Abstract
Background
Mesenchymal stem cells (MSCs) from human adipose tissue and bone marrow have a great potential for use in cell therapy due to their ease of isolation, expansion, and differentiation. Our intention was to isolate and promote in vitro expansion and differentiation of MSCs from human adipose and bone marrow tissue into cells with a pancreatic endocrine phenotype and to compare the potency of these cells together.
Methods and results
MSCs were pre-induced with nicotinamide, mercaptoethanol, B-27 and b-FGF in L-DMEM for 2 days and re-induced again in supplemented H-DMEM for another 3 days. Expression of five genes in differentiated beta cells was evaluated by Real-time PCR and western blotting and the potency of insulin release in response to glucose stimulation was evaluated by insulin and C-peptide ELISA kit. The differentiated cells were evaluated by immunocytochemistry staining for Insulin and PDX-1. Quantitative RT-PCR results showed up-regulation of four genes in differentiated beta-islet cells (Insulin, Ngn-3, Pax-4 and Pdx-1) compared with the control. Western blot analysis showed that MSCs cells mainly produced proinsulin and insulin after differentiation but nestin was more expressed in pre-differentiated stem cells. Glucose and insulin secretion assay showed that insulin levels and C-peptide secretion were significantly increased in response to 10 mM glucose.
Conclusions
Our study showed that both adipose and bone marrow stem cells could differentiate into functional beta-islet cells but it seems that adipose stem cells could be a better choice for treatment of diabetes mellitus according to their higher potency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin dependent diabetes mellitus (IDDM) is one of the most common chronic diseases that is caused usually by a defect in insulin-secreting pancreatic β-islet cells due to self-immune attack of T-lymphocytes [1]. Although daily insulin injections as main treatment of IDDM can control blood glucose level, exogenous insulin does not completely imply the physiology of natural insulin secretion, therefore it may lead to complications such as severe hypoglycemia and coma [2,3,4]. The replacement of beta cells by pancreatic transplant to the patient is another method which is associated with major problems, including limitation in the pancreatic donor and immunologic rejection of the transplant [5,6,7]. Several sources of stem cells with different potency and function has been found. Pluripotent cells are embryonic stem cells derived from the inner cell mass of the embryo. This type of cells is able to make cells from all three basic germ layers (endoderm, mesoderm, and ectoderm). Multipotent stem cells have ability to differentiate into cell types derived from a single germ layer such as mesenchymal stem cells (MSCs) which form adipose tissue, bone, and cartilage. Oligopotent stem cells such as myeloid cells have restricted potency to differentiate and they can form terminally differentiated cells of a specific tissue. In fact, over the years, various types of stem cells, including embryonic stem cells (ESCs) [8, 9] and mesenchymal stem cells (MSCs) [10], have the ability to differentiate into insulin producing cells. Due to some controversial processes such as destroying human embryo in its early stages of development, using embryonic stem cells (ESCs) involved ethical issues. MSCs are multipotent stem cells that have been introduced as new sources of insulin-producing cells and do not have the ethical concerns related to ESCs [11, 12]. MSCs have ability to homing in damaged tissues [13], and naturally produce cytokines and growth factors, such as IL-6, M-CSF, IL-10, HGF, TGF-β and PGE2 that prevent apoptosis of beta-pancreatic cells and increase their reengineering and angiogenesis [14,15,16]. Furthermore, through various immunomodulatory pathways, in a non-MHC restricted manner these cells can suppress active immune reactions and have emerged as an interesting therapeutic option [17, 18]. CD antigens or cluster of differentiation cell surface markers were used to figure out characteristics of stem cells and they can be used to exclude undifferentiated cells from other populations. The most common surface markers used to describe MSCs are positive surface markers such as CD29, CD44, CD54, CD105 whereas negative antigens are CD11, CD18, CD19, and CD45. These markers are different and naturally expressed on stem cell precursors, endothelial or epithelial cells, natural killer cells and etc. MSCs can be obtained from variety of sources including bone marrow, adipose tissue, umbilical cord, endometrium, peripheral blood monocytes, and liver cells [19]. But the adipose tissue and bone marrow have preferable advantage in terms of availability and abundance. The clinical applicability of bone marrow stem cells (BMSCs) may be limited due to the invasive procedure required for sample collection. On the other hand, for adipose stem cells (ADSCs) liposuction aspirates are widely available and many studies reported that adipose tissue contain a significantly greater number of mesenchymal stem cells than bone marrow per unit weight and should not be wasted. Collectively, the properties of ADSCs render them well suited for applications in regenerative medicine and can provide a viable alternative for BMSCs. In this study we aimed to compare the potency of human ADSCs and BMSCs to differentiate into functional beta-islet pancreatic cells for in vitro insulin production. Although there have been many reports of successful induction of adipose and bone marrow mesenchymal stem into insulin producing cells but more data and investigation needed to compare adipose and bone marrow stem cell together in human for potential differentiation to beta islet like cells. So, this study could be a controversial evaluation for both of these two human cell types and it may offer a potent and effective therapeutic approach in cell therapy.
