Abstract
Background
Pluripotent stem cells (PSCs) produced by somatic cell reprogramming self-renew in culture and can differentiate into any cell type, representing a powerful tool for disease modeling, drug screening, regenerative medicine, and the discovery of personalized therapies to treat tissue-specific pathologies. We previously reported the directed differentiation of human PSCs into epidermal stem and progenitor cells (ESPCs) and 3D epidermis to model the inherited syndrome Fanconi anemia (FA), wherein epidermal cell-junctional defects discovered using this system were validated in patient populations. Here, we describe in detail the corresponding protocol for generating PSC-derived keratinocytes using a distinct, normal PSC line (209.2 PSC).
Methods and results
Our approach modifies previous protocols to minimize spontaneous cell death and terminal differentiation, eliminate cell stress-inducing keratinocyte selection steps, and reduce total protocol duration and cost. Independent donor-derived PSC lines were converted into ESPCs through the addition of relevant morphogens and a ROCK inhibitor. Results for the 209.2 PSC line highlight consistencies in 2D and also variable features in 3D epidermis compared to the previously published FA-PSC lines. 209.2 PSC-derived ESPCs exhibited a basal cell phenotype while maintaining the capacity to form epidermal organotypic rafts with morphology consistent with fetal epidermis. Transcriptional analyses demonstrated 209.2 ESPCs express epidermis-selective markers and not early endoderm markers, thus supporting an immature stage of p63+ epidermal development.
Conclusions
This protocol provides an accelerated path for the generation of human ESPCs and 3D epidermal models to study normal epidermal development and homeostasis, elucidate mechanisms of epidermal disease pathogenesis, and provides a platform for developing personalized therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human epidermis comprises the outermost layer of the skin and has essential functions in thermoregulation, protection against excessive water loss, and as a key barrier against physical, chemical, environmental, and infectious insults [1, 2]. The epidermis is capable of continued regeneration throughout the life of the organism and this is driven by epidermal stem and progenitor cells (ESPCs) in the basal cell layer. This basal compartment harbors keratinocytes with unique self-renewal and proliferative capacity to regenerate the entire stratified squamous epithelium [3]. Due to substantial differences in tissue structure between mouse and human skin [4,5,6], isolation of stem and progenitor keratinocytes directly from human donors has been critical to advance the field of human skin engineering and therapeutic transplantation, and to take advantage of technological advances in gene editing and genetic profiling to elucidate molecular, cellular, and structural mechanisms underlying dermatologic diseases [7,8,9]. However, relevant methodologies can be hampered by donor-specific variability and a limited capacity to culture keratinocytes from patients with select diseases [4, 10, 11]. Existing methodologies also do not recapitulate the stepwise progression of embryonic development to gain insights into the pathogenesis of inherited diseases [4, 10]. A suitable alternative approach to generate an unlimited source of donor-specific ESPCs is provided by reprogramming somatic cells into induced pluripotent stem cells (PSCs) via expression of four transcription factors: OCT3/4 (POU5F1), SOX2, KLF4, and c-MYC [12]. These embryonic stem cell (ESC)-like cells possess unlimited self-renewal capacity and the ability to differentiate into cell types derived from all three germ layers of the human embryo: ectoderm, endoderm, and mesoderm. After gastrulation, these layers undergo patterning and differentiation to generate distinct functional organs. The endoderm gives rise to the digestive and respiratory systems and internal organs. The mesoderm gives rise to skeletal, muscle, and connective tissues, whereas the ectoderm gives rise to the nervous system and epidermis. With regard to the skin, several groups have focused their efforts on developing protocols for the directed differentiation of PSCs into keratinocyte lineages [4, 13,14,15] by activating relevant signaling pathways that drive epidermal development and morphogenesis [14, 16, 17].
