Abstract
Chronic inflammation is a major factor in tumor growth and progression. Cancer cells secrete C-X-C chemokine ligand 8 (CXCL8) along with its receptor C-X-C chemokine receptor 1 (CXCR1) and chemokine receptor 2 (CXCR2). It plays a significant role in the activation and trafficking of inflammatory mediators, tumor proliferation and interferes in breast cancer development by controlling cell adhesion, proliferation, migration, and metastasis. This axis also plays a significant role in driving different cancers and melanomas, including breast cancer progression, by controlling stem cell masses. Few small-molecule CXCR1/2 inhibitors and CXCL8 releasing inhibitors have been identified in the past two decades that bind these receptors in their inactive forms and blocks their signaling as well as the biological activities associated with inflammation. Inhibitors of certain inflammatory molecules are projected to be more efficient in different inflammatory diseases. Preclinical trials indicate that patients may be benefitted from combined treatment with targeted drugs, chemotherapies, and immunotherapies. Thus, targeting the CXCL8-CXCR1/2 signaling axis in breast cancer could be a promising approach for its therapeutics. This review examines the roles of the CXCL8-CXCR1/2 signaling axis and how it is implicated in the tumor microenvironment in breast cancer. In addition, we also discuss the potential role of the CXCL8-CXCR1/2 axis in targeted therapeutics for breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemokines and their cognate receptors are involved in a wide range of pathological conditions due to their engagement in hematopoiesis, stimulation of immune cells, and maturation of precursor cells [1, 2]. CXCL8 one of the leading chemokines that promote inflammatory pathways by attracting and accelerating neutrophils and granulocytes [3]. CXCL8 exhibits its biological functions after binding with GPCR receptors, CXCR1 and CXCR2 [4]. CXCL8-CXCR1/2 axis has been reported to mediate the initiation and progression of multiple human cancers, including prostate, breast, lung cancer, colon, and skin cancer [5]. This axis is considered an attractive target due to its diverse contribution to proliferation, blood vessel differentiation, incursion, and metastases of cancer [3], particularly in breast cancer treatment [6]. Generation of tumors by cancer stem cells (CSCs) has been evaluated in immunocompromised mice using cell surface marker CD44+CD24−/low lineage [7].
Although the pathways of CXCL8-CXCR1/2 have been explored widely in the case of tumor proliferation and apoptosis, very few studies are available regarding its function in the development and metastasis of breast cancer [5, 8]. Several studies have revealed the impact of the CXCL8-CXCR1/2 axis on CSCs, indicating future opportunities to use it as a therapeutic cancer target [6]. Though CXCR1 has significant therapeutic capability in breast cancer via the articulation of ALDH, its involvement and activities in tumor cells such as non-CSC are yet unidentified [9]. The combined effect of docetaxel and reparixin (CXCR1/2) on the implanted mice cell with human breast cancer cells has found effective downsizing tumors rather than their single treatment [10]. CXCL8 levels were evaluated in malignant pleural effusion due to breast cancer and/or ascites in where restoration and in vitro proliferation of tumor cells was observed [10]. It was found that most mammosphere cells expressed outer CXCR1 while the outgrowth of CXCL8 was obstructed by the CXCR1/2 inhibitor [11]. Both independent and integrated effects of inhibiting CXCR1/2 in the association of prevailing drugs have been evaluated, aiming for recovery from lung diseases and metastatic breast cancer [1]. However, further research is necessary to evaluate the diagnostic and clinical efficacy of CXCL8 signaling in breast cancer prior to their extensive application.
Configuration of CXCL8 chemokine and CXCR1/2 receptors
Interleukin-8 (IL-8) or C-X-C motif ligand 8 (CXCL8) was previously known as a neutrophil-activating factor (NAF), the earliest chemokine to be clarified and sequenced in 1987 [12]. CXCL8 is a member of elastin-like recombinamer (ELR)+ CXC chemokine [13]. The peptide of CXCL8 forms the C-terminal of 57–72 residues in association along with one α-helix and three β-strands [14]. Cys9–Cys50 and Cys7–Cys34 are two stable disulfide bonds and fix the configuration of the CXCL8-CXCR1/2 axis. The receptor shows a higher inclination to CXCL8 monomer compare to dimer, and such variations in the binding sites may be the reason behind diversified signaling cascades [15, 16].
CXCL8 binds with two GPCR (G protein-coupled receptors), i.e., CXCR1 and CXCR2, that help express its signals. These two receptors have 76% similarity in their sequence and shackle to CXCL8 with similar impulses (Kd ≈ 4 nM) [17, 18]. Chemokine ligands CXCL6 and CXCL8 are mostly bound with chemokine receptor CXCR1. However, CXCL6 and CXCL8 also have a strong propensity to CXCR2 with other chemokine ligands like CXCL1, CXCL2, CXCL3, CXCL5, and CXCL7 [19]. The N-terminal of CXCR1 incorporates CXCL8 and nourishes tumor cells, where CXCL8/CXCR2 fosters angiogenesis in prostate cancer [20].
Secretion and expression of CXCL8 and CXCR1/2
Interleukin-8 (IL-8, or CXCL8), a chemokine, is now known to possess tumorigenic and pro-angiogenic properties [21]. Different types of cells, including epithelial, phagocyte, endothelial, and muscle cells, are the releasing sites of CXCL8 [21]. A protein consisting of 99 amino acids construct the basics of CXCL8, which then transform into either CXCL8 isozymes retaining 77 or 72 amino acid and exhibit monocytic and phagocytic behavior [13]. IL-8RA and IL-8RB consist of 7 transmembrane domains [22]. Chromosome 4q13-q21 are the carriers of the relevant genes of CXCL8 [23], whereas chromosome 2q33-q36 recruits the IL-8RA, IL-8RB, and IL8RBP genes [24]. Up-regulation of CXCL8 expression is regulated by the combination of any of the three approaches such as superimposed of CXCL8 promoter, CXCL8 mRNA steadfast via the p38 MAPK pathway, and enhanced expression of CXCL8 through c-Jun N-terminal kinase (JNK) and NF-κB signaling pathway [25]. Although CXCL8 is nearly unidentified in un-stimulated cells, its multiples cytokines such as CXCL1, CXCL6, CXCL12, TNFα, hypoxia, and ROS, AP-1, bacterial particles, and other environmental factors advances CXCL8 expression [13, 26]. Such stimulation intensifies the up-regulation of CXCL8 expression up to 10–100 times [27]. The N-terminal, extracellular loop 2 (ECL2), and transmembrane domain 4 (TM4) of CXCR1/2 are key for the detection of the rate of receptor internalization, expression of ligand binding, and their explicitness [28, 29]. However, CXCR2 ligands bind extensively but have site-specificity on CXCR2, indicating un-ambivalent binding and activation of receptor sites [30].