Materials and methods
Isolation and culture of human adipose and bone marrow stem cells
Human adipose tissue was collected by needle biopsy or liposuction aspiration at surgery ward of Shariati Hospital, Tehran, Iran. The protocol was performed in accordance with the Declaration of Helsinki and approved by the Cellular Transplantation Ethics Committee of Iranian public hospitals and also Zabol University of Medical Sciences ethics committee. All participants were informed about the proposed intervention and we emphasized their roles in our decision-making process and also all of the participants were informed about the risks of the proposed intervention. Adipose tissue was washed extensively with sterile phosphate-buffered saline (PBS) and 1% penicillin–streptomycin to remove red blood cells. Washed tissue fragments were placed in a sterile tissue culture plate with 0.075% Collagenase Type I (Sigma-Aldrich, USA) prepared in PBS containing 2% penicillin–streptomycin for tissue digestion. After incubation for 30 min at 37 °C and 5% CO2, collagenase activity was neutralized by adding 5 ml of α-MEM containing 20% heat inactivated fetal bovine serum (Gibco, Thermo Fisher Scientific, USA) and centrifuged in 4000×g for 10 min. To lyse remaining red blood cells, the pellet was re-suspended in 160 mM NH4Cl at room temperature for 10 min and then centrifuged at 1200×g for 10 min to pellet the MSC-rich dense cell fraction. Afterward, the collected cells were filtered through 100-μm cell strainer (Grainer, USA) and incubated for 48 h at 37 °C in 5% CO2 in culture medium with serum and antibiotics. Bone marrow was isolated from one patient in the hematology ward of Shariati hospital, Tehran, Iran under aseptic condition and dispersed into single cell suspension, L-DMEM cells were cultured in a density of 1.5 × 105/cm2 in alpha-MEM (Sigma, USA), 10% FBS, 1% penicillin–streptomycin and incubated at 37 °C and 5% CO2 for 3 days and later the medium was refreshed every 48 h until confluence was reached.
Characterization of cultured adipose and bone marrow stem cells
Flow cytometry analysis was used to determine positive (CD29 and CD105) and negative (CD18 and CD34) markers. The cells were washed with PBS containing 2% FBS. FITC conjugated anti human CD18, CD29, CD34 and CD105 monoclonal antibodies (Cell Signaling Technology, USA). The fluorescence of the cells was immediately determined by a FACS-Calibur flow cytometry (Becton Dickinson, San Jose, USA). The FACs flow cytometry data were analyzed using freely available WinMDI software ver.2.9 (http://facs.scripps.edu/software.html).
