no significant difference in fungal biomass was observed among the ERF3-loss-of-function mutants. The expression profiling of pathogenesis-related genes (StPR1 and StNPR1) was carried out in wild type, and CRISPR/Cas9 targeted potato plants to analyze the impact and contribution of the StERF3 gene in the regulation of disease response. We observed the significantly high relative expression of StPR1 and StNPR1 genes in knockouts compared to their corresponding control (Fig. 3b).
Real-time quantitative PCR (RT-qPCR) analysis: a Quantification of fungal biomass using RT-qPCR analysis revealed less fungal biomass in ERF3-loss-of-function mutants (PTP2 & PTP3) compared to wild-type (WT) b Expression profiling of pathogenesis-related gene (StPR1 and StNPR1 genes) showed high relative expression of StPR1 and StNPR1 genes in mutants in comparison to their corresponding control (WT)
Discussion
Precise genome editing in vegetatively propagated highly heterozygous tetraploid potato crop was challenging. However, CRISPR/Cas9 has been successfully adopted with ease of performance to edit the potato genome [23, 40]. For StERF3 gene targeting through CRISPR/Cas9, we adopted the strategy explained by Ma et al. [24]. The ubiquitin promoter derived from Oryza sativa, OsU6a [24], was used to develop a sgRNA expression cassette. Johansen et al. [41] used potato endogenous U6 promoter to derive genome editing in potatoes and found 35% allelic efficiency. However, Ma et al. [24] explained the higher expression of sgRNA under the OsU6a promoter and its effectiveness in deriving genome editing. We chose the OsU6a promoter, with 33% full allelic editing efficiency observed with OsU6a-derived CRISPR/Cas9 gene targeting. These results were similar to Johansen et al. [41], who found 35% editing efficiency with the CRISPR/Cas9 targeting in potatoes. Promoter and sgRNA sequences were synthesized in the pUC57 vector (intermediate vector). The transcription start site of target-sgRNA contains guanine (G) [24]. The sgRNA expression cassettes containing target sites were developed through overlapping PCR and cloned into pYLCRISPR/Cas9Pubi-B binary vector using Golden gate assembly [24, 38]. The CRISPR/Cas9 expression vector contains the basta selection gene and two BsaI sites for cloning the CRISPR/Cas9-sgRNA expression cassette [24]. We transformed CRISPR/Cas9 construct into a potato through Agrobacterium-mediated potato transformation.
Potato is a tetraploid crop containing four sets of homologous chromosomes; the full allelic mutation (in all four alleles) can produce StERF3-loss-of-function mutants. Out of six (6) putative targeted plants, we obtained two (2) StERF3 full allelic edited potato plants, PTP2 and PTP3. The insertion/deletions were analyzed using IDAA. Though other strategies of mutation detection are also employed, including enzyme mismatch cleavage (EMC) assay (T4E7 and T7E1) and DSDecodeM, etc., for mutation detection, IDAA is a more effective tool for indel detection in polyploid crops [41]. EMC is low cost but produces less sensitive, less accurate, or unreliable indels (insertions/deletions) detection [42]. Moreover, larger indels may be easily detected through EMC, but single base indel is not detected in this way [43]. DSDecodeM is inefficient for detecting multiallelic-editing events in polyploidy crops. The IDAA addresses these issues and gives more precise and reliable results in polyploid crop plants. It combines amplicon labeling and capillary electrophoresis [44]. It provides a quick and direct assessment of insertions/deletions with a sensitivity of ± 1 bp.
Previously, it was demonstrated that laboratory studies through detached leaf assay for assessing late blight disease is a quick and accurate method [45, 46]. Tian et al. [14] explained P. infestans inoculation on detached leaves of RNAi transgenic plant at 4dpi and 5dpi with lesser disease symptoms than the control plant. While our study of StERF3 full genetic knockouts showed infinitesimal symptoms in the start days of post-inoculation. Even after 7dpi, only 15% of disease symptoms were observed on StERF3 edited plants. However, lab experiment does not give proper disease assessment because of fewer disease component study; thereby, greenhouse experimentation is preferred by Dorrance and Inglis [47]. Therefore, our results were further verified in a greenhouse assessment that encompassed the resistance attributes of StERF3-loss-of-function plants. The full allelic mutated plants showed significantly increased late blight resistance to potato.
To gain insight into the role of the StERF3 gene in stress response, we performed a quantitative analysis (RT-qPCR) of defense-related marker genes. The Salicylic acid (SA) mediated marker gene, PR1 (Pathogenesis related protein 1), and SA regulatory gene, NPR1 (Nonexpressor of PR1), having GCC promoter, showed remarkably higher gene expression, similar to Tian et al. [14]. Moreover, quantitative measurement of fungal biomass from infected leaves through RT-qPCR showed low expression of P. infestans specific PiO8 gene [48] that lower the establishment of fungal biomass. These results suggested knocking out the StERF3 gene that negatively regulates P. infestans resistance in potatoes, resulting in a higher expression of SA-defense marker genes (PR1 and NPR1) which control disease progression.
