Abstract
Background
The liver has a solid inbuilt antioxidant defense system to regulate oxidative stress. However, exposure to an excessive level of ROS causes liver injury. This study examined the cytoprotective effect of neoxanthin, a xanthophyll antioxidant molecule isolated from Solanum trilobatum in stress-induced HepG2 cells.
Methods and results
The cytotoxic effect of H2O2 and cytoprotective potential of β-carotene, lutein, and neoxanthin was analyzed by WST-1 assay. The intracellular ROS level and mitochondrial membrane potential (MMP) were measured using DCFH-DA (2′, 7′-dichlorofluorescin diacetate) and JC-10 MMP assay. The expression of anti-oxidant and apoptotic markers was measured by western blot analysis. Neoxanthin pretreatment exhibited better protection than β-carotene and lutein against cell death caused by H2O2. It significantly arrested H2O2-mediated elevation of intracellular ROS levels and protected MMP. The intracellular antioxidant enzymes HO-1 and SOD-2 were upregulated by neoxanthin pretreatment. Neoxanthin also activated the protein expression of redox-sensitive transactivation factors, Nrf2 and NF-kB. The cytoprotective effect of neoxanthin was associated with increased expression of the anti-apoptotic protein, Bcl-2 and decreased pro-apoptotic protein Bax.
Conclusions
For the first time, our results demonstrate that neoxanthin offers adequate protection against stress-mediated cytotoxicity in hepatocytes by activating the intracellular antioxidant defense system and blocking apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver, the major detoxifying organ of the human body, comprises specialized cells called hepatocytes. The liver receives the priority cause for oxidative stress because of energy metabolism and toxins removal that produce plenty of reactive oxygen species (ROS), leading to liver damage if not removed from the system. Oxidative stress is the primary cause of many chronic disorders, including liver diseases, due to the accumulation of excessive intracellular ROS and an imbalance in the anti-oxidative defense mechanisms [1]. Liver diseases take the lead in the cause of death in many countries, and evidence showed an increased incidence rate of liver diseases in the United States and many countries of Europe and Asia [2]. According to previous studies, hepatitis infection and non-alcoholic fatty liver disease are the primary cause of liver cirrhosis, resulting in hepatocellular carcinoma and subsequently death if left untreated [3]. Severe oxidative stress and failure of the antioxidant system lead to disruption of redox balance and biological homeostasis.
The ROS under a controlled level does not harm the system as it is scavenged by membrane metalloproteases such as superoxide dismutase (SOD-2) and heme oxygenase (HO-1). However, excessive ROS leads to lipid peroxidation, DNA damage, and even cell death [4]. Superoxide anion (O2−) and non-radical hydrogen peroxide (H2O2) are the critical molecules primarily involved in liver damage [5]. Superoxide anion is produced from an unstable singlet electron which subsequently forms H2O2 by antioxidant enzymes. H2O2 lacks an ionic charge and freely diffuses across cell membrane, causing intracellular damage.
Moreover, H2O2 is a byproduct of mitochondrial electron chain reaction, and NADPH is the primary enzyme that generates H2O2. It was reported that a total of 31 intracellular enzymatic reactions release H2O2 as a byproduct of metabolic response [6]. H2O2 up to 0.1 mM concentration is scavenged by endogenous antioxidant machinery, thus maintaining a steady intracellular state devoid of oxidative stress. But, a level above 0.1 mM disrupts the redox signaling by elevated oxidative stress [7]. H2O2 at 0.2 mM concentration was reported to induce apoptosis, and a higher level (2 mM) cause necrosis [8]. Through silencing or activating specific genes or transcription factors that regulate the intracellular antioxidant system, we could maintain cellular redox balance and prevent the cells from damage caused by oxidative stress [9, 10]. Alternatively, dietary antioxidants that activate antioxidant signaling cascade in cells could be presumed to impart protection against oxidative damage.
Carotenoids are a group of tetraterpenoids synthesized in all photosynthetic organisms and some non-photosynthetic organisms such as yeast, molds, and bacteria [11]. Naturally existing colorful fruits, vegetables, bacteria, and even marine algae are rich sources of diverse carotenoids with different structures and functions [12,13,14]. Among other phytochemicals, carotenoids play a pivotal role in mitigating many metabolic abnormalities such as insulin resistance, glucose intolerance, and oxidative stress [15, 16]. Though humans cannot synthesize carotenoids de-novo, their presence in the human system is through the dietary intake of carotenoids-rich foods. The intestinal absorption of carotenoids and their transportation into blood circulation is through a sequential mechanism similar to dietary fat [17].
In plants, xanthophyll carotenoids act as a first-line defense in removing excess ROS generated as byproducts during respiration and photosynthesis, and protecting the cells from photo-oxidative damage. They inhibit oxidation reactions as they get effectively oxidized by scavenging excited ROS in the cellular system. According to the earlier reports, anti-cancer/anti-invasive efficacy of carotenoids varies depending on their conjugated double bonds and functional groups [12, 18]. In this view, neoxanthin possesses a characteristic 5, 6-monoepoxide and allenic bond and is known to have solid anti-proliferative and anti-cancer properties [19, 20]. This study isolated and purified neoxanthin from Solanum trilobatum, a well-known medicinal plant in the Indian Ayurvedic and Siddha systems [21]. The purified neoxanthin was subjected to examine the hepatoprotective effect for the first time in stress (H2O2)-induced hepatocellular carcinoma (HepG2) cells, a widely used in vitro model to study the mechanisms underlying dysfunctions of liver cells, intracellular transport of molecules, and liver toxicity and metabolism [22, 23]. Further, we examined the possible molecular mechanism through which neoxanthin imparts/offers protection against oxidative damage in HepG2 cells.