Differentiation of MSCs into functional islet beta cells
At passage 3, adipose and bone marrow derived stem cells were induced to differentiate into functional pancreatic cells. According to previous reports about beta cell differentiation, we used design of experiment (DOE) to find cause-and-effect relationships and to determine the relationship between factors affecting our differentiation process. Among our designed experiments we decided to choose our best quality method. Therefore, cells were plated in L-DMEM medium supplemented with 20% FBS and 1% penicillin–streptomycin for 10 days at 37 °C and 5% CO2. Second, the cells were cultured in differentiation medium containing L-DMEM supplemented with 10% FBS, 2% B27, b-FGF, and 10 mM nicotinamide (all purchased from Sigma-Aldrich) for 7 days and finally, the cells were cultured in induction medium containing H-DMEM supplemented with 2% B27, b-FGF, 10 ng/ml activin A, 10 mM nicotinamide, and 1 mM 2-mercaptoethanol (Sigma-Aldrich) for another 6 days. Cells cultured in L-DMEM medium without an inducer were used as controls.
Real-time PCR analysis
Total RNA was isolated utilizing the high pure RNA isolation kit (Roche, Mannheim, Germany), according to the manufacturer’s instructions. For synthesis of first strand cDNA, total RNA (0.2 µg) from each sample, before and after differentiation was used in a random primed reverse transcription (RT) reaction utilizing the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, Lithuania). Quantitative real-time PCR using custom made gene specific primers (Invitrogen, Carlsbad, CA) was performed to evaluate mRNA expression before and after differentiation. Target genes and the primer sequences used for qRT-PCR were described in Table 1. Quantitative real-time PCR (qPCR) reactions were carried out using the HOT MOLPol EvaGreen qPCR Mix Plus (Solis BioDyne, Estonia) in a total volume of 20 µl on ABI Prism 7900 Sequence Detector System (Applied Biosystems) according to the manufacturer’s instructions. The following thermal profile was used: One cycle at 95 °C for 15 min and 40 cycles of denaturation at 95 °C for 15 s, annealing at 60–65 °C for 20 s and elongation at 72 °C for 20 s. All qRT-PCR results were normalized to GAPDH gene as the reference gene. The results were expressed as the target/reference ratio of differentiated cells divided by the target/reference ratio of the calibrators (undifferentiated control samples) [20].
Western blot analysis
Protein expression of nestin and insulin as two markers of ADSCs and BMSCs before and after differentiation to beta-islet cells was evaluated by western blot analysis. Cells before and after differentiation were homogenized in a lysis buffer containing 50 mM Tris (pH 7.4), 2 mM EDTA, 2 mM EGTA, 2 mM NaF, 1 mM Na3VO4, 10 mM B-glycerophosphate, 10 mM 2-mercaptoethanol, sodium desoxycholate, and 1 μl protease and phosphatase inhibitor cocktail and 1 mM phenylmethanesulfonyl. Proteins were separated on a 12% SDS-PAGE under reducing conditions at a constant voltage (120 v) for 1:45 h. The separated proteins were electro-transferred to the polyvinylidene difluoride (PVDF, Bio Rad) membrane. To block non-specific binding sites, membranes were incubated in 5% non-fat dry milk in TBST buffer (20 mM Tris–HCl pH 7.5, 137 mM NaCl, 0.5% Tween 20) for 1 h at room temperature. After blocking, the membranes were incubated overnight at 4 °C with primary antibodies against nestin (1/1000), insulin (1/1000) and β-actin (1/1000). The blots were then incubated for 1 h at room temperature with a HRP conjugated secondary antibody (1:3000) (All antibodies were purchased from Abcam, USA). Protein bands were visualized using the enhanced chemiluminescence method (ECL detection kit, Pierce Biotech, Inc.) and Alliance 4.7 Gel Doc (UVTEC, Cambridge). Band intensities were quantified using UV BAND image analysis software (UVTEC, Cambridge).