References
Tunio MH, Gao J, Shaikh SA, Lakhiar IA, Qureshi WA, Solangi KA, Chandio FA (2020) Potato production in aeroponics: an emerging food growing system in sustainable agriculture for food security. Chil J Agric Res 80:118–132
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194
Erwin DC, Ribeiro OK (1996) Phytophthora diseases worldwide. American Phytopathological Society Press, Saint Paul
Rahman MM, Dey TK, Ali MA, Khalequzzaman KM, Hussain MA (2008) Control of late blight disease of potato by using new fungicides. Int J Sustain Crop Prod 3:10–15
Chen Y, Etheridge N, Schaller GE (2005) Ethylene signal transduction. Ann Bot 95:901–915
Sharma MK, Kumar R, Solanke AU, Sharma R, Tyagi AK, Sharma AK (2010) Identification, phylogeny, and transcript profiling of ERF family genes during development and abiotic stress treatments in tomato. Mol Genet Genom 284:455–475
Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13:1959–1968
Ohta M, Ohme-Takagi M, Shinshi H (2000) Three ethylene-responsive transcription factors in tobacco with distinct transactivation functions. Plant J 22:29–38
Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH (2001) Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13:1035–1046
Zhou J, Tang X, Martin GB (1997) The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes. EMBO J 16:3207–3218
He P, Warren RF, Zhao T, Shan L, Zhu L, Tang X, Zhou JM (2001) Overexpression of Pti5 in tomato potentiates pathogen-induced defense gene expression and enhances disease resistance to Pseudomonas syringae pv. tomato. Mol Plant-Microbe Interact 14:1453–1457
Wang H, Huang Z, Chen Q, Zhang Z, Zhang H, Wu Y, Huang D, Huang R (2004) Ectopic overexpression of tomato JERF3 in tobacco activates downstream gene expression and enhances salt tolerance. Plant Mol Biol 55:183–192
Singh KB, Foley RC, Oñate-Sánchez L (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5:430–436
Tian Z, He Q, Wang H, Liu Y, Zhang Y, Shao F, Xie C (2015) The potato ERF transcription factor StERF3 negatively regulates resistance to Phytophthora infestans and salt tolerance in potato. Plant Cell Physiol 56:992–1005
Saurabh S, Vidyarthi AS, Prasad D (2014) RNA interference: concept to reality in crop improvement. Planta 239:543–564
Mezzetti B, Smagghe G, Arpaia S, Christiaens O, Dietz-Pfeilstetter A, Jones H, Kostov K, Sabbadini S, Opsahl-Sorteberg HG, Ventura V, Taning CNT (2020) RNAi: what is its position in agriculture. J Pest Sci 93:1125–1130
Chen SJ (2019) Minimizing off-target effects in CRISPR-Cas9 genome editing. Cell Biol Toxicol 35:399–401
Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C, Qiu JL (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotech 32:947–951
Feng C, Yuan J, Wang R, Liu Y, Birchler JA, Han F (2016) Efficient targeted genome modification in maize using CRISPR/Cas9 system. J Genet Genomics 43:37–43
Ikeda T, Tanaka W, Mikami M, Endo M, Hirano HY (2016) Generation of artificial drooping leaf mutants by CRISPR-Cas9 technology in rice. Genes Genet Syst 90:231–235
Du H, Zeng X, Zhao M, Cui X, Wang Q, Yang H, Cheng H, Yu D (2016) Efficient targeted mutagenesis in soybean by TALENs and CRISPR/Cas9. J Biotechnol 217:90–97
Ito Y, Nishizawa-Yokoi A, Endo M, Mikami M, Toki S (2015) CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem Biophys Res Commun 467:76–82
Wang S, Zhang S, Wang W, Xiong X, Meng F, Cui X (2015) Efficient targeted mutagenesis in potato by the CRISPR/Cas9 system. Plant Cell Rep 34:1473–1476
Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Lin Y, Xie Y (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8:1274–1284
Razzaq HA, Ijaz S, Awan FS, Ul HI (2021) Establishment of in vitro regeneration system for genome editing in potato cv. Lady Rosetta. Pak J Agri Sci 58:1795–1804
Kieu NP, Lenman M, Wang ES, Petersen BL, Andreasson E (2021) Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci Rep 11:1–12
Irzhansky I, Cohen Y (2006) Inheritance of resistance against Phytophthora infestans in Lycopersicon pimpenellifolium L3707. Euphytica 149:309–316
Seifu YW (2017) Reducing severity of late blight (Phytophthora infestans) and improving potato (Solanum tuberosum L.) tuber yield with pre-harvest application of calcium nutrients. Agronomy 7:69
Llorente B, Bravo-Almonacid F, Cvitanich C, Orlowska E, Torres HN, Flawia MM, Alonso GD (2010) A quantitative real-time PCR method for in planta monitoring of Phytophthora infestans growth. Lett App Microbiol 51:603–610
Yan HZ, Liou RF (2006) Selection of internal control genes for real-time quantitative RT-PCR assays in the oomycete plant pathogen Phytophthora parasitica. Fungal Genet Biol 43:430–438