Materials and methods
Chemicals and reagents
Dimethyl sulfoxide (DMSO), silica gel, bovine serum albumin (BSA), and 2′, 7′ -dichlorofluorescein diacetate (DCFH-DA) were purchased from Sigma-Aldrich Co., St Louis, USA. The mitochondrial membrane potential (MMP) assay kit and caspase-3 assay kit were obtained from Abcam Inc., Cambridge, UK. The water-soluble tetrazolium-1-(WST-1) reagent was purchased from Roche Life Science, Connecticut, USA. The cell culture media and supplements were purchased from Life Technologies, California, USA. HepG2 cells were obtained from ATCC, Virginia, USA. The DC protein assay kit was procured from Bio-Rad Laboratories, California, USA. Primary antibodies to nuclear factor erythroid 2-related factor 2 (Nrf2), HO-1, B cell lymphoma 2 (Bcl-2), Bcl2 associated X (Bax), and β-actin were purchased from Cell Signaling Technology Inc., Massachusetts, USA, and the antibodies to SOD-2 and nuclear factor kappa B (NF-kB) were procured from Abcam Inc., Cambridge, UK. The secondary antibody, enzyme-conjugated anti-rabbit IgG was purchased from Cell Signaling Technology Inc., Massachusetts, USA. Solvents were of HPLC grade from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. All other reagents and chemicals were of analytical quality commercially available. In all experiments, Milli-Q (Merck Millipore Corporation, Massachusetts, USA) water was used.
Sample preparation
The total carotenoids were extracted from the leaves of S. trilobatum as described in Kavalappa et al. [24]. The neoxanthin-rich fraction was separated by open column chromatography using hexane and acetone at a 60:30 (v/v) ratio. The column-separated neoxanthin-rich fraction was subjected to HPLC (Shimadzu Corporation, Kyoto, Japan) system equipped with a reverse phase C-18 column. The sample was eluted with a binary gradient mobile phase [Acetonitrile: methanol: water (75:15:10, v/v/v) and Ethyl acetate: methanol (70:30, v/v)] with the flow rate of 1 mL/min for 35 min. The absorption spectrum of neoxanthin was measured using a photodiode array detector (SPD-M10A, Shimadzu Corporation, Kyoto, Japan) attached to the HPLC system. Lutein and β-carotene were purified from Spinacia oleracea described by Sowmya Shree et al. [13] and Shivarudrappa and Ponesakki [16]. The purified carotenoids (purity ≥ 95%) were stored at low temperatures (− 80 °C) for further experimentation. The concentrations of carotenoids used for the experiments are set considering the physiologically attainable levels [25].
Cell culture
The HepG2 cell line was cultured in a Dulbecco’s modified eagle medium (DMEM) containing antibiotic solution [penicillin (100 U/mL) and streptomycin (100 µg/mL)] supplemented with 10% heat-inactivated fetal bovine serum (FBS) and non-essential amino acids. Cells were maintained in a humidified atmosphere at 37 °C with 5% CO2. The sub-culturing was performed by trypsinizing the cells with 0.25% trypsin- ethylenediaminetetraacetic acid (EDTA) solution. In all the experiments, HepG2 cells were pretreated with carotenoid at noted concentrations for 3 h and were then challenged to oxidative stress with the addition of H2O2 into the culture media. The purified carotenoids used for experimentation were grouped into N (neoxanthin), L (lutein) and B (β-carotene). The cells treated with H2O2 alone were grouped as H2O2, and the cells maintained in media alone were grouped as control.
Cell viability and morphology
The HepG2 cells seeded at a density of 5 × 103 cells/mL were treated with 0.5 mM H2O2 to examine the cytotoxic effect and incubated at 37 °C for different time points (2 h, 4 h, and 6 h). This concentration disrupted the redox signaling by elevated oxidative stress in the earlier study [7]. On the other hand, to determine the cytoprotective effect of carotenoids, the cells (seeding density of 5 × 103 cells/mL) were pretreated with neoxanthin (N), lutein (L), and β-carotene (B) each at two different concentrations (0.05 and 0.1 µM) for 3 h followed by treatment with H2O2 (0.5 mM) for 4 h. DMSO was used as a vehicle with the final concentration of 0.1% (v/v) in the culture medium. After treatment, the WST-1 reagent (10 µl) was added to each well and incubated at 37 °C for 1 h. The absorbance of resulted reaction was measured at 450 nm. The morphology of cells of the entire experimental group was observed under a Phase contrast microscope (Model 1X73, Olympus Corporation, Tokyo, Japan). The images captured were analyzed for cell density and morphology. Since neoxanthin exhibited better cytoprotection than other carotenoids against H2O2-mediated growth insult, further experiments were conducted.
ROS assay
Intracellular ROS level was measured using a chemically reduced form of fluorescein, DCFH-DA. Briefly, HepG2 cells (5 × 103 cells/mL) seeded in 96-well clear bottom black plate (Eppendorf Pvt. Ltd., Hamburg, Germany) were pretreated with neoxanthin at 0.05 and 0.1 µM (N-0.05 µM and N-0.1 µM) for 3 h followed by H2O2 exposure for 4 h. Media containing DMSO alone served as control. After 4 h of H2O2 exposure, DCFH-DA (10 µM) was added to each well and incubated for 30 min. The fluorescence intensity was measured using a multimode plate reader (Infinite-M200 Pro, Tecan, Männedorf, Switzerland) at an excitation wavelength of 485 nm and an emission of 530 nm. DCFH-DA is a fluorogenic dye that reacts with ROS within the cell and forms a fluorescent product, dichlorofluorescein (DCF) which reflects the intracellular level of ROS. The inhibitory effect of neoxanthin on H2O2-mediated oxidative stress was visualized under an inverted fluorescent microscope and the images of treated cells were compared with control (Model 1X73, Olympus Corporation, Tokyo, Japan).