In breast cancer, expression and secretion of IL-8 upregulated both in vitro and in vivo [31, 32]. The IL-8 receptors CXCR1 and CXCR2 contribute to IL-8-mediated tumorigenic and angiogenic responses, both of which are G-protein coupled, and the Duffy antigen receptor for cytokines [33, 34]. In a recent study, it has been suggested that the human Dachshund homolog 1 (DACH1) is a crucial factor to inversely regulate CXCL8 to repress tumorigenesis of lung adenocarcinoma and improve prognosis [34]. Other studies have shown that vorinostat induces CXCL8 expression in ovarian cancer (OC) cells, which increases OC cell survival [35].
Intracellular signaling pathways of CXCL8/or activation and inhibition of CXCL8
PI3K-AKT (phosphatidylinositol 3‑kinase/protein kinase B) signaling pathway, also known as AKT signaling pathway, catalyzes the phosphorylation of phosphatidylinositol 3‑kinase (PI3K) and controls cell stability, angiogenesis, and mobility. Thus, it becomes a key driver of the downstream intracellular signal of CXCL8 [36]. CXCL8 can also stimulate the AKT synthesis mechanism in androgen-independent prostate cancer cells [3]. Despite the subsistence of several serine or threonine kinases linked in mitogen-activated protein kinase (MAPK or MAP kinase), the Ras/Raf/MEK/ERK signaling pathway is unique. This cellular system is greatly affected by CXCL8 and, therefore, regulates oncogenesis [37,38,39]. CXCL8 functionalizes MAPK and Ras-GTPase by stimulating the PI3K and EGFR, respectively [38, 39]. MAPK driven signal function is significantly restrained by dabrafenib, trametinib, vemurafenib, sorafenib, PD184352, SCH772984, and XMD8–92 [40], whereas PI3K is restrained by GDC-0941, LY294002, and BEZ235 [41]. Transcription of phosphorylation of protein kinase C has occurred when CXCL8 activates PLC signaling. Moreover, CXCL8 induces a PLC-dependent PKC signaling mechanism for effective migration of human cancer cells [42] as well as monitors cyclin D1 expression via activation of PKC [37]. In vivo repression of PKC is triggered by the sotrastaurin, Go 6983, enzastaurin, staurosporine, and quercetin [5].
Contribution of CXCL8 and CXCR1/2 pathway in breast cancer
In breast cancer patients, elevated levels of CXCL8 were reported as healthier ones, while concentration was variable with the state of pathogenesis [43]. For example, upregulation of CXCL8 has been observed among affected people who experienced bone metastasis [44]. Elevated CXCL8 retard disease prognostic and heighten the risk of cancer [45, 46]. CXCL8 can decline cells' immune capacity against cancer and thus create a favorable microenvironment towards tumorigenesis, whereas facilitation of a suitable microenvironment is predominant in breast cancer. The expression of CXCR1 and CXCR2 is vivid in all types of breast cancer cells [6, 33]. The elevated level of CXCL8 was also demonstrated in HER2-positive breast cancers, estrogen receptor (ER) and/or progesterone receptor (PR) negative breast cancers [47, 48], while transactivation of HER2 was extremely proliferated by CXCL8 [49, 50]. Generally, EGFR/HER2 signaling is activated by CXCL8 with the influence of SRC, PI3K, and MEK in CSCs, along with the capability of cluster creation of these cells. Blockade of colony formation with upgraded productiveness of tyrosine kinase inhibitor was the combined effect of CXCR1/2 inhibition with SCH563705 [11]. Neovascularization that triggers rapid outgrowth and transformation of tumor cells has become a major service of CXCL8 in the tumor microenvironment [5]. Vascular endothelial growth factor (VEGF) incorporates with CXCL8 derivative from breast cancer cell to generate, promote and elongate tumor neovasculature [51]. Simultaneously, any default in the endoplasmic reticulum and depletion of glucose are considered the upregulatory components of VEGF and CXCL8 [52].
In contrast, the microvessel density (MVD) of ER-negative tumors can be reduced due to the downregulation of CXCL8 without influencing acceleration and cell division of cancer cells [53]. Both the generation and multiplication of breast cancer along with blood circulation for remote metastasis are greatly benefitted by tumor neovascularization. The abnormal outgrowth of CXCL8 instigated via IL-1β and TNF-α can nurture angiogenesis and neutrophils, thus the secretion of enzymes causing tissue rupture and tumor development [54]. CXCL8 available in ER-negative breast cancer because cyclooxygenase-2 (COX-2) is potent enough for confirmation of human osteoclast and bone endurance [55]. In addition, newly discovered tumor inhibitors, such as Dachshund 1 (DACH1), that incorporate the AP-1 and NF-kB, were identified as inhibitors of breast cancer cell delocalization and transformation [56]. The formation of tumor clusters and distant metastasis are the initial clinical signs of increased serum levels of CXCL8 [43]. It was reported that CXCR2 knock-down in the mammal tumor cell, i.e., C166, 4T1 through short hairpin RNA (shRNA) liable to dwindling cell invasion without affecting cell multiplication. In contrast, normal lung metastasis up to 40% was diminished by CXCR2 knock-down in the case of implanted mice models with the cell lines (C166, 4T1). CXCL1, CXCL3, CXCL5, and CXCL7 were studied for their effect on CXCR2 in the case of anti-drug breast cancer cells, and massive alteration was observed in the xenograft experiment [57]. Expression of CXCR1 and CXCR2 are reported in all breast cancer cells [33]. CXCL8 is also associated with growth receptors expressed on the surface of breast cancer cells. Increased CXCL8 has been primarily detected in triple-negative breast cancer (ER−, PR−, and HER2−) [33, 48]. In a study that compared triple-negative breast cancer patients, CXCL8 was identified as the top-ranked gene linked with poor prognosis [58]. Human cytokine antibody arrays confirmed that CXCL8 expression is inversely associated with ER status but positively associated with the metastatic potential of ER− breast cancer cells [59]. Moreover, CXCL8 has the potential to increase the invasiveness of ER + breast cancer cells and increase the activity of breast cancer stem-like cells (CSCs) by transactivating HER2 [50].