Insulin and C-peptide secretion assay
To determine whether the differentiated ADSCs and BMSCs were responsive to glucose stimulation, insulin release was measured after exposure to high glucose level using human insulin ELISA kit (Abcam, Cambridge, UK). After differentiation of adipose and bone marrow stem cells, they were washed twice with PBS and incubated in low glucose DMEM culture media for 3 h and then the medium was collected and stored at − 20 °C. In next step, cells were washed twice with PBS and incubated for 3 h in high glucose DMEM culture media and again, the medium was collected and stored at − 20 °C. C-peptide content of culture medium was determined by using C-peptide ELISA kits (Abcam, Cambridge, UK) according to the manufacturer’s instructions. Briefly, 50 µl of samples was added to 100 µl C-peptide HRP and biotin conjugate in a 96-well plate and incubated for 2 h at room temperature. After washing three times with 300 µl washing solution, 100 µl tetramethylbenzidine (TMB) was added and incubated for 15 min at room temperature in the dark. Then, 100 µl stop solution was added to the wells and mixed for 10 s in shaker. Finally, the absorbance of the sample at 450 nm within 5 min of addition of the stop solution against a reference wavelength of 620–630 nm was measured.
Immunofluorescence assay
The differentiated cells were further characterized by showing specific protein localization by immunocytochemistry (ICC) technique. Differentiated adipose and bone marrow stem cells were fixed in 4% paraformaldehyde, permeabilized using chilled 100% methanol for 10 min, blocked with 5% normal goat serum for 60 min at RT and incubated overnight in the primary antibodies against Insulin (#ab181547, Abcam, Cambridge, MA, UK) and Pdx1 (#ab134150, Abcam) at 4 °C. Subsequently, the cells were incubated with biotinylated secondary antibody for 2 h at RT. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI, 0.1 μg/ml) (blue color) (Vector Laboratories, Burlingame, CA), and the resultant immunofluorescence was viewed under a fluorescent microscope (Leica Microsystems GmbH, Germany). MetaXpress (Molecular Devices, Sunnyvale, CA, USA) software was used to determine the proportion of insulin-positive cells.
Statistical analysis
The results obtained from the experiment were presented as means ± SD. Each experiment was repeated 3 times. Student’s t-test was used to compare the means of two groups. P value of less than, or equal to 0.05 was considered to be statistically significant. The statistical analysis was performed using the SPSS 16.0 software program (Statistics Package for Social Sciences, SPSS Inc. Chicago, Illinois, USA).
Results
Characterization and differentiation of adipose and bone marrow stem cells
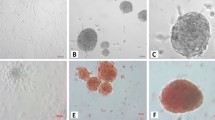
After ADSC and BMSC isolation and cell culture process, they rapidly proliferated and were confluent after 4–5 days of stimulation. Flow cytometry method was used to analyze the expression pattern of cell surface markers and the results showed that these cells displayed CD29 and CD105 as positive surface markers but they did not express CD18 and CD34 as negative surface markers (Fig. 1). During differentiation, morphological changes of ADSCs and BMSCs to beta-cell-like cells were investigated by an invert microscope. Before differentiation, ADSCs and BMSCs exhibited a fibroblast and spindle-like morphology but after differentiation they formed clusters with a round shape morphology.
Gene expression analysis of genes involved during ADSCs and BMSCs differentiation
To determine whether the adipose and bone marrow stem cells had differentiated to beta-cell-like cells, the expression of nine genes related to the pancreatic endocrine development and function were evaluated by real-time PCR analysis. Pdx-1 (pancreas duodenum homeobox-1) a pancreatic master gene; Pax-4 and Ngn-3, two genes important in the early pancreatic development; NeuroD1, a gene that regulates expression of the insulin gene and contributes to the regulation of several cell differentiation pathways like endocrine islet cells of the pancreas; Insulin-1 gene encodes insulin specially after differentiation of MSCs to beta-cell-like cells and finally, Nestin is a gene required for survival, renewal and mitogen-stimulated proliferation of neural progenitor cells but also important as a pre-marker for islet cell differentiation. NKX6.1 gene is necessary to confer beta cell identity to differentiating endocrine precursors in the embryo and raising the possibility to maintain the differentiated state of mature beta cells. The prohormone convertase PC1/3 is endopeptidase found in islet cells that convert the prohormones to mature hormone. Islet cells express other secreted peptides such as islet amyloid polypeptide (IAPP). In adult islet, IAPP is produced by β-cells and some δ-cells and is expressed in the developing endocrine pancreas. According to Fig. 2a that is related to gene expression after differentiation, except for Pdx-1 and Nestin, a significant difference (For Ngn-3; P < 0.001, For Insulin; P < 0.01 and for Pax-4, NKX6.1, PC1/3, IAPP and NeuroD1; P < 0.05) in gene expression between adipose and bone marrow stem cells was obviously cleared. For example, Ngn-3 was 10.9 times more expressed in adipose stem cells compare to bone marrow stem cells. Figure 2b Shows that except Nestin gene that was downregulated after differentiation, all of the other genes were upregulated in both type of stem cells. According to Fig. 3, gene expression of all genes mentioned previously was evaluated before and after differentiation in adipose and bone marrow stem cells. Results showed that Nestin gene expression was significantly (P < 0.001) decreased after differentiation, on the other hand the expression of genes involved in differentiated state of adult and mature beta cells (NKX6.1, PC1/3 and IAPP) was significantly increased (P < 0.01).