Mitochondrial membrane potential (MMP) assay
The MMP assay was performed using JC-10 MMP assay kit by following the manufacture's protocol. Briefly, HepG2 cells (5 × 103 cells/mL) seeded in 96-well clear bottom black plate (Eppendorf Pvt. Ltd., Hamburg, Germany) were pretreated with neoxanthin at 0.05 and 0.1 µM (N-0.05 µM and N-0.1 µM) for 3 h followed by H2O2 exposure for 4 h. After treatment, JC-10 loading dye solution was added to each well. The plate was incubated for 30 min under dark conditions. After incubation, assay buffer B was added. The fluorescence intensity of the reaction mixture was measured at 490/525 nm (Green) and 540/590 nm (Red) using a multimode reader (Infinite-M200 Pro, Tecan, Männedorf, Switzerland). The ratio of red/green was subjected to calculate the MMP. Carbonyl cyanide m-chlorophenylhydrazone (CCCP) was used as a positive control.
Western blotting
In this protocol, the total cellular proteins of all experimental groups seed at a density of 5 × 103 cells/mL were extracted in lysis buffer (20 mM Tris-buffered saline, 1% Triton-X100, protease inhibitor cocktail, 50 mM sodium fluoride, and 1 mM sodium orthovanadate) and clarified by centrifugation at 15,000 × g for 25 min at 4 °C. The concentration of soluble proteins in the cell lysate was quantified using Bradford’s method. Protein at a concentration of 30 µg from each sample was subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoretic (SDS-PAGE) separation using 10 or 12.5% acrylamide gel. Separated proteins on the gel were transferred onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories, California, USA), and were blocked using 3% skimmed milk powder for 2 h. The membrane was then probed with a specific primary antibody and subsequently with a secondary antibody for 1 h. Finally, the bands of respective proteins were visualized under Bio-Rad visualizer (Bio-Rad Laboratories, California, USA) using Clarity™ western ECL substrate (Bio-Rad Laboratories, California, USA). The beta-actin was used as the internal control for all the proteins.
Caspase-3 activity
A caspase-3 colorimetric assay kit (Abcam Inc., Cambridge, UK) was used to measure the activity of caspase-3 in HepG2 cells of all experimental groups. Briefly, cells (seeded at a density of 5 × 103 cells/mL) after neoxanthin pretreatment followed by H2O2 exposure, the cells were washed with phosphate-buffered saline (PBS) and were then lysed using cell lysis buffer. The soluble proteins were clarified by centrifugation and quantified by Bradford’s method. The protein concentration of 100 μg in 50 μl cell lysis buffer from each sample was mixed with 50 μl reaction buffer, and the absorbance of the reaction mixture was measured at 400 nm. Then, 5 μl caspase-3 substrate (p-nitro aniline chromophore, DEVD-pNA) was added to the sample mixture and incubated at 37 °C for 1 h. The absorbance of the final reaction mixture was measured at the same wavelength and calculated for caspase-3 activity.
Statistical analysis
Values are presented as means of the data from three independent experiments ± SD. Data were analyzed for significant differences (p < 0.05) by one-way (ANOVA) analysis of variance using the Tukey–Kramer post-hoc test.
Results
Isolation and purification of neoxanthin
The solvent extract of S. trilobatum leaves was examined for its carotenoids profile using a reverse-phase HPLC system. The carotenoids profile of S. trilobatum leaves is shown in Fig. 1A. The carotenoid peaks on the chromatogram were identified based on the absorption spectrum (λmax), and the retention time (RT). The pigment profile of S. trilobatum showed major peaks corresponding to neoxanthin at 10 min with λmax 437 nm, lutein at 16 min with λmax 446 nm and β-carotene at 36 min with λmax 449 nm. The HPLC-purified neoxanthin (Fig. 1B) exhibited the same absorption spectrum of 437 nm (Fig. 1C). The present study, for the first time highlights the carotenoid profile of S. trilobatum.
Cytoprotective effect of carotenoids on stress-induced HepG2 cells
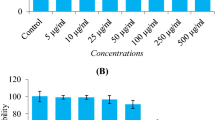
The WST-1 assay was carried out to measure the cytotoxic effect of H2O2 on the viability of HepG2 cells and examine the protective efficacy of carotenoids. The effect of H2O2 on the viability of HepG2 cells is presented in Fig. 2A. The results indicated that H2O2 (0.5 mM) significantly inhibits HepG2 cell viability at all the three-time intervals (2, 4, and 6 h) tested. The cytoprotective ability of neoxanthin, lutein, and β-carotene against H2O2-mediated growth inhibition in HepG2 cells is presented in Fig. 2B. The vehicle DMSO used to dissolve carotenoids did not affect the viability of HepG2 cells. Pretreatment with neoxanthin for 3 h exhibited superior cytoprotection against H2O2 treatment than lutein and β-carotene, as noticed with a significant (p < 0.05) increase in cell viability even at 0.05 µM concentration (N0.05 µM). However, pretreatment with lutein and β-carotene had significant cytoprotection only at 0.1 µM concentration. The same observation was noticed in microscopic images of carotenoid pretreated HepG2 cells challenged with H2O2 (Fig. 2C). As shown in Fig. 2C, the cell density in the image of the H2O2-treated group is reduced compared to the vehicle. But, cell density in carotenoid pretreated groups showed a dose-dependent increase. It indicates that those carotenoids possess cytoprotective potential against H2O2-mediated cell death.