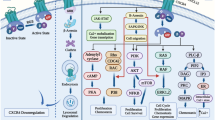
Owing to the effects of the CXCL8 (− 251) A allele and CXCR2 (+ 1208) T allele, the single nucleotide polymorphism (SNP) has identified that Tunisian people are highly prone to breast cancer [60]. On the other hand, CXCL8 A allele is responsible for a higher risk of breast cancer among Asian people but a lower risk to Africans [61]. However, the efficacy of SNPs to evaluate the impacts of CXCL8 (− 251) strain in breast cancer remains challenging [5]. Activation of the CXCL8-CXCR1/2 axis in breast cancer is depicted in Fig. 1.

Copyright 2016 Elsevier). CXCL8 interacts with G protein-coupled receptors (GPCRs), such as CXCR1 or CXCR2, leading to G protein activation at the cellular level. Heterotrimeric Ga and bg subunits activate the major effectors PLC and PI3K to induce phosphorylation of PKC and Akt, respectively. The two signaling pathways have been shown to activate transcription factors involved in tumor cell survival, angiogenesis, and migration. CXCL8 also promotes cell proliferation, survival, motility, and invasion by activating non-receptor tyrosine kinases (e.g., Src and FAK) and members of the RhoGTPase family. Cell proliferation and survival are aided by an activated Raf-1/MAP/Erk signaling cascade. Dashed arrows show unconfirmed pathways implicated in the CXCL8 signaling axis
The diagram summarizes the major signaling pathways of CXCL8 in cancers (adapted with permission from Ref. [5],
Role of CXCL8-CXCR1/2 signaling axis in the tumor microenvironment
One of the first chemokines that are investigated for their pro-inflammatory role in the body is CXCL8. In the late 1980s. Neutrophil-activating factor (NAF)is a secretory protein released by LPS-stimulated blood monocytes stirred exocytosis of neutrophils and rapid release of reactive oxygen species, also known as an oxidative burst [62]. Chemokines such as CXCL1, CXCL5, CXCL7, and CXCL8 are produced and released by different forms of oncogenic cells and function in a paracrine manner by facilitating the proliferation and migration of cancer cells [63]. The expression of chemokines in human tumors also indicates tumor grade and metastatic progression. For example, patients with prostate cancer show marked release in serum CXCL8 levels compared to healthy controls, which correlates with stages of metastasis [61].
CXCR1/2, along with their ligand CXCL8, is proven to be essential for the function and operation of inflammatory mediators, tumor development, and metastasis. The signaling axis CXCL8-CXCR1/2 also involves in the pathogenesis of various disorders, including asthma, cystic fibrosis, and cancers. Complementary signaling in the tumor microenvironment between CXCL8 on cancer cells and CXCR1/2 on surrounding stromal cells is essential to cancer progression and metastasis and inhibits signaling. This axis may help control the proliferation of cancer stem cells (CSCs) and their self-renewal [1]. The signal plays a key role in the development of initial and adaptive immune responses as well as the maturation of precursor cells and hematopoiesis [2].
The tumor microenvironment is important in tumor development and metastasis. Tumor-associated macrophages, adipocytes, fibroblasts, and other types of non-malignant stromal cells are part of the tumor microenvironment, tumor development, and metastasis [64]. Oncogenic cells secrete boosted levels of CXCR1/2 ligands even when the expression of the receptors is amiss. This indicates CXCR1/2 ligands may act as a paracrine-like factor in the tumor microenvironment. In addition, the CXCR1/2 ligands played key roles in the infiltration of neutrophil tumors that facilitated the proliferation, invasion, and chemoresistance of cancer cells through increased angiogenic levels and growth factors produced by the neutrophils associated with the tumors [64]. Being the most copious cytokines in tumor tissues, CXCL8 facilitates tumor-associated macrophages (TAMs) dependent papillary thyroid carcinoma (PTC) invasion and tumor metastasis [65]. Adipose stromal cells (ASC) associated with obesity, which augments some types of cancer, such as prostate cancer, are conscripted by the CXCL1/8-CXCR1/2 axis into the tumor microenvironment [66]. In the blood, CXCR2 signaling controls the development and metastasis of breast cancer. Female mice that lack the CXCR2 gene show diminution in angiogenesis, the development of tumor cells, metastasis, and apoptosis [67]. Fluorescence imaging, both optical and laser-induced studies, shows the favorable correlation of tumor burden in the tumor microenvironment and expression of CXCR2 [68]. Within the tumor microenvironment of colon cancer, it was seen thatCXCL8 and CXCR2 facilitated cell development, progression, and metastasis of the same [69]. KrasLA1 mouse-derived LKR-13 lung adenocarcinoma cells have an immunogenic response to in-vitro anti-CXCR2 antibodies. The same genetically engineered cells developed into the human tumor in vaccinated mice. However, they are responsive to the antibody, suggesting the CXCR2 receptor plays a role in microenvironmental health related to the tumor. CXCL1 was identified in stromal cells to have mediated paracrine functions in the tumor microenvironment of an osteogenic prostate tumor via the CXCR2 receptor [70]. In a mixed cell xenograft model, therapeutic intervention with an inhibitor of CXCR2 had a delayed effect on tumor development. These findings culminated in the understanding that it is essential for stromal cells and cancer cells to interact for tumor cells to form, invade, and metastasis. As well as validate the interaction between CXCL8 and CXCR2 as a crucial therapeutic target [71].