Western blot analysis for ADSCs and BMSCs
Western blot analysis was performed to test insulin or nestin protein expressions in MSCs. It was expected that after differentiation process, stem cells produce insulin but in different quantity in ADSC and BMSCs. After differentiation culture, the cells mainly produced proinsulin and insulin with detectable bands corresponding to 7 and 5.5 kDa, respectively. However, MSCs cultured in media alone (before differentiation), showed no (pro)-insulin reactivity. Nestin as an important pre-marker for islet cell differentiation, and its expression was tested. Western-blotting using anti-nestin showed a single band at 220 kDa in pre-differentiated stem cells while nestin expression in differentiated islet-like cells was decreased significantly. Our results showed that insulin and nestin proteins were more expressed in adipose stem cells compared to bone-marrow stem cells (Fig. 4).
a Western blot analysis of insulin and nestin proteins in adipose stem cells and b in bone-marrow stem cells before and after differentiation to beta-cells. Insulin and nestin were quantified with densitometry of immune-reactive bands and these proteins were expressed as average density to β-actin in c adipose stem cells and d in bone-marrow stem cells
Glucose-induced insulin and C-peptide secretion analysis
ADSCs and BMSCs after differentiation were checked because of their viability. The viability of ADSCs and BMSCs at the end of differentiation were 95 and 93%, respectively. Insulin and C-peptide secretion in response to different concentration of glucose was assessed in differentiated cells. The amount of insulin and C-peptide secretion in adipose and bone marrow stem cells, before and after exposure of these cells with different concentration of glucose (5 mM, 10 mM and 20 mM) were evaluated and results were shown in Table 2. According to the results (Table 2), insulin levels and C-peptide secretion were significantly increased in response to 10 mM glucose but an increase of glucose concentration to 20 mM did not elevate insulin and C-peptide secretion significantly more.
Immunofluorescence assay for Insulin and PDX-1 proteins
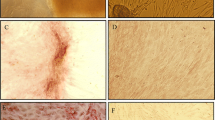
To identify all nuclei and assess for production of PDX-1 and insulin, we used the Multi Wavelength Cell Scoring module in MetaXpress. Data are expressed as the percentage of differentiated cells producing insulin and PDX-1 among total cells as defined by DAPI nuclear staining. Immunofluorescence analysis detected cytoplasmic localization of Insulin and PDX-1 protein in differentiated adipose and bone marrow stem cells on day 25 (Fig. 5). The results showed that the proportion of insulin-positive cells to total cells ranged between 2.3 and 7.1% in adipose stem cells and between 1.1 and 2.5% in bone marrow stem cells. Our results showed significant difference between adipose and bone marrow stem cells to produce insulin (P < 0.05). But for PDX-1, this proportion ranged between 1.1 and 2.9 for adipose and between 0.7 and 1.7 for bone marrow stem cells and results showed that no significant differences by statistical analysis (P > 0.05).