Cytoprotective effect of carotenoids (N, neoxanthin; L, lutein; B, β-carotene) on the viability of stress-induced HepG2 cells. A The cell viability of HepG2 cells treated with H2O2 (0.5 mM) for 2 h, 4 h and 6 h were analyzed by WST-1 assay. Values are mean ± SD (n = 3). B The HepG2 cells pretreated with neoxanthin (N), lutein (L) or β-carotene (B) at each 0.05 and 0.1 µM concentrations for 3 h were exposed to stress by the addition of H2O2 for 4 h. After incubation, the viability was analyzed by WST-1 assay. Values are mean ± SD (n = 3). C The morphology of control (media alone), vehicle (media with DMSO), H2O2 exposed (media with DMSO and H2O2) and carotenoid pretreated (media with carotenoid, DMSO and H2O2) HepG2 cells were observed under a phase-contrast microscope, and the representative picture is presented. Bars with an asterisk indicate a significant difference (p < 0.05) with the respective control
Neoxanthin prevents oxidative stress induced by H2O2 in HepG2 cells
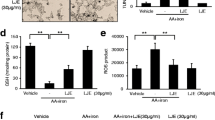
Oxidative stress in cells is traced by measuring the intracellular ROS levels. Thus, we intend to examine the preventive effect of neoxanthin on H2O2-mediated elevation of ROS in HepG2 cells by using DCFH-DA (Fig. 3). As shown in Fig. 3A, the addition of H2O2 triggered intracellular ROS levels in HepG2 cells, while neoxanthin pretreatment inhibited this H2O2-mediated ROS generation in a concentration-dependent manner. H2O2-treatment increased the ROS levels by 38% compared to control (p < 0.05). Neoxanthin pretreatment at 0.05 and 0.1 µM concentration (N-0.05 µM and N-0.1 µM) suppressed H2O2-induced ROS levels by 12 and 24%, respectively in HepG2 cells. These findings were further confirmed by observing the cells under a fluorescent microscope (Fig. 3B). We noticed an increased number of green fluorescence saturated cells in the H2O2 alone treated group compared to the control group, while the number is reduced in the neoxanthin pretreated group.
Protective effect of neoxanthin against H2O2-induced oxidative stress in HepG2 cells. A The intracellular ROS levels were measured as indicated in the materials and methods section after pretreatment of HepG2 cells with neoxanthin (N-0.05 µM & N-0.1 µM) for 3 h followed by H2O2 exposure (0.5 mM) for 4 h. Values are mean ± SD (n = 3). B The microscopic images of the intensity of DCF fluorescence of the respective experimental group. Cells cultured in media with DMSO (0.1%) served as control. C The mitochondrial membrane potential (MMP) was analyzed in HepG2 cells pretreated with neoxanthin (N-0.05 µM & N-0.1 µM) for 3 h followed by H2O2 exposure (0.5 mM) for 4 h using a JC-10 assay kit as indicated in materials and methods. The quantitative analysis of MMP was calculated using the ratio of red/green fluorescence intensity. Values are mean ± SD (n = 3). CCCP, carbonyl cyanide m-chlorophenylhydrazone served as a positive control. Bars with an asterisk indicate a significant difference (p < 0.05) with the respective control
As MMP plays a vital role in controlling ROS levels within the cell, we measured its status in H2O2-treated HepG2 cells using JC-10 MMP assay kit and subsequently examined the role of neoxanthin pretreatment on MMP (Fig. 3C). As expected, the addition of H2O2 significantly reduced MMP in HepG2 cells, whereas neoxanthin pretreatment at both 0.05 and 0.1 µM concentrations (N-0.05 µM and N-0.1 µM) significantly blocked this H2O2-mediated MMP loss. The positive control, CCCP, a mitochondrial uncoupler used in our study, exhibited a drastic reduction in MMP. Collectively, our results suggest that the protective efficacy of neoxanthin against oxidative damage in liver cells might be through its potent antioxidant activity.
Neoxanthin activates endogenous antioxidant machinery in stress-induced HepG2 cells
Carotenoids are widely known for their antioxidant potential, and a large number of research studies demonstrate that they also regulate several signaling molecules in almost all the cells [16, 26, 27]. Whether the suppressive effect of neoxanthin observed against H2O2-mediated oxidative stress in HepG2 cells is related to the protection of the antioxidant defense system, we measured the protein expression of major endogenous detoxifying enzymes, HO-1 and SOD-2 through western blot analysis. Correlating with our hypothesis, we noticed a reduction in the protein expression of HO-1 by 30% in H2O2-treated cells, whereas increased expression of the protein by 17% and 22% in 0.05 µM and 0.1 µM neoxanthin-treated (N-0.05 µM and N-0.1 µM) cells, respectively compared to H2O2 treatment (Fig. 4A). The addition of H2O2 also decreased SOD-2 expression by 51% while neoxanthin pretreatment at 0.05 µM and 0.1 µM (N-0.05 µM and N-0.1 µM) preserved the level by 21 and 35%, respectively (Fig. 4B). Similarly, the expression of significant transactivation factors, Nrf2, that regulate the transcription of HO-1 and SOD-2 was significantly (p < 0.05) suppressed by H2O2, while increased protein expression was noticed in carotenoid pretreated HepG2 cells (Fig. 4C). A maximum increase in the expression level of Nrf2 (42%) was observed at a neoxanthin concentration of 0.1 µM (N-0.1 µM). It is essential to notice that the results observed in this section depict an additional role of neoxanthin as an effective modulator of endogenous antioxidant protein expression in stress-induced liver cells.