Therapeutic potential of targeting CXCL8-CXCR1/2 axis in breast cancer
With the increase in gene copy numbers in CXCL1/2, CXCL1/2 expression is also high in breast cancer cells. The resultant paracrine loop of CXCL1/2 promotes chemoresistance and metastasis in breast cancer [72]. The formation of thrombin triggers CXCL1 and CXCR6 expression and secretion in the tumor. Against CXCL1, antibodies are produced that inhibit endothelial tube formation. Tumor formation, angiogenesis, and metastasis reduced by CXCL1 depletion in 4T1 cells were due to lower CXCL1 and higher CXCR2 expression in malignant ones than in premalignant ones [73]. In the epithelium of breast cancer cells, fibroblast growth factor receptors (FGFRs) are activated, resulting in the downregulation of transforming growth factor-beta1 (TGF-β1)/SMAD3 pathways in tumor tissue macrophages that have associated with increased CXCL1/CXCL2 expression [5]. A series of chemokines stimulated the invasive potential of breast cancer cells while inhibiting their invasiveness [74]. In order to promote breast cancer metastasis, the multipotent mesenchymal stem cells produce CXCL1 and CXCL5 for the recruitment of cancer cells. The inhibition by CXCL1, CXCL5, and CXCR2 antibodies, as well as by SB265610, was observed during this phase [6]. Mesenchymal stem cells formed CXCL1 and CXCL5 and caused breast cancer cells to metastasize to/in bone [11]. Antibodies that act against CXCL1, CXCL5, and CXCR2, along with SB265610, have been demonstrated to inhibit this process. CXCR2 shRNA (HCC827, 4T1) decreased cell invasion in human breast cancer cell lines but did not affect cell proliferation. CXCR2 knock-down in an orthotopic mouse model led to a decrease of spontaneous lung metastases by 40%. In the in vitro and in vivo mouse models, transfection with shRNA knock-down cells has also boosted paclitaxel and doxorubicin efficacy [11]. Xenograft studies featuring reparaxin resulted in reduced tumor growth and metastasis. In the breast cancer stem cells of human-derived metastatic cancer and invasive breast cancer, CXCL8 induces SRC, PI3 K, and MEK mediated EGFR/HER2 signaling pathways as well as boost these cells' growth capacity [11]. Correlating to the disease stage, the available levels of these chemical entities in breast cancer patients were higher than in healthy volunteers [43]. Increased expressions of CXCR2 ligands CXCL1, CXCL3, CXCL5, and CXCL7 were observed in breast cancer cells with drug resistance but with less metastatic potential in the xenograft mouse model [55]. When a person receives a microbial infection, macrophages at the site secrete CXCL8, attracting neutrophils and expressing CXCR1/2 at the infection target. CXCR2 is responsible for recruiting neutrophils at the target site of infection, whereas CXCR1 battles microbes by facilitating granule release and oxidative burst. The role of CXCL8 and CXCR1/2 in breast cancer is given in Table 1.
In breast cancer, the expression of either ALDH or CD24/CD44++ is characterized by tumor cells that shape tumors in immunocompromised mice [7]. A phenotype that represents two predominantly non-overlapping populations of cells, which was developed as a drug target for breast cancer stem cells (BCSCs), its expression was almost untraceable in greater amounts of (i.e., non-CSCs) tumor cells [9]. BCSCs have been shown to multiply in response to the inclusion of endogenous CXCL8, but limited molecular weight CXCR1/2 antagonists (reparixin) have been shown to multiply in response to the addition of endogenous CXCL8 [79]. Both monoclonal antibodies were able to inhibit the migration of stem cells into CSCs. When a FAS-FASL signal was added, a number of bulk tumor cells were killed, which indicates that synergistic effects are possible with chemotherapy. The application of weekly docetaxel and reparixin resulted in more sustained tumor growth inhibition than either treatment alone in in-vitro and in-vivo models of breast cancer. However, although reparixin was used along with chemotherapy, much lower amounts of CSCs were observed in the mice tumor when compared to mice treated with chemotherapy alone. This study finds that CXCL8 and FASL are released from cells within the tumor bulk that are dying during chemotherapy treatment [10]. CXCR1 on CSCs produces a survival signal on the surface that enables CSCs to withstand FASL-supplied apoptotic signals. Conversely, these cells experience programmed cell death by Fas-dependent apoptosis when CXCR1 signaling on CSCs is inhibited by reparixin.
By altering pathways linked with apoptosis and multidrug resistance, the CXCL8–CXCR1/2 signaling axis directly modulates tumor cell chemosensitivity [76, 80]. In vitro and in vivo studies reported deviant expression of CXCL8, which linked to progression, VEGF-independent angiogenesis, and chemoresistance of colorectal cancer [81, 82]. Similarly, CXCL8 in renal cancer cells induces the migration of mesenchymal stem cells, which play a key role in the development, metastasis, and drug resistance of cancers [83]. Resistance to kinase inhibitors like sunitinib is associated with increased expression of tumor-derived CXCL8 in clear cell renal cell carcinoma [84]. Anti-CXCL8 might make tumors more sensitive to sunitinib therapy in a nude mouse model [84]. In breast cancer, prostate cancer, and colorectal carcinoma, the CXCL8–CXCR1/2 signaling axis has been linked to chemotherapy resistance. In an in vitro study on colorectal cancer, the CXCL8-CXCR1/2 role in chemoresistance has been well characterized in colorectal cancer, where CXCL8 has been demonstrated to reduce oxaliplatin sensitivity [81]. In ovarian cancer, CXCL8 signaling has also been linked to enhanced resistance to cisplatin and paclitaxel [85]. In addition, a CXCR2 antagonist (SCH527123) has been demonstrated to make cells more sensitive to oxaliplatin in vivo [86].