Immunofluorescence of differentiated adipose (A1–A3) and bone marrow (B1–B3) stem cells. Immunofluorescence staining: Insulin (Red), PDX-1 (Green) and nucleus (DAPI, Blue). The red color depicts Insulin protein which is expressed in the cytoplasm and the green color depicts PDX-1 protein which is located in the nucleus and cytoplasm, although mostly in the cytoplasm. Although the control cells (undifferentiated cells) were negative for insulin and PDX-1 staining. All images were acquired with a Leica Microsystem fluorescent microscope. Magnification, ×400. (Color figure online)
Discussion
Treatment of diabetes mellitus by pancreatic islet transplantation combined with severe shortage of donor pancreas, have forced scientists to find a new prospect to generate insulin-secreting beta cells from various progenitor populations. Because of self-renewal and multipotent characteristics, multipotent stem cells (MSCs) have attracted a great deal of attention as potential candidates for transplantation [21]. MSCs have been isolated from different adult tissues, such as bone marrow, adipose, liver, lung, etc. Adipose and bone marrow derived MSCs have a good proliferative capacity, as well as the advantage of being autologous sources. Due to some clinical applicability of BMSCs, it may prefer to use adipose stem cells instead of bone marrow stem cells [22, 23]. On the other hand, liposuction aspirates are widely available and attractive features, including availability, abundance, 500-fold higher frequency of mesenchymal stem cells (MSCs) and higher immunomodulatory capacity when compared to bone marrow-derived counterparts [24]. We used flowcytometry method to characterize adipose and bone marrow stem cell from other cell types. CD105 is a positive surface marker in adipose and bone marrow stem cells but it has been shown that CD105, function as a TGF-β1 co-receptor and TGF-β1 is an inhibitor of mesenchymal cell osteogenic differentiation. Therefore, cells with lower CD105 expression and thus diminished TGF-β1 signaling would be expected to more rapidly differentiate to osteoblasts [25]. Our flowcytometric results showed that CD105 is positive and we concluded that our MSCs not differentiated to osteoblast and bone formation. In this study we used nicotinamide, beta mercaptoethanol, B27 and b-FGF in DMEM media to differentiate MSCs to beta-cell-like cells. Nicotinamide as an inducer of endocrine differentiation could increase the mitotic indices of beta cells. It was previously reported that using low glucose medium (5 mM) with nicotinamide supplementation together, increased insulin content approximately 20-fold. It seems that β-mercaptoethanol could increase the potency of nicotinamide [26, 27]. B27 supplement is a mix of hormones and other molecules that were empirically added to media to promote growth and proliferation of cells. During pancreatic islet development, activin A as a member of the transforming growth factor beta superfamily, has been shown to induce embryonic stem cells to pancreatic precursors [28]. According to results of our study, after differentiation of MSCs to beta-cell-like cells, the cells lost nestin expression but on the other hand, they turned on mRNA expression of transcription factors such as Pax-4, Pdx-1, NeuroD1 and Insulin. These genes play an important role in the development and formation of pancreatic beta-cell-like cells. In this study, we observed that except Pdx-1 and nestin, there were significant differences between expression level of other genes in adipose and bone marrow stem cells (P < 0.05 for Pax-4 and NeuroD1; P < 0.01 for Insulin and P < 0.001 for Ngn3). Our results clarified that adipose stem cells expressed significantly more levels of Pax-4, NeuroD1, Insulin and Ngn-3 compared to bone marrow stem cells but the expression of NKX6.1, PC1/3 and IAPP genes was not significantly different. Many reports have shown that exendin-4 upregulated Pdx-1 during ß-cell regeneration. Therefore, low expression of Pdx-1 gene in our study in comparison with other genes could be the result of not using of exendin-4 in our media [29]. Nestin was regarded as an important pre-marker for islet cell differentiation and this marker is transiently expressed in early stages in many tissues, including pancreas [30]. So, down regulation of nestin