Preventive effect of neoxanthin on H2O2-mediated blockage of the expression of antioxidant signaling proteins. Protein expression of HO-1 (A), SOD-2 (B) and Nrf2 (C) in the cellular extract of HepG2 pretreated with neoxanthin (N-0.05 and N-0.1 µM) for 3 h followed by H2O2 (0.5 mM) exposure for 4 h was detected by western blotting as described in materials and methods. Values are mean ± SD (n = 3). Bars with an asterisk indicate a significant difference (p < 0.05) with the respective control
Neoxanthin halts programmed cell death in stress-induced HepG2 cells
We hypothesized that disrupted antioxidant defense due to oxidative stress might subject the cells to undergo apoptosis. Bcl family proteins play a significant role in regulating intrinsic and extrinsic signaling pathways of apoptosis. Therefore, this study is interested to measure the protein expression of Bax and Bcl-2 by western blotting. As speculated, H2O2 treated HepG2 cells had elevated expression of a pro-apoptotic protein, Bax (60%), and decreased anti-apoptotic protein, Bcl-2 (50%). But, neoxanthin pretreatment for 3 h blocked this stress-mediated increase in Bax expression (Fig. 5A), and prevented Bcl-2 expression (Fig. 5B) from a descent. The maximum reversible effect of 51% and 44% were shown by neoxanthin at 0.1 µM concentration (N-0.1 µM) on the expression of Bax and Bcl-2, respectively. A redox-sensitive transcription factor, NF-kB that plays a vital role in protecting the cells from apoptosis under stressed conditions, was upregulated by neoxanthin pretreatment, which was repressed by H2O2 (Fig. 5C). Increased activity of caspase-3 noticed in H2O2-treated cells confirms apoptosis induction, and reduction in the activity shown by neoxanthin pretreatment indicates its protective efficacy against ROS-mediated cell death (Fig. 5D).
Neoxanthin pretreatment halts H2O2-induced apoptosis in HepG2 cells. Protein expression of Bax (A), Bcl-2 (B) and NF-kB (C) in the cellular extract of HepG2 pretreated with neoxanthin (N-0.05 and N-0.1 µM) for 3 h followed by H2O2 (0.5 mM) exposure for 4 h was detected by western blotting as described in materials and methods. Values are mean ± SD (n = 3). D A colorimetric assay analysed Caspase-3 activity. Values are means ± SD (n = 3). Bars with an asterisk indicate a significant difference (p < 0.05) with the respective control
Discussion
The present study highlights the protective role of neoxanthin isolated from S. trilobatum on H2O2-induced oxidative stress in HepG2 cells. A large number of studies used H2O2 as a potent oxidant to examine the antioxidant potential of bioactive in various cell types such as myocytes [28], mouse hippocampal neural cells (HT22) [29], Retinal pigment epithelial cells (RPE) [30], human hepatocellular carcinoma cells (BEL-7402 and HepG2) [31, 32], human fetal lung fibroblast cells (IMR-90) [33] and human umbilical vein endothelial cells (HUVEC) [34]. In this study, we demonstrate that neoxanthin at 0.05 and 0.1 µM concentrations effectively protects HepG2 cells from H2O2-created oxidative stress by scavenging ROS, preventing MMP loss, and enhancing the expression of intracellular antioxidant enzymes. It is also noticed that neoxanthin effectively blocks H2O2-induced apoptosis by upregulating an anti-apoptotic marker, Bcl2, and downregulating a pro-apoptotic marker, Bax.
S. trilobatum, a thorny, branched, straggling shrub that belongs to the family Solanaceae, is found mostly in the southern part of India. It is a long-known medicinal plant used in the Indian Ayurvedic and Siddha systems of medicine, mainly for the treatment of respiratory diseases [35]. Reports demonstrated that alcoholic and aqueous extracts of S. trilobatum possess anti-inflammatory [36, 37], antidiabetic [38], antitumor [39] and hepatoprotective [40]. But those studies subjecting extracts provide a lack of pharmacokinetic strategies, mechanisms of action, interactions, and adverse effects. Though few studies had reported the anti-inflammatory and chemoprotective efficacy of two constituents, sobatum and solasodine isolated from S. trilobatum, they did not reveal the underlying molecular mechanism [36, 41]. In the current study, we studied the carotenoids profile of S. trilobatum where we found neoxanthin as one of the carotenoids along with lutein and β-carotene. Neoxanthin is a unique epoxy, trihydroxy xanthophyll carotenoid present in leek, arugula, and lamb’s lettuce [11], and its hepatoprotective effect is not well understood. We report for the first time that neoxanthin possesses protection against H2O2-mediated inhibition in HepG2 cell viability, which is comparable with lutein and β-carotene as shown in Fig. 2B. Previous reports emphasized that the bioavailability of epoxy carotenoids like neoxanthin and fucoxanthin is lower than non-epoxy carotenoids like β-carotene and lutein; however, the cellular uptake and metabolic conversion of carotenoids are greatly affected by divalent ions and phospholipids [42,43,44]. On the other hand, neoxanthin showed the highest anti-proliferative effect in human prostate cancer cells compared to other non-epoxy carotenoids like β-carotene and lutein [45]. Thus, it could be speculated that an in vivo study with a suitable model is necessary to understand the biological activity better.
Previous reports showed that H2O2, a highly reactive free radical, readily diffuses into the cytoplasm and damages the cell by reacting with macromolecules such as DNA, proteins, and lipids [46]. In our study, the addition of H2O2 (0.5 mM) elevated intracellular ROS levels by 38% (Fig. 3A) compared to control, which corresponds to a loss of cell viability by around 25% (Fig. 2B). It was reported that ROS-driven oxidative stress impairs mitochondria by inducing rapid depolarization of MMP [47]. Consistent with the findings, H2O2 addition in our study drastically reduced the MMP. More interestingly, neoxanthin, even at 0.05 µM (N-0.05 µM) concentration, effectively neutralized intracellular ROS and subsequently improved the loss of MMP. Earlier workers observed similar effects with other carotenoids such as lycopene, astaxanthin, fucoxanthin, lutein, and β-carotene at varying concentrations ranging from 1–20 µM in various cell models [48,49,50,51,52]. Although the inhibitory concentration of carotenoids varied with different cell models, neoxanthin in our study exerted a similar effect at a significantly lower concentration (N-0.1 µM) compared to other carotenoids. Our data signify that the antioxidant potency of carotenoids varies on their chemical alignment.