Clinical trials in breast cancer
In a phase Ib study (NCT02001974) [87, 88], to evaluate the safety and define the pharmacokinetic (PK) profile of orally administered Reparixin in combination with Paclitaxel in HER 2 (Human epidermal growth factor receptor-2) negative metastatic breast cancer patients. The secondary objectives were to evaluate the effects of orally administered reparixin on cancer stem cell (CSC) markers, evaluate peripheral blood samples for enumeration of circulating tumor cells (CTCs), and assess disease response for indication of efficacy. In this study, 33 participants were involved, divided into three groups. Group 1 participants in this clinical trial receive Paclitaxel 80 mg/m2 i.v. (Days 1, 8, and 15 of a 28-day cycle) and Reparixin oral 400 mg three times daily (t.i.d.) 3 weeks on 1 week off (three to six patients). Group 2 participants in this clinical trial receive Paclitaxel 80 mg/m2 i.v. (Days 1, 8, and 15 of a 28-day cycle) and Reparixin oral 100% increase to 800 mg. Group 3 participants in this clinical trial receive Paclitaxel 80 mg/m2 i.v. (Days 1, 8, and 15 of a 28-day cycle) and Reparixin oral 50% increase to 1200 mg. All three groups receive the same intervention, association of Paclitaxel at fixed dosage with three increasing dosages of Reparixin. The first results of this study were posted on April 19, 2021. Weekly Paclitaxel and Reparixin combination in metastatic breast cancer (MBC) appeared to be safe and tolerable, with demonstrated responses in the enrolled population for this study in this clinical trial phase. Considering the safety data from the above clinical trial, a pilot study (NCT01861054) of single-agent reparixin was started to evaluate the safety and biological effects of orally administered reparixin in early breast cancer [89]. The primary objectives of this study were to evaluate the effects of orally administered reparixin on CSCs in the primary tumor, and the tumoral microenvironment in an early breast cancer population and the safety of oral reparixin administered three times daily (t.i.d.) for 21 consecutive days. The secondary objective was to define the pharmacokinetic (PK) profile of orally administered reparixin. In this study, 20 participants were involved divided into two groups assignment. Patients of Group A (ER+ and/or PR+/HER2−) were given Reparixin 1000 mg (two 500 mg tablets) for 21 consecutive days. Reparixin was administered orally, every 6–8 h t.i.d. preferably with food. Patients of Group B (ER−/PR−/HER2−) were given Reparixin 1000 mg (two 500 mg tablets) for 21 consecutive days. Reparixin was administered orally, every 6–8 h t.i.d. preferably with food. The first results of this study were posted on March 25, 2021. Reparixin appeared safe and well-tolerated. CSCs were reduced in several patients as measured by flow cytometry, suggesting targeting of CXCR1 on CSC. Flow cytometry indicated activity in the majority of patients [90]. However, later published evidence that the two breast cancer CSC populations investigated (i.e., ALDH+ and CD24/CD44+) reside in different areas of primary breast tumors and can transition from one phenotype to the other [91] may have an impact on the accuracy of CSC counts in this patient population. In general, the therapeutic significance of a ≥ 20% reduction in CSC after a single 21-day course of reparixin in this patient cohort is unknown, and it was outside the scope of this study. Also, in this trial, reparixin looked to be well-tolerated in terms of safety, with 10/20 participants suffering one or more adverse drug reactions (ADRs), all of which were of grade ≤ 2. Neither treatment-emergent adverse events (TEAE) leading to treatment cessation nor TEAE-related surgical delays were documented. However, the effect of therapies on neutrophil migration was not established in the research mentioned above, and it has to be determined in the clinical setting.
Conclusion
The CXCL8-CXCR1/2 axis function in inflammatory disorders ranging from cystic fibrosis, asthma, alzheimer's disease, and various cancer is well known. CXCL8 plays a significant role in initiating inflammatory immune responses. A variety of signaling channels are involved in the CXCL8-induced cellular signals. The axis is considered significant to the growth of different types of cancer. Genes involved in this signaling system are key players in the tumor microenvironment, promoting tumor growth and leading to the tumor's capacity to spread and metastasize. The combination of chemotherapy along with anti-CXCR2 agents has proven to be more successful than any treatment alone. Rather than hampering the expression or secretion of CXCL8, targeting the CXCL8 along with its receptors, CXCR1/2 may be the most successful strategy, which could still result in receptor activation from other chemokines. Clinical studies to establish and confirm inhibitors and antibodies targeting CXCL8-CXCR1/2 signaling pathways for inflammatory diseases are underway. While reparixin is found to be well-tolerated, a very low number of CSCs in typical primary breast tissues form an obstruction to effective assessment. Identification of clinical and cellular or molecular biomarkers of breast cancer gives way to a future direction of research in the (neo)adjuvant setting. The axis of CXCL8-CXCR1/2 performs the dual responsibility of promoting tumor formation and suppression of tumors. All the extensive evidence suggests that the targeted inhibition of CXCL8 could prove valuable in sensitizing tumor cells to chemotherapeutic drugs and increasing the survival of patients who have breast cancer. Close consideration should be paid to cultivating either inhibitors or stimulators of this axis. Overall assessment of the prognostic, analytical, and therapeutic value of the CXCL8-CXCR1/2 signaling axis in breast cancer is required.