after ADSCs and BMSCs differentiation to beta-cell-like cells is a logical event. The results of western blot analysis for insulin and nestin expression in our experiment validated the results of qPCR. Considering nestin expression in pre-differentiated MSCs, MSCs might differentiate into pancreatic islet-like cells through intermediate neurocyte stage. NKX6.1, PC1/3 and IAPP genes involved in developing of endocrine pancreas and their gene expression were investigated to confirm the ability of functional beta islet-like cells to produce insulin. Results of our study showed a significant increase (P < 0.01) in expression of these related genes after differentiation to beta-like cells. C-peptide is an indicator of the insulin secretion function of pancreatic islet cells and an increased level of C-peptide is proportional to insulin secretion [31]. Results of insulin and C-peptide secretion analysis showed that they could respond to different glucose concentrations, among which 20 mM glucose stimulated a significant increase in release of insulin and C-peptide in both ADSCs and BMSCs. However, there is no significant differences between insulin and C-peptide secretion in response to 10 mM and 20 mM glucose concentration. Several studies reported that beta cells replicated more when they were induced by increasing glucose levels but at extremely hyperglycemic conditions, beta cell replication was reduced or stopped [32]. On the other hand, according to the results of immunofluorescent assay the presence of PDX-1 protein in nucleus and cytoplasm and also insulin protein in the cytoplasm of both adipose and bone marrow stem cells may confirms the intrinsic synthesis of insulin in our differentiated stem cells. More expression rate of insulin and PDX-1 protein in adipose stem cells compare to bone marrow stem cells could verified the potency of adipose stem cells in treatment of diabetes mellitus.
In conclusion, our study showed that both adipose and bone marrow stem cells had advantage of being autologous sources and they could differentiate into functional beta-cell-like cells but according to the results of this study, by comparing these two kinds of cells, it seems that adipose stem cells could be a good choice for treatment of diabetes mellitus according to their higher potency. Further research is required in terms of in vivo evaluation of glucose levels in diabetic model as well as their immune reaction. To prove the functionality of stem cell-derived beta-cell-like cells, it’s required to transplant these differentiated cells into streptozocin-mediated diabetic mice. Such analyses would confirm the nature of the differentiated insulin+ cells.
References
Carlsson P-O, Schwarcz E, Korsgren O, Le Blanc K (2015) Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 64(2):587–592
Vanikar A, Dave S, Thakkar U, Trivedi H (2010) Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: a novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. https://doi.org/10.4061/2010/582382
Karges B, Kapellen T, Wagner VM, Steigleder-Schweiger C, Karges W, Holl RW et al (2017) Glycated hemoglobin A1c as a risk factor for severe hypoglycemia in pediatric type 1 diabetes. Pediatr Diabetes 18(1):51–58
Brod M, Christensen T, Thomsen TL, Bushnell DM (2011) The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health 14(5):665–671
Vergani A, Fotino C, D’Addio F, Tezza S, Podetta M, Gatti F et al (2013) Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes 62(5):1665–1675
Bertuzzi F, Ricordi C (2007) Beta-cell replacement in immunosuppressed recipients: old and new clinical indications. Acta Diabetol 44(4):171–176
Scharfmann R (2003) Alternative sources of beta cells for cell therapy of diabetes. Eur J Clin Investig 33(7):595–600
Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH et al (2014) Generation of functional human pancreatic β cells in vitro. Cell 159(2):428–439
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A et al (2014) Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 32(11):1121–1133
Chao KC, Chao KF, Fu YS, Liu SH (2008) Islet-like clusters derived from mesenchymal stem cells in Wharton’s jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS ONE 3(1):e1451
Aguayo-Mazzucato C, Bonner-Weir S (2010) Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol 6(3):139–148