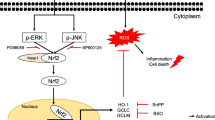
Our curiosity to understand the underlying molecular mechanism, we investigated the potential pathways contributing to neoxanthin’s protective effect against oxidative damage in HepG2 cells. It is known that cells utilize intricate intracellular antioxidant-defense mechanisms to protect themselves from elevated ROS-induced damage. Many studies demonstrated that activation of Nrf2/ARE pathway increases the expression of antioxidant enzymes that protect the cells from oxidative damage [16, 53]. Earlier studies showed that few carotenoids attenuate the harmful effect of H2O2 through activating the Nrf2-ARE pathway in various cell types [50,51,52]. As expected, neoxanthin pretreatment effectively enhanced the protein expression of Nrf2, and antioxidant enzymes, SOD-2, and HO-1. From these results, we speculate that neoxanthin may stimulate the nuclear translocation of Nrf2, thereby promoted the expressions of its regulatory genes, SOD-2 and HO-1.
Disrupted MMP and reduced cell viability in H2O2 exposed HepG2 cells and recovery of those effects in neoxanthin pretreated cells added our interest to examine the apoptotic pathway. Bcl-2 family proteins are the primarily targeted apoptotic markers by carotenoids in the earlier studies [12, 13, 54]. Consistent with the previous evidence showing ROS as a potential inducer of apoptosis [55], H2O2 in our study significantly upregulated the protein expression of a pro-apoptotic factor, Bax, and downregulated the expression of an anti-apoptotic protein marker, Bcl-2. Interestingly, neoxanthin was found to be an effective scavenger of ROS in our study reversed H2O2-mediated changes in the expression of those apoptotic marker proteins. Similarly, lycopene in earlier studies was found to reverse H2O2-mediated changes in the expression of Bcl-2 and Bax in human SH-SY5Y neuroblastoma and bovine mammary epithelial cells [52, 54]. The Bcl-2 family proteins play an essential role in stabilizing the MMP and regulating the release of cytochrome c into the cytoplasm [55]. The loss of MMP promotes the release of cytochrome c, which in turn activates the executioner caspases. Therefore, disrupted MMP in H2O2 exposed cells might be due to altered expression of Bcl-2 and Bax, resulting in increased caspase-3 activity. As shown in Fig. 5, neoxanthin pretreatment efficiently prevented those H2O2-mediated inductions of apoptotic signaling markers. A previous report revealed that NF-kB is a potential suppressor of apoptosis by targeting the transcription of anti-apoptotic proteins [56]. We noticed a decreased protein expression of NF-kB in H2O2 exposed cells which were blocked by neoxanthin pretreatment. The modulatory effect of carotenoids on NF-kB expression and its association with cell survival has been documented [14, 24, 57]. Though neoxanthin can act as a direct antioxidant, its cytoprotective function in this study appears to rest also in enhanced expression of a cell survival marker, NF-kB.
Our study emphasizes the highest potential of neoxanthin as a powerful antioxidant in comparison with lutein and β-carotene. We do reveal the presence of neoxanthin as one of the carotenoids in S. trilobatum having a hepatoprotective role, which provides a piece of supportive evidence to this medicinal plant that is being used in Ayurvedic medicine. From our study, it is apparent that neoxanthin at low concentration prevents oxidative stress-induced HepG2 cell death. We demonstrated the efficacy of neoxanthin in alleviating oxidative stress by reverting the loss of MMP, increasing the expression of antioxidant enzymes, and preventing apoptosis. At the molecular level, the observed protective role of neoxanthin against oxidative stress-mediated cell death may contribute partly through the upregulated expression of Nrf2 and NF-kB, the key regulators of intracellular antioxidant defense and apoptosis, respectively. Overall, the present study elucidated the molecular mechanism underlying the cytoprotective role of neoxanthin isolated from S. trilobatum against H2O2-induced HepG2 cell death, which warrants further in vivo experiments using liver injury models. This study also sets a virtual platform for future investigations for exploiting this dietary molecule against widespread oxidative stress-associated disease models.