References
Ha H, Debnath B, Neamati N (2017) Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics 7(6):1543–1588. https://doi.org/10.7150/thno.15625
Le Y, Zhou Y, Iribarren P, Wang J (2004) Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol 1(2):95–104
Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14(21):6735–6741. https://doi.org/10.1158/1078-0432.CCR-07-4843
Zlotnik A, Yoshie O (2012) The chemokine superfamily revisited. Immunity 36(5):705–716. https://doi.org/10.1016/j.immuni.2012.05.008
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, Wu K (2016) The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev 31:61–71. https://doi.org/10.1016/j.cytogfr.2016.08.002
Ruffini PA (2019) The CXCL8-CXCR1/2 axis as a therapeutic target in breast cancer stem-like cells. Front Oncol 9:40. https://doi.org/10.3389/fonc.2019.00040
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988. https://doi.org/10.1073/pnas.0530291100
Azenshtein E, Meshel T, Shina S, Barak N, Keydar I, Ben-Baruch A (2005) The angiogenic factors CXCL8 and VEGF in breast cancer: regulation by an array of pro-malignancy factors. Cancer Lett 217(1):73–86. https://doi.org/10.1016/j.canlet.2004.05.024
Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS (2009) Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 69(4):1302–1313. https://doi.org/10.1158/0008-5472.CAN-08-2741
Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS (2010) CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest 120(2):485–497. https://doi.org/10.1172/JCI39397
Singh JK, Farnie G, Bundred NJ, Simões BM, Shergill A, Landberg G, Howell SJ, Clarke RB (2013) Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and-independent mechanisms. Clin Cancer Res 19(3):643–656. https://doi.org/10.1158/1078-0432
Schröder J, Mrowietz U, Morita E, Christophers E (1987) Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol 139(10):3474–3483
Brat DJ, Bellail AC, Van Meir EG (2005) The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol 7(2):122–133. https://doi.org/10.1215/S1152851704001061
Baldwin ET, Weber IT, St Charles R, Xuan JC, Appella E, Yamada M, Matsushima K, Edwards BF, Clore GM, Gronenborn AM et al (1991) Crystal structure of interleukin 8: symbiosis of NMR and crystallography. Proc Natl Acad Sci USA 88(2):502–506. https://doi.org/10.1073/pnas.88.2.502
Nasser MW, Raghuwanshi SK, Grant DJ, Jala VR, Rajarathnam K, Richardson RM (2009) Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol 183(5):3425–3432. https://doi.org/10.4049/jimmunol.0900305
Joseph PRB, Rajarathnam K (2015) Solution NMR characterization of WT CXCL 8 monomer and dimer binding to CXCR 1 N-terminal domain. Protein Sci 24(1):81–92
Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI (1991) Structure and functional expression of a human interleukin-8 receptor. Science 253(5025):1278–1280. https://doi.org/10.1126/science.1840701
Kunsch C, Rosen CA (1993) NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 13(10):6137–6146. https://doi.org/10.1128/mcb.13.10.6137
Matsuo Y, Raimondo M, Woodward TA, Wallace MB, Gill KR, Tong Z, Burdick MD, Yang Z, Strieter RM, Hoffman RM, Guha S (2009) CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int J Cancer 125(5):1027–1037. https://doi.org/10.1002/ijc.24383
Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, Lokeshwar VB, Lokeshwar BL (2007) Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res 67(14):6854–6862. https://doi.org/10.1158/0008-5472.CAN-07-1162
Matsushima K, Baldwin ET, Mukaida N (1992) Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem Immunol 51:236–265. https://doi.org/10.1159/000319091
Park SH, Das BB, Casagrande F, Tian Y, Nothnagel HJ, Chu M, Kiefer H, Maier K, De Angelis AA, Marassi FM, Opella SJ (2012) Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature 491(7426):779–783. https://doi.org/10.1038/nature11580
Modi WS, Dean M, Seuanez HN, Mukaida N, Matsushima K, O’Brien SJ (1990) Monocyte-derived neutrophil chemotactic factor (MDNCF/IL-8) resides in a gene cluster along with several other members of the platelet factor 4 gene superfamily. Hum Genet 84(2):185–187. https://doi.org/10.1007/BF00208938
Ahuja SK, Özçelik T, Milatovitch A, Francke U, Murphy PM (1992) Molecular evolution of the human interleukin-8 receptor gene cluster. Nat Genet 2(1):31–36. https://doi.org/10.1038/ng0992-31
Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M (2002) Multiple control of interleukin-8 gene expression. J Leukoc Biol 72(5):847–855. https://doi.org/10.1189/jlb.72.5.847
Wald O, Shapira OM, Izhar U (2013) CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics 3(1):26–33. https://doi.org/10.7150/thno.4922
Podolin PL, Bolognese BJ, Foley JJ, Schmidt DB, Buckley PT, Widdowson KL, Jin Q, White JR, Lee JM, Goodman RB, Hagen TR, Kajikawa O, Marshall LA, Hay DW, Sarau HM (2002) A potent and selective nonpeptide antagonist of CXCR2 inhibits acute and chronic models of arthritis in the rabbit. J Immunol 169(11):6435–6444. https://doi.org/10.4049/jimmunol.169.11.6435
Catusse J, Liotard A, Loillier B, Pruneau D, Paquet JL (2003) Characterization of the molecular interactions of interleukin-8 (CXCL8), growth related oncogen alpha (CXCL1) and a non-peptide antagonist (SB 225002) with the human CXCR2. Biochem Pharmacol 65(5):813–821. https://doi.org/10.1016/s0006-2952(02)01619-2
Prado GN, Suetomi K, Shumate D, Maxwell C, Ravindran A, Rajarathnam K, Navarro J (2007) Chemokine signaling specificity: essential role for the N-terminal domain of chemokine receptors. Biochemistry 46(31):8961–8968. https://doi.org/10.1021/bi7004043
Ahuja SK, Lee JC, Murphy PM (1996) CXC chemokines bind to unique sets of selectivity determinants that can function independently and are broadly distributed on multiple domains of human interleukin-8 receptor B: determinants of high affinity binding and receptor activation are distinct. J Biol Chem 271(1):225–232. https://doi.org/10.1074/jbc.271.1.225
Bendrik C, Dabrosin C (2009) Estradiol increases IL-8 secretion of normal human breast tissue and breast cancer in vivo. J Immunol 182(1):371–378
Shao N, Lu Z, Zhang Y, Wang M, Li W, Hu Z, Wang S, Lin Y (2015) Interleukin-8 upregulates integrin β3 expression and promotes estrogen receptor-negative breast cancer cell invasion by activating the PI3K/Akt/NF-κB pathway. Cancer Lett 364(2):165–172
de Campos Zuccari DAP, Leonel C, Castro R, Gelaleti GB, Jardim BV, Moscheta MG, Regiani VR, Ferreira LC, Lopes JR, de Santi ND (2012) An immunohistochemical study of interleukin-8 (IL-8) in breast cancer. Acta Histochem 114(6):571–576. https://doi.org/10.1016/j.acthis.2011.10.007