Santana A, Enseñat-Waser R, Arribas MI, Reig J, Roche E (2006) Insulin-producing cells derived from stem cells: recent progress and future directions. J Cell Mol Med 10(4):852–868
Valina C, Pinkernell K, Song Y-H, Bai X, Sadat S, Campeau RJ et al (2007) Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J 28(21):2667–2677
Rahavi H, Hashemi SM, Soleimani M, Mohammadi J, Tajik N (2015) Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res. https://doi.org/10.1155/2015/878535
Soleymaninejadian E, Pramanik K, Samadian E (2012) Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol 67(1):1–8
Mabed M, Shahin M (2012) Mesenchymal stem cell-based therapy for the treatment of type 1 diabetes mellitus. Curr Stem Cell Res Ther 7(3):179–190
Nauta AJ, Fibbe WE (2007) Immunomodulatory properties of mesenchymal stromal cells. Blood 110(10):3499–3506
McIntosh KR, Frazier T, Rowan BG, Gimble JM (2013) Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert Rev Clin Immunol 9(2):175–184
Gabr MM, Zakaria MM, Refaie AF, Abdel-Rahman EA, Reda AM, Ali SS et al (2017) From human mesenchymal stem cells to insulin-producing cells: comparison between bone marrow-and adipose tissue-derived cells. BioMed Res Int. https://doi.org/10.1155/2017/3854232
Entezari Heravi R, Hadizadeh F, Sankian M, Tavakol Afshari J, Behravan J (2012) Cyclooxygenase-2 inhibition by novel bisaryl imidazolyl imidazole derivatives increases Bax/Bcl-2 ratio and upregulates caspase-3 gene expression in caco-2 colorectal cancer cell line. Genes Genomics 34(2):199–220
Zhang YH, Wang HF, Liu W, Wei B, Bing LJ, Gao YM (2009) Insulin-producing cells derived from rat bone marrow and their autologous transplantation in the duodenal wall for treating diabetes. Anat Rec 292:728–735
Päth G, Perakakis N, Mantzoros CS et al (2019) Stem cells in the treatment of diabetes mellitus—focus on mesenchymal stem cells. Metabolism 90:1–15
Tetsuya I, Kazunori T, Yuma W, Luping G, Katsuki M et al (2020) Adipose tissue from type 1 diabetes mellitus patients can be used to generate insulin-producing cells. Pancreas 49:1225–1231
Strem BM, Hicok KC, Zhu M et al (2005) Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54(3):132–141
Levi B, Wan DC, Glotzbach JP, Hyun J, Januszyk M, Montoro D (2011) CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor β1 (TGF-β1) signaling. J Biol Chem 286:39497–39509
Soria B, Roche E, Berna G, Leon-Quinto T, Reig JA, Martin F (2000) Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 49:157–162
Chen LB, Jiang XB, Yang L (2004) Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol 10(20):3016–3020
Bonfanti P, Nobecourt E, Oshima M, Albagli-Curiel O, Laurysens V, Stangé G et al (2015) Ex vivo expansion and differentiation of human and mouse fetal pancreatic progenitors are modulated by epidermal growth factor. Stem Cells Dev 24:1766–1778
Mahboob VS, Subrahmanyam G (2017) Exendin-4, nicotinamide and β-mercaptoethanol based induction differentiation of Wharton’s jelly tissue mesenchymal stem cells in to Pdx-1 mediated insulin producing cells. J Cell Tissue Res 17(2):6205–6215
Milanesi A, Lee JW, Xu Q, Perin L, Yu JS (2011) Differentiation of nestin-positive cells derived from bone marrow into pancreatic endocrine and ductal cells in vitro. J Endocrinol 209:193–201
Li Y, Wang F, Liang H, Tang D, Huang M, Zhao J et al (2021) Efficacy of mesenchymal stem cell transplantation therapy for type 1 and type 2 diabetes mellitus: a meta-analysis. Stem Cell Res Ther. https://doi.org/10.1186/s13287-021-02342-5
Ben-Othman N, Courtney M, Vieira A, Pfeifer A, Druelle N, Gjernes E et al (2013) From pancreatic islet formation to beta-cell regeneration. Diabetes Res Clin Pract 101(1):1–9
Acknowledgements
This work was supported by the Research Committee of Zabol University of Medical Sciences, Zabol, Iran. We would like to give special thanks to Medical Biotechnology Center of CinnaGen Co. for their support to publish this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eydian, Z., Mohammad Ghasemi, A., Ansari, S. et al. Differentiation of multipotent stem cells to insulin-producing cells for treatment of diabetes mellitus: bone marrow- and adipose tissue-derived cells comparison. Mol Biol Rep 49, 3539–3548 (2022). https://doi.org/10.1007/s11033-022-07194-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07194-7