Wang X, El H, Adam ALR, Daayf F (2008) Differential activation and suppression of potato defense responses by Phytophthora infestans isolates representing US-1 and US-8 genotypes. Plant Pathol 57:1026–1037
Machinandiarena MF, Lobato MC, Feldman ML, Daleo GR, Andreu AB (2012) Potassium phosphite primes defense responses in potato against Phytophthora infestans. J Plant Physiol 169:1417–1424
Lehtonen MJ, Somervuo P, Valkonen JPT (2008) Infection with Rhizoctonia solani induces defense genes and systemic resistance in potato sprouts grown without light. Phytopathology 98:1190–1198
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotech 31:827–832
Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF, Van Der Oost J (2011) Evolution and classification of the CRISPR–Cas systems. Nat Rev Microbiol 9:467–477
Tsai SQ, Zheng Z, Nguyen NT, Liebers M, Topkar VV, Thapar V, Wyvekens N, Khayter C, Iafrate AJ, Le LP, Aryee MJ (2015) GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotech 33:187–197
Xie K, Yang Y (2013) RNA-guided genome editing in plants using a CRISPR–Cas system. Mol Plant 6:1975–1983
Engler C, Kandzia R, Marillonnet S (2008) A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 3:3647
Veillet F, Perrot L, Chauvin L, Kermarrec MP, Guyon-Debast A, Chauvin JE, Nogué F, Mazier M (2019) Transgene-free genome editing in tomato and potato plants using agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int J Mol Sci 20:402
Nakayasu M, Akiyama R, Lee HJ, Osakabe K, Osakabe Y, Watanabe B, Sugimoto Y, Umemoto N, Saito K, Muranaka T, Mizutani M (2018) Generation of α-solanine-free hairy roots of potato by CRISPR/Cas9 mediated genome editing of the St16DOX gene. Plant Physiol Biochem 131:70–77
Johansen IE, Liu Y, Jørgensen B, Bennett EP, Andreasson E, Nielsen KL, Blennow A, Petersen BL (2019) High efficacy full allelic CRISPR/Cas9 gene editing in tetraploid potato. Sci Rep 9:1–7
Huang MC, Cheong WC, Lim LS, Li MH (2012) A simple, high sensitivity mutation screening using Ampligase mediated T7 endonuclease I and surveyor nuclease with microfluidic capillary electrophoresis. Electrophoresis 33:788–796
Yang Z, Steentoft C, Hauge C, Hansen L, Thomsen AL, Niola F, Vester-Christensen MB, Frödin M, Clausen H, Wandall HH, Bennett EP (2015) Fast and sensitive detection of indels induced by precise gene targeting. Nucleic acids Res 43:59–59
Andersen PS, Jespersgaard C, Vuust J, Christiansen M, Larsen LA (2003) Capillary electrophoresis-based single strand DNA conformation analysis in high-throughput mutation screening. Hum Mutat 21:455–465
Foolad MR, Sullenberger MT, Ashrafi H (2015) Detached-leaflet evaluation of tomato germplasm for late blight resistance and its correspondence to field and greenhouse screenings. Plant Dis 99:718–722
Majeed A, Muhammad Z, Rehmanullah Z, Haq CI (2019) Comparative assessment of detached leaflet and tuber disc assays for studying the aggressiveness of different isolates of Phytophthora Infestans. Pak J Bot 51:699–703
Dorrance AE, Inglis DA (1997) Assessment of greenhouse and laboratory screening methods for evaluating potato foliage for resistance to late blight. Plant Dis 81:1206–1213
Wang C, Gao H, Chu Z, Ji C, Xu Y, Cao W, Zhou S, Song Y, Liu H, Zhu C (2021) A nonspecific lipid transfer protein, StLTP10, mediates resistance to Phytophthora infestans in potato. Mol Plant Pathol 22:48–63
Acknowledgements
This work was supported by Dr. Siddra Ijaz Laboratory, “Molecular Biology of Plant Disease Resistance Lab” of Centre of Agricultural Biochemistry and Biotechnology (CABB), University of Agriculture Faisalabad, and Dr. Imran Ul Haq Laboratory, “Fungal Molecular Biology Lab,” Department of Plant Pathology, University of Agriculture Faisalabad. We acquired the characterized culture of Phytophthora infestans from the Fungal Molecular Biology Laboratory-Culture Collection (FMB-CC-UAF) affiliated member of “The World Data Centre for Microorganisms (WDCM).”
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
SI, IUH, and IAK conceived, designed, and developed the experiment. HAR experimented, and SI and IUH supervised the experimentation. HAR, SI, IUH, and IAK analyzed and interpreted the data and drafted and revised the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to publish
The authors affirm the publication of the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Razzaq, H.A., Ijaz, S., Haq, I.U. et al. Functional inhibition of the StERF3 gene by dual targeting through CRISPR/Cas9 enhances resistance to the late blight disease in Solanum tuberosum L.. Mol Biol Rep 49, 11675–11684 (2022). https://doi.org/10.1007/s11033-022-07958-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07958-1