References
Prieto I, Monsalve M (2017) ROS homeostasis, a key determinant in liver ischemic-preconditioning. Redox Biol 12:1020–1025
Asrani SK, Devarbhavi H, Eaton J, Kamath PS (2019) Burden of liver diseases in the world. J Hepatol 70:151–171
Grewal US, Walia G, Bakshi R, Chopra S (2018) Hepatitis B and C viruses, their coinfection and correlations in chronic liver disease patients: a tertiary care hospital study. Int J Appl Basic Med Res 8:204
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. BBA-Mol Cell Res 1863:2977–2992
Luangmonkong T, Suriguga S, Mutsaers HA, Groothuis GM, Olinga P, Boersema M (2018) Targeting oxidative stress for the treatment of liver fibrosis. Rev Physiol Biochem Pharmacol 175:71–102
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122:877–902
Subedi RP, Vartak RR, Kale PG (2017) Management of stress exerted by hydrogen peroxide in Drosophila melanogaster using Abhrak bhasma. J Appl Pharm Sci 7:065–071
Singh M, Sharma H, Singh N (2007) Hydrogen peroxide induces apoptosis in HeLa cells through the mitochondrial pathway. Mitochondrion 7:367–373
Marinho HS, Real C, Cyrne L, Soares H, Antunes F (2014) Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2:535–562
Kohlgrüber S, Upadhye A, Dyballa-Rukes N, McNamara CA, Altschmied J (2017) Regulation of transcription factors by reactive oxygen species and nitric oxide in vascular physiology and pathology. Antioxid Redox Signal 26:679–699
Stephen NM, Gayathri R, Niranjana R, Prasad Y, Das AK, Baskaran V, Ganesan P (2017) Carotenoids: types, sources, and biosynthesis. Plant secondary metabolites, vol 2. Apple Academic Press, Palm Bay, pp 103–132
Ganesan P, Noda K, Manabe Y, Ohkubo T, Tanaka Y, Maoka T et al (2011) Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. BBA-Gen Subj 1810:497–503
Sowmya Shree G, Prasad KY, Arpitha HS, Deepika UR, Kumar KN, Mondal P, Ganesan P (2017) β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells. Mol Cell Biochem 436:1–12
Kavalappa YP, Gopal SS, Ponesakki G (2021) Lutein inhibits breast cancer cell growth by suppressing antioxidant and cell survival signals and induces apoptosis. J Cell Physiol 236:1798–1809
Gopal SS, Maradgi T, Ponesakki G (2019) Anti-obese properties of carotenoids: an overview of underlying molecular mechanisms. Carotenoids: properties, processing and applications. Elsevier Academic Press, Cambridge
Shivarudrappa AH, Ponesakki G (2020) Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells. J Cell Commun Signal 14:207–221
Berni P, Chitchumroonchokchai C, Canniatti-Brazaca SG, De Moura FF, Failla ML (2015) Comparison of content and in vitro bioaccessibility of provitamin A carotenoids in home cooked and commercially processed orange fleshed sweet potato (Ipomea batatas Lam). Plant Foods Hum Nutr 70:1–8
Gopal SS, Eligar SM, Vallikannan B, Ponesakki G (2021) Inhibitory efficacy of lutein on adipogenesis is associated with blockage of early phase regulators of adipocyte differentiation. BBA-Mol Cell Biol Lipids 1866:158812
Kotake-Nara E, Asai A, Nagao A (2005) Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett 220:75–84
Kotake-Nara E, Sugawara T, Nagao A (2005) Antiproliferative effect of neoxanthin and fucoxanthin on cultured cells. Fish Sci 71:459–461
Kirtikar KR, Basu BS (1935) Solanacea. In: Blatler E, Cauis JC, Mhaskar KS (eds) Indian Medicinal Plants , vol 3, 2nd edn. Oriental Enterprises, Dehradun, pp 403–407
Chiu Y, Lo H, Huang H, Chao P, Hwang L, Huang P et al (2013) The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal 21:253–260
Ma Z, Li C, Qiao Y, Lu C, Li J, Song W et al (2016) Safflower yellow B suppresses HepG2 cell injury induced by oxidative stress through the AKT/Nrf2 pathway. Int J Mol Med 37:603–612
Kavalappa YP, Rudresh DU, Gopal SS, Shivarudrappa AH, Stephen NM, Rangiah K, Ponesakki G (2019) β-carotene isolated from the marine red alga, Gracillaria sp. potently attenuates the growth of hepatocellular carcinoma (HepG2) cells by modulating multiple molecular pathways. J Funct Foods 52:165–176
Böhm V, Lietz G, Olmedilla-Alonso B, Phelan D, Reboul E, Bánati D et al (2021) From carotenoid intake to carotenoid blood and tissue concentrations–implications for dietary intake recommendations. Nutr Rev 79:544–573
Niranjana R, Gayathri R, Mol SN, Sugawara T, Hirata T, Miyashita K, Ganesan P (2015) Carotenoids modulate the hallmarks of cancer cells. J Funct Foods 18:968–985
Cheng J, Liu D, Zhao J, Li X, Yan Y, Wu Z et al (2019) Lutein attenuates oxidative stress and inhibits lipid accumulation in free fatty acids-induced HepG2 cells by activating the AMPK pathway. J Funct Foods 60:103445
Liu Y, Zhang Y, Lin K, Zhang DX, Tian M, Guo HY et al (2014) Protective effect of piperine on electrophysiology abnormalities of left atrial myocytes induced by hydrogen peroxide in rabbits. Life Sci 94(2):99–105
Rao W, Zhang L, Su N, Wang K, Hui H, Wang L et al (2013) Blockade of SOCE protects HT22 cells from hydrogen peroxide-induced apoptosis. Biochem Biophy Res Commun 441:351–356
Xu X, Hang L, Huang B, Wei Y, Zheng S, Li W (2013) Efficacy of ethanol extract of Fructus lycii and its constituent’s lutein/zeaxanthin in protecting retinal pigment epithelium cells against oxidative stress: in vivo and in vitro models of age-related macular degeneration. J Opthamol 2013:862806
Li W, Zhang J, An W (2010) The conserved CXXC motif of hepatic stimulator substance is essential for its role in mitochondrial protection in H2O2-induced cell apoptosis. FEBS Lett 584:3929–3935
Qi G, Mi Y, Fan R, Li R, Wang Y, Li X et al (2017) Tea polyphenols ameliorate hydrogen peroxide and constant darkness-triggered oxidative stress via modulating the Keap1/Nrf2 transcriptional signaling pathway in HepG2 cells and mice liver. RSC Adv 7:32198