Liu Q, Li A, Yu S, Qin S, Han N, Pestell RG, Han X, Wu K (2018) DACH1 antagonizes CXCL8 to repress tumorigenesis of lung adenocarcinoma and improve prognosis. J Hematol Oncol 11(1):1–16
Gatla HR, Zou Y, Uddin MM, Singha B, Bu P, Vancura A, Vancurova I (2017) Histone deacetylase (HDAC) inhibition induces IκB kinase (IKK)-dependent interleukin-8/CXCL8 expression in ovarian cancer cells. J Biol Chem 292(12):5043–5054
Wang L-H, Cheng GZ, Park S, Shu S, He L, Kong W, Zhang W, Yuan Z, Cheng JQ (2008) Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Curr Cancer Drug Targets 8(1):2–6. https://doi.org/10.2174/156800908783497159
MacManus CF, Pettigrew J, Seaton A, Wilson C, Maxwell PJ, Berlingeri S, Purcell C, McGurk M, Johnston PG, Waugh DJ (2007) Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res 5(7):737–748. https://doi.org/10.1158/1541-7786.MCR-07-0032
Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL (1996) Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem 271(5):2832–2838. https://doi.org/10.1074/jbc.271.5.2832
Luppi F, Longo A, De Boer W, Rabe K, Hiemstra P (2007) Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer 56(1):25–33. https://doi.org/10.1016/j.lungcan.2006.11.014
Murphy C, McGurk M, Pettigrew J, Santinelli A, Mazzucchelli R, Johnston PG, Montironi R, Waugh DJ (2005) Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res 11(11):4117–4127. https://doi.org/10.1158/1078-0432.CCR-04-1518
Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, Hernandez-Ramirez D, Bockenstedt PL, Liaw PC, Cabral AR, Knight JS (2015) Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol 67(11):2990–3003. https://doi.org/10.1002/art.39247
Lang K, Niggemann B, Zanker KS, Entschladen F (2002) Signal processing in migrating T24 human bladder carcinoma cells: role of the autocrine interleukin-8 loop. Int J Cancer 99(5):673–680. https://doi.org/10.1002/ijc.10424
Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, Vermeulen PB, Dirix LY (2004) Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res 10(21):7157–7162. https://doi.org/10.1158/1078-0432.CCR-04-0812
Kamalakar A, Bendre MS, Washam CL, Fowler TW, Carver A, Dilley JD, Bracey JW, Akel NS, Margulies AG, Skinner RA, Swain FL, Hogue WR, Montgomery CO, Lahiji P, Maher JJ, Leitzel KE, Ali SM, Lipton A, Nicholas RW, Gaddy D, Suva LJ (2014) Circulating interleukin-8 levels explain breast cancer osteolysis in mice and humans. Bone 61:176–185. https://doi.org/10.1016/j.bone.2014.01.015
Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso NE, Harris CC (2011) Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst 103(14):1112–1122. https://doi.org/10.1093/jnci/djr216
Bălăşoiu M, Bălăşoiu AT, Mogoantă SŞ, Bărbălan A, Stepan AE, Ciurea RN, Alexandru DO, Enescu A, Mogoantă L (2014) Serum and tumor microenvironment IL-8 values in different stages of colorectal cancer. Rom J Morphol Embryol 55(2 Suppl):575–578
David JM, Dominguez C, Hamilton DH, Palena C (2016) The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines (Basel) 4(3):22. https://doi.org/10.3390/vaccines4030022
Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, Huang RP (2004) Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer 109(4):507–515. https://doi.org/10.1002/ijc.11724
Singh JK, Simoes BM, Clarke RB, Bundred NJ (2013) Targeting IL-8 signalling to inhibit breast cancer stem cell activity. Expert Opin Ther Targets 17(11):1235–1241. https://doi.org/10.1517/14728222.2013.835398
Singh JK, Simões BM, Howell SJ, Farnie G, Clarke RB (2013) Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res 15(4):1–9. https://doi.org/10.1186/bcr3436
Chelouche-Lev D, Miller CP, Tellez C, Ruiz M, Bar-Eli M, Price JE (2004) Different signalling pathways regulate VEGF and IL-8 expression in breast cancer: implications for therapy. Eur J Cancer 40(16):2509–2518. https://doi.org/10.1016/j.ejca.2004.05.024
Marjon PL, Bobrovnikova-Marjon EV, Abcouwer SF (2004) Expression of the pro-angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress. Mol Cancer 3(1):1–12. https://doi.org/10.1186/1476-4598-3-4
Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, Zhu YF, Wang SM (2007) Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer 121(9):1949–1957. https://doi.org/10.1002/ijc.22930
De Larco JE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC, Furcht LT (2001) A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol 158(2):639–646. https://doi.org/10.1016/S0002-9440(10)64005-9
Singh B, Berry JA, Vincent LE, Lucci A (2006) Involvement of IL-8 in COX-2-mediated bone metastases from breast cancer. J Surg Res 134(1):44–51. https://doi.org/10.1016/j.jss.2006.03.018
Wu K, Katiyar S, Li A, Liu M, Ju X, Popov VM, Jiao X, Lisanti MP, Casola A, Pestell RG (2008) Dachshund inhibits oncogene-induced breast cancer cellular migration and invasion through suppression of interleukin-8. Proc Natl Acad Sci USA 105(19):6924–6929. https://doi.org/10.1073/pnas.0802085105
Sharma B, Varney ML, Saxena S, Wu L, Singh RK (2016) Induction of CXCR2 ligands, stem cell-like phenotype, and metastasis in chemotherapy-resistant breast cancer cells. Cancer Lett 372(2):192–200. https://doi.org/10.1016/j.canlet.2015.12.011
Rody A, Karn T, Liedtke C, Pusztai L, Ruckhaeberle E, Hanker L, Gaetje R, Solbach C, Ahr A, Metzler D (2011) A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res 13(5):1–12
Freund A, Jolivel V, Durand S, Kersual N, Chalbos D, Chavey C, Vignon F, Lazennec G (2004) Mechanisms underlying differential expression of interleukin-8 in breast cancer cells. Oncogene 23:6105–6114
Snoussi K, Mahfoudh W, Bouaouina N, Fekih M, Khairi H, Helal AN, Chouchane L (2010) Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness. BMC Cancer 10(1):283. https://doi.org/10.1186/1471-2407-10-283
Huang J, Yao JL, Zhang L, Bourne PA, Quinn AM, di Sant’Agnese PA, Reeder JE (2005) Differential expression of interleukin-8 and its receptors in the neuroendocrine and non-neuroendocrine compartments of prostate cancer. Am J Pathol 166(6):1807–1815. https://doi.org/10.1016/S0002-9440(10)62490-X
Peveri P, Walz A, Dewald B, Baggiolini M (1988) A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med 167(5):1547–1559. https://doi.org/10.1084/jem.167.5.1547
Sun H, Chung W-C, Ryu S-H, Ju Z, Tran HT, Kim E, Kurie JM, Koo JS (2008) Cyclic AMP-responsive element binding protein- and nuclear factor-κB–regulated CXC chemokine gene expression in lung carcinogenesis. Cancer Prev Res 1(5):316–328. https://doi.org/10.1158/1940-6207.CAPR-07-0002