Overall, successful differentiation of PSCs into keratinocytes consists of four main steps: initiation, selection/specification, enrichment, and expansion. Initiation of ectodermal differentiation with concomitant inhibition of neural fate is induced by culturing cells in the presence of retinoic acid (RA) and bone morphogenic protein-4 (BMP4) [4, 13, 14, 16]. Selection/specification of cells with an epidermal cell fate is achieved by incubating cells in defined keratinocyte culture media [4, 13] or by plating cells on an extracellular matrix that mimics the basement membrane environment [14]. Enrichment for stem cell populations is carried out by selective attachment to collagen type IV and/or type I (Col IV and/or Col I)-coated surfaces, followed by a 1–2 week culture period for cells to reach sufficient confluency [13, 14]. Expansion of the stem cell population occurs in serum-free epidermal keratinocyte media using Col IV, Col I, or fibronectin-coated plates. Upon application of various protocols for the generation of ESPCs, we noted spontaneous cell death and differentiation after Day 10 of the differentiation process as a limiting factor. Therefore, we built on previously published strategies [4, 13, 14] and exploited reported roles for the Rho-associated protein kinase (ROCK) inhibitor Y-27632 [18,19,20,21] to develop a simplified, efficient, and cost-effective protocol for the directed differentiation of PSCs into ESPCs that eliminates the need for feeder cells and stress-prone selection steps as well as minimizes spontaneous cell death resulting in a renewable source of ESPCs with shortened generation time. While ROCK generally functions as a critical regulator of actin cytoskeleton organization, cell adhesion, migration, and motility in many cell types [19, 20, 22, 23], we took advantage of unique functions of ROCK1 and ROCK2 isoforms in keratinocytes to support PSC directed differentiation. ROCK1 and ROCK2 have opposing roles in keratinocytes [18, 20]; ROCK1 inhibits whereas ROCK2 promotes keratinocyte terminal differentiation as has been demonstrated by genetic loss-of-function studies [20]. While Y-27632 inhibits both ROCK isoforms [20, 24], the net effect of keratinocyte treatment with this drug is inhibition of terminal differentiation with enhanced cell proliferation, growth, and stemness [18,19,20], possibly due to the more prominent functional role of ROCK2 in keratinocytes as compared to ROCK1 [18]. We also hypothesized that Y-27632 treatment would enhance keratinocyte cell survival since it has been reported to protect ESCs from apoptotic cell death while, importantly, not affecting their differentiation potential [19, 21].
Itoh et al. [4] were the first to report the generation of functional keratinocytes from normal and epidermolysis bullosa patient-derived PSCs within 30 days. Therein, PSCs were maintained on mitomycin C-treated mouse embryonic fibroblast (MEF) feeder layers and seeded at low density on matrigel-coated plates in the absence of feeders prior to keratinocyte differentiation. The PSCs were then cultured in MEF-conditioned ESC media for 1 day, followed by an initiation step where Defined Keratinocyte Serum-Free Media (DKSFM) supplemented with RA (1 μM) and BMP4 (10 ng/mL) was added for 4 days. Cells were then cultured in DKSFM media until Day 30 for the selection of epidermal-like cells, and flow sorted for integrin α6high/β4high cells to enrich the Keratin 14-positive (K14+) population followed by plating on fibronectin and collagen-coated dishes for expansion. Another study from Kogut et al. [13] reported efficient differentiation of feeder-free PSCs into K14/K5-positive keratinocytes capable of generating epidermis when transplanted into immunodeficient mice. In the corresponding 24 day-protocol, low density PSCs were seeded on Geltrex and Col I-coated dishes and cultured in N2B27 media for 1 day at low oxygen conditions (5%). Cells were then switched to atmospheric oxygen (20%) at the initiation step, which was performed as in the previous study but using higher concentrations of BMP4. Epidermal-like cells were then selected by culturing the cells first in DKSFM media and then in CnT07 media. On day 24, K14+ cells were enriched by selective attachment to Col IV/Col I-coated plates followed by expansion on Col I-coated plates after 2 weeks in CnT07 media. Another sophisticated approach to differentiate PSCs into a keratinocyte-lineage was reported by Petrova et al. [14]. In this 24 day-protocol, PSCs were cultured on matrigel-coated dishes and allowed to grow for 3 days at low oxygen conditions (5%). Dense, undifferentiated cells were then switched to atmospheric oxygen (20%) at the initiation step, which differed from the previous studies as it required incubation in mTeSR1 media for 7 days. The selection step was performed by plating cells on a 3D decellularized human dermal fibroblast extracellular matrix for 7 days with extended addition of RA and BMP4. The resulting cells were then enriched by rapid adherence to Col IV-coated plates as in the previous study but were cultured in DKSFM media supplemented with RA for 7 days, followed by expansion in Epi Life media.
Our protocol is described in detail below. Briefly, feeder-free PSCs were cultured in mTeSR1 media and then switched to a defined keratinocyte media containing RA (1 μM) and BMP4 (25 ng/mL) to induce ectodermal differentiation. Cells were then cultured in the progenitor cell promoting media CnT07 that was later supplemented with Y-27632 for the remainder of the differentiation process to prevent terminal differentiation and stimulate cell growth and survival [18,19,20,21]. At day 20, ESPCs were expanded on Col IV-coated plates, in the absence of any additional enrichment by rapid cell attachment (Fig. 1). Our protocol includes important modifications to previously published approaches. We used RA and high concentrations of BMP4 only during the initiation step [13, 14], eliminating the need for continuous addition of these morphogens in subsequent phases [14], thus reducing cost and enhancing protocol ease. We avoided successive changes in PSC culture conditions and the use of feeder cells entirely, thus increasing translational applicability. Plating small PSC colonies at low density prevented the risk of spontaneous differentiation. We eliminated cell stress-inducing conditions such as hyperoxia, frequent sequential passaging, and ESPC flow sorting, thus maximizing cell viability. The use of CnT07 media for sustaining keratinocyte fate, later supplemented with Y-27632 to maximize stemness [18,19,20,21], replaced DKSFM media entirely and resulted in greater ESPC yields by decreasing cell death. Finally, our protocol does not require the additional steps to enrich for the K14+ population, thus decreasing the overall protocol time 20%–33% as compared to other published protocols resulting in the generation of differentiated keratinocytes in 20 days.
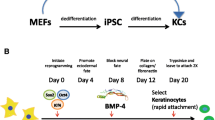
Directed differentiation of PSCs into ESPCs. A Schematic overview of the differentiation strategy used for the differentiation of PSCs into ESPCs. Media and reagents added at each stage are indicated. Y-27632 is added on Day 10 and provided continuously from this point forward. At Day 20, cells are grown on Col IV-coated plates in the absence of the rapid cell attachment step. B Bright-field image of PSCs at day -1 before passaging (70–80% confluency). C Bright-field image of confluent PSC-derived ESPCs at passage 1 showing the formation of cohesive sheets of cells. At this point, cells can be passaged to new Col IV-coated plates. D and E After the first passage (not shown), 4 × 105 cells per well were seeded in 6-well plates, harvested after 4 days, counted, and re-seeded at 4 × 105 cells per well. Population doubling time (D) defined as \(PDT = \frac{4 \times \log (2)}{{{\text{log}}\left( {\frac{ Total\ cell\ number }{{400,000}}} \right)}}\) and number of generations (E) defined as \(n = \frac{4}{{{\text{PDT}}}}\) were calculated for PSC-derived ESPCs during the final four passages [33, 34]. n = 3 technical replicates. Error bars represent standard deviation. Total number of passages = 5
This simplified protocol has been successfully used to culture keratinocytes from five distinct PSC lines derived from five independent donors, including two PSC lines previously published [10]. Therein we provided evidence that this approach can be used as a robust and simplified model to study normal skin development and donor-specific disease pathogenesis with relevance to human skin, as well as a personalized platform for testing candidate treatments. 209.2 PSC-derived ESPCs generated with this protocol maintained the capacity to be cultured for a minimum of four passages using serum-free conditions (Fig. 1C, D) while retaining a basal cell phenotype as determined by expression of the basal cell markers p63, integrin α6 (INTα6), integrin β4 (INTβ4), and Collagen type XVII alpha 1 (COL17A1) as well as lacking expression of the pluripotency markers SOX2 and OCT4 (Fig. 2A, B). A previous report wherein p63+ cells were derived from human ESCs did not conclusively identify these as keratinocytes or even as ectodermal cells, suggesting that the cells may represent an incomplete or immature form of p63+ epithelial lineage development [25]. We thus performed additional studies demonstrating that the p63+ ESPCs derived with the current protocol contained features of the epidermal lineage not shared by other epithelial tissues, namely the expression of skin-enriched genes C1ORF68 and LCE2B [26, 27]. We also show that ESPCs express TGM1 and TGM2, proteins involved in the formation of the cornified cell envelope that are not expressed in endodermally-derived oral mucosal epithelia [28]. Finally, we show that the ESPCs lack expression of GATA6, which is expressed in primitive endoderm but not ectodermally-derived epidermal cells [29, 30] (Fig. 2C). The ESPCs generated using the currently described protocol, were capable of forming PSC-derived epidermal organotypic rafts (PSC-EORs) when subjected to organotypic epithelial raft culture [31]. Interestingly, in contrast to the terminal differentiation into cornified epidermal cells observed with the PSC lines used for the FA studies [10], 209.2 PSC-derived EORs showed immature morphology consistent with fetal epidermis (Fig. 2D, E), thus representing an immature stage of epidermal keratinocyte development which will be valuable for determining critical factors that induce (or inhibit) terminal keratinocyte differentiation [32].
Basal characteristics of PSC-derived ESPCS. A Relative expression of the pluripotency markers OCT4 and SOX2 in ESPCs normalized to the expression in PSCs and relative expression of the basal cell markers ΔNp63, INTα6, and INTβ4 in ESPCs normalized to the expression in near-diploid immortalized keratinocytes that form skin (NIKS) [35]. n = 2–3 biological replicates. Error bars represent standard error of mean. *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001 using one-way ANOVA with Tukey’s test. B Confocal analysis confirming the proliferating basal cell identity of PSC-derived ESPCs at passage 2 by the expression of Ki67, COL17A1, and p63. E-cadherin (epithelial marker) was expressed whereas Vimentin (mesenchymal marker) and Filaggrin (suprabasal marker) expression were absent. Scale bar, 50 μm. C Relative expression of skin-enriched genes C1ORF68, LCE2B, TGM1, and TGM2 normalized to the expression in NIKS and relative expression of the primitive endoderm marker GATA6 normalized to the expression in normal oral keratinocytes (NOKS) [26,27,28,29,30]. Since distinct geographies of the mouth derive from ectoderm or endoderm [36], NOKS isolated from gingival tissue [37] were used as positive control for endoderm. ND = not detected. n = 1–3 biological replicates. Error bars represent standard error of mean. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001 using one-way ANOVA with Tukey’s test. D Histological section of 3D engineered epidermis generated using PSC-derived ESPCs. Scale bar, 100 μm. E 3D stratified architecture and cellular organization of distinct keratinocyte cell types comprising the PSC-EORs confirmed by staining for the basal cell marker COL17A1 and the suprabasal cell differentiation marker, K10. Scale bar, 50 μm
Materials
Equipment
-
1.
37 °C beads bath
-
2.
37 °C, 5% CO2 incubator
-
3.
Biological safety hood
-
4.
Cell culture centrifuge (room temperature)
-
5.
Laminar flow hood
-
6.
Dissection microscope
-
7.
Inverted microscope
-
8.
Nutating mixer
-
9.
Cell lifter (Corning, Cat#3008)
-
10.
6-well plates (Falcon, Cat#353046)
-
11.
60 mm tissue culture treated plates (Corning, Cat#430166)
Reagents
-
1.
The human 209.2 PSC line was generated by and obtained from the Cincinnati Children’s Hospital Medical Center Pluripotent Stem Cell Facility
-
2.
DMEM/F12 (Thermo Fisher Scientific, Cat#11330-302)
-
3.
hESC-qualified Matrigel (BD Biosciences, Cat#354277)
-
4.
mTeSR1 media (Stem Cell Technologies, Cat#5850)
-
5.
Dispase (Thermo Fisher Scientific, Cat#17105-041)
-
6.
Dulbecco’s phosphate-buffered saline (PBS) (Thermo Fisher Scientific, Cat#14200-075)
-
7.
1 × Defined Keratinocyte-SFM media (DKSFM) (Thermo Fisher Scientific, Cat#10744-019)
-
8.
Retinoic acid (RA) (Sigma-Aldrich, Cat#R2625)
-
9.
Human recombinant bone morphogenetic protein-4 (BMP4) (StemGent, Cat#03-0007)
-
10.
PCT Epidermal Keratinocyte Medium, Defined (CnT07) (CELLnTEC, Cat#CnT-07)
-
11.
Y-27632 Dihydrochloride (Enzo Life Sciences, Cat#ALX-270-333-M005)
-
12.
Glacial acetic acid (Fisher Scientific, Cat#A38-500)
-
13.
Collagen Type IV (Col IV) (Sigma-Aldrich, Cat#C5533-5MG)
-
14.
0.25% Trypsin–EDTA (Thermo Fisher Scientific, Cat#25200-056)
-
15.
DMEM (Thermo Fisher Scientific, Cat#11965-092)
-
16.
Fetal bovine serum (FBS) (GE Healthcare, Cat#SH30396.03)
-
17.
Fungizone/Amphotericin B (Omega Scientific, Cat#FG-70)
-
18.
Penicillin–Streptomycin, (Thermo Fisher Scientific, Cat#15140-122)
-
19.
Dimethyl sulfoxide (DMSO) (Sigma, Cat#D8418-250ML)
-
20.
Hydrochloric acid (HCl) (Fisher Scientific, Cat#A144-500)
-
21.
Antibodies: Rabbit p63α D2K8X (Cell Signaling, Cat#13109S); Mouse Keratin 10 (K10) RKSE60 (abcam, Cat#ab9025); Mouse Vimentin V9 (Santa Cruz, Cat#sc-6260); Goat E-cadherin (R&D Systems, Cat#AF648); Rabbit Ki67 (abcam, Cat#ab15580); Rabbit Collagen type XVII alpha 1 (abcam, Cat#ab184996); Mouse Filaggrin AKH1 (Santa Cruz, Cat#sc-66192); AlexaFluor 488 Donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories, Cat#711-545-152); AlexaFluor 488 Donkey anti-goat IgG (Jackson Immunoresearch Laboratories, Cat#705-545-147); Donkey anti-mouse IgG Rhodamine Red (Jackson Immunoresearch Laboratories, Cat#715-295-150); AlexaFluor 647 Donkey anti-goat IgG (Jackson Immunoresearch Laboratories, Cat#705-605-147); AlexaFluor 647 Donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories, Cat#711-605-152); ProLong Gold antifade/DAPI (Thermo Fisher Scientific, Cat#P36931).
-
22.
RT-qPCR primers: β-actin Forward 5′-AGAGCTACGAGCTGCCTGAC-3′ and Reverse 5′-AGCACTGTGTTGGCGTACAG-3′; ΔNp63 Forward 5′-ATTGTAAGGGTCTCGGGGTGGG-3′ and Reverse 5′-GAGTCTGGGCATTGTTTTCCAGGT-3′; Integrin α6 Forward 5′-ATTCGGGAGTACCTTGGTGGAT-3′ and Reverse 5′-TTCTCTTGAAGAAACCACACTTC-3′; Integrin β4 Forward 5′-GGGTCCAGGAAGATCCATTT-3′ and Reverse 5′-AGTCGCAATACGGGTACAGG-3′; POU5F1 (OCT4) Forward 5′-AGTGCCCGAAACCCACACTGC-3′ and Reverse 5′-CGCTGCTTGATCGCTTGCCC-3′; SOX2 Forward 5′-GCTTAGCCTCGTCGATGAAC-3′ and Reverse 5′-AACCCCAAGATGCACAACTC-3′; C1ORF68 Forward 5′-GAAAGGTTCGGGACTAGGGG-3′ and Reverse 5′-GGCCTGTCACACAGACAGTT-3′; LCE2B Forward 5′-CTACAGCCTGATGCTTAACCC-3′ and Reverse 5′-TGAGTCTTTGTGGTCGCTGTC-3′; TGM1 Forward 5′-GAACGACTGCTGGATGAAGAGG-3′ and Reverse 5′-CTTGATGGACTCCACAGAGCAG-3′; TGM2 Forward 5′-TGTGGCACCAAGTACCTGCTCA-3′ and Reverse 5′-GCACCTTGATGAGGTTGGACTC-3′; GATA6 Forward 5′