Chen C, Liu T, Chen C, Wong CH, Chen C, Lu F, Chen SC (2007) The efficacy of protective effects of tannic acid, gallic acid, ellagic acid, and propyl gallate against hydrogen peroxide-induced oxidative stress and DNA damages in IMR-90 cells. Mol Nutr Food Res 51:962–968
Park WH (2013) The effects of exogenous H2O2 on cell death, reactive oxygen species and glutathione levels in calf pulmonary artery and human umbilical vein endothelial cells. Int J Mol Med 31:471–476
Ramya S, Rajasekaran C, Sivaperumal R, Krishnan A, Jayakumararaj R (2008) Ethnomedicinal perspectives of botanicals used by Malayali Tribes in Vattal Hills of Dharmapuri (TN), India. Ethnobot Leafl 12:1054–1060
Emmanuel S, Ignacimuthu S, Perumalsamy R, Amalraj T (2006) Anti-inflammatory activity of Solanum trilobatum. Fitoterapia 77:611–612
Ranjith MS, Ranjitsingh AJA, Shankar SG, Vijayalaksmi GS, Deepa K, Babu K, Sidhu HS (2010) Solanum trilobatum in the management of atopy: through inhibition of mast cell degranulation and moderation of release of interleukins. Pharmacogn Res 2:10–14
Ahmed Z, Syed K, Ahmed Sidhra SZ, Ponmurugan P, Kumar BS (2016) Ameliorative potential of Solanum trilobatum leaf extract and fractions on lipid profile and oxidative stress in experimental diabetes. Pak J Pharm Sci 29:1578
Shahjahan M, Vani G, Shyamaladevi CS (2005) Effect of Solanum trilobatum on the antioxidant status during diethyl nitrosamine induced and phenobarbital promoted hepatocarcinogenesis in rat. Chem Biol Interact 156:113–123
Ganesan K, Sukalingam K, Xu B (2017) Solanum trilobatum L. ameliorate thioacetamide-induced oxidative stress and hepatic damage in albino rats. Antioxidants 6:68
Vijaimohan K, Devi CS, Mallika J (2010) Chemoprotective effect of sobatum against lithium-induced oxidative damage in rats. J Young Pharm 2:68–73
Sugawara T, Kushiro M, Zhang H, Nara E, Ono H, Nagao A (2001) Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr 131:2921–2927
Asai A, Yonekura L, Nagao A (2008) Low bioavailability of dietary epoxyxanthophylls in humans. Br J Nutr 100:273–277
Biehler E, Hoffmann L, Krause E, Bohn T (2011) Divalent minerals decrease micellarization and uptake of carotenoids and digestion products into Caco-2 cells. J Nutr 141:1769–1776
Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A (2001) Carotenoids affect proliferation of human prostate cancer cells. J Nutr 131:3303–3306
D’Andrea T, Hellmold H, Jonsson C, Zhivotovsky B, Hofer T, Wärngård L, Cotgreave I (2004) The transcriptosomal response of human A549 lung cells to a hydrogen peroxide-generating system: relationship to DNA damage, cell cycle arrest, and caspase activation. Free Radic Biol Med 36:881–896
Park J, Lee J, Choi C (2011) Mitochondrial network determines intracellular ROS dynamics and sensitivity to oxidative stress through switching inter-mitochondrial messengers. PloS one 6:e23211
Heo SJ, Ko SC, Kang SM, Kang HS, Kim JP, Kim SH et al (2008) Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur Food Res Technol 228:145–151
Kim Y, Seo JH, Kim H (2011) β-Carotene and lutein inhibit hydrogen peroxide-induced activation of NF-κB and IL-8 expression in gastric epithelial AGS cells. J Nutr Sci Vitaminol 57:216–223
Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y et al (2013) Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis 19:1656
Wang X, Cui YJ, Qi J, Zhu MM, Zhang TL, Cheng M, Liu SM, Wang GC (2018) Fucoxanthin exerts cytoprotective effects against hydrogen peroxide-induced oxidative damage in L02 cells. Bio Med Res Int 2018:1–11
Sun X, Jia H, Xu Q, Zhao C, Xu C (2019) Lycopene alleviates H2O2-induced oxidative stress, inflammation and apoptosis in bovine mammary epithelial cells via the NFE2L2 signaling pathway. Food Funct 10:6276–6285
Lin J, Xia J, Zhao HS, Hou R, Talukder M, Yu L et al (2018) Lycopene triggers Nrf2–AMPK cross talk to alleviate atrazine-induced nephrotoxicity in mice. J Agric Food Chem 66:12385–12394
Feng C, Luo T, Zhang S, Liu K, Zhang Y, Luo Y, Ge P (2016) Lycopene protects human SH-SY5Y neuroblastoma cells against hydrogen peroxide-induced death via inhibition of oxidative stress and mitochondria-associated apoptotic pathways. Mol Med Rep 13:4205–4214
Levine B, Sinha SC, Kroemer G (2008) Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4:600–606
Fan Y, Dutta J, Gupta N, Fan G, Gélinas C (2008) Regulation of programmed cell death by NF-κB and its role in tumorigenesis and therapy. Programmed cell death in cancer progression and therapy. Springer, Dordrecht, pp 223–250
Bohn T (2019) Carotenoids and markers of oxidative stress in human observational studies and intervention trials: Implications for chronic diseases. Antioxidants 8:179
Acknowledgements
The author, TM, acknowledges the Department of Biotechnology (DBT), New Delhi, India, for granting a research fellowship. The authors thank the Director, CSIR-CFTRI, for the constant support to carry out this work.
Author information
Authors and Affiliations
Contributions
GP, DUR, NMS and TM designed the study; DUR, TM and NMS performed the experiments; GP, DUR, TM and NMS wrote the manuscript; AN and KNR contributed to data analysis and editing of the manuscript, and all the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Udayawara Rudresh, D., Maradagi, T., Stephen, N.M. et al. Neoxanthin prevents H2O2-induced cytotoxicity in HepG2 cells by activating endogenous antioxidant signals and suppressing apoptosis signals. Mol Biol Rep 48, 6923–6934 (2021). https://doi.org/10.1007/s11033-021-06695-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06695-1