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4(1):71–78. https://doi.org/10.1038/nrc1256
Singh S, Sadanandam A, Varney ML, Nannuru KC, Singh RK (2010) Small interfering RNA-mediated CXCR1 or CXCR2 knock-down inhibits melanoma tumor growth and invasion. Int J Cancer 126(2):328–336. https://doi.org/10.1002/ijc.24714
Fang W, Ye L, Shen L, Cai J, Huang F, Wei Q, Fei X, Chen X, Guan H, Wang W, Li X, Ning G (2014) Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis 35(8):1780–1787. https://doi.org/10.1093/carcin/bgu060
Sharma B, Nannuru KC, Varney ML, Singh RK (2015) Host Cxcr2-dependent regulation of mammary tumor growth and metastasis. Clin Exp Metastasis 32(1):65–72. https://doi.org/10.1007/s10585-014-9691-0
Leung SJ, Rice PS, Barton JK (2015) In vivo molecular mapping of the tumor microenvironment in an azoxymethane-treated mouse model of colon carcinogenesis. Lasers Surg Med 47(1):40–49. https://doi.org/10.1002/lsm.22309
Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D, Chung HK, Choi D, LaBonte MJ, Ladner RD, Nagulapalli Venkata KC, Rosenberg DO, Petasis NA, Lenz HJ, Hong YK (2012) Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer 106(11):1833–1841. https://doi.org/10.1038/bjc.2012.177
Wislez M, Fujimoto N, Izzo JG, Hanna AE, Cody DD, Langley RR, Tang H, Burdick MD, Sato M, Minna JD, Mao L, Wistuba I, Strieter RM, Kurie JM (2006) High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res 66(8):4198–4207. https://doi.org/10.1158/0008-5472.CAN-05-3842
Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, Mohri D, Miyabayashi K, Asaoka Y, Maeda S, Ikenoue T, Tateishi K, Wright CV, Koike K, Omata M, Moses HL (2011) Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest 121(10):4106–4117. https://doi.org/10.1172/JCI42754
Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE (2012) A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150(1):165–178. https://doi.org/10.1016/j.cell.2012.04.042
Tang Z, Yu M, Miller F, Berk RS, Tromp G, Kosir MA (2008) Increased invasion through basement membrane by CXCL7-transfected breast cells. Am J Surg 196(5):690–696. https://doi.org/10.1016/j.amjsurg.2008.08.001
Bohrer LR, Schwertfeger KL (2012) Macrophages promote fibroblast growth factor receptor-driven tumor cell migration and invasion in a CXCR2-dependent manner. Mol Cancer Res 10(10):1294–1305. https://doi.org/10.1158/1541-7786.MCR-12-0275
Shao N, Chen LH, Ye RY, Lin Y, Wang SM (2013) The depletion of interleukin-8 causes cell cycle arrest and increases the efficacy of docetaxel in breast cancer cells. Biochem Biophys Res Commun 431(3):535–541. https://doi.org/10.1016/j.bbrc.2013.01.022
Shi Z, Yang WM, Chen LP, Yang DH, Zhou Q, Zhu J, Chen JJ, Huang RC, Chen ZS, Huang RP (2012) Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat 135(3):737–747. https://doi.org/10.1007/s10549-012-2196-0
Sharma B, Nawandar DM, Nannuru KC, Varney ML, Singh RK (2013) Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Mol Cancer Ther 12(5):799–808. https://doi.org/10.1158/1535-7163.MCT-12-0529
Nannuru KC, Sharma B, Varney ML, Singh RK (2011) Role of chemokine receptor CXCR2 expression in mammary tumor growth, angiogenesis and metastasis. J Carcinogenes 10:40. https://doi.org/10.4103/1477-3163.92308
Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G, Cervellera MN, Di Cioccio V, Cesta MC, Galliera E (2004) Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci USA 101(32):11791–11796
Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ (2008) Interleukin-8 signaling attenuates TRAIL-and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther 7(9):2649–2661
Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM (2011) Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer 128(9):2038–2049
Ueda T, Shimada E, Urakawa T (1994) Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol 29(4):423–429
Liang-kuan B, Nan Z, Cheng L, Fu-Ding L, Tian-Xin L, Xu-Jun X, Chun J, Jin-Li H, Hai H, Cai-Xia Z (2014) Kidney cancer cells secrete IL-8 to activate Akt and promote migration of mesenchymal stem cells. In: Urologic oncology: seminars and original investigations, vol 5. Elsevier, Amsterdam, pp 607–612
Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian C-N, Kahnoski R, Futreal PA, Furge KA, Teh BT (2010) Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 70(3):1063–1071
Wang Y, Qu Y, Niu XL, Sun WJ, Zhang XL, Li LZ (2011) Autocrine production of interleukin-8 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cytokine 56(2):365–375
Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D, Benhaim L, Paez D, Rosenberg DO, Venkata KCN (2012) The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther 11(6):1353–1364
Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, Perez RP, Kato G, Wicha M (2017) Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2–negative metastatic breast cancer. Clin Cancer Res 23(18):5358–5365
Lim S, Park J, Shim MK, Um W, Yoon HY, Ryu JH, Lim D-K, Kim K (2019) Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 9(25):7906
Goldstein L, Sparano J, Perez R, Vito C, Reuben J, Landis M, McCanna S, Ruffini P, Cristofanilli M, Chang J (2013) Abstract OT2-6-03: a single arm, preoperative, pilot study to evaluate the safety and biological effects of orally administered reparixin in early breast cancer patients who are candidates for surgery. AACR
Goldstein LJ, Perez RP, Yardley DA, Han LK, Reuben JM, McCanna S, Butler B, Ruffini PA, Chang JC (2016) Abstract CT057: a single-arm, preoperative, pilot study to evaluate the safety and biological effects of orally administered reparixin in early breast cancer patients who are candidates for surgery. AACR
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD (2014) Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep 2(1):78–91
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AM, and NN conducted the literature search and did writing. KHS contributed to writing. JM, and VT conceptualized the ideas and contributed to writing and preparing the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent obtained from all individual participants was included in the study. All authors have read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mishra, A., Suman, K.H., Nair, N. et al. An updated review on the role of the CXCL8-CXCR1/2 axis in the progression and metastasis of breast cancer. Mol Biol Rep 48, 6551–6561 (2021). https://doi.org/10.1007/s11033-021-06648-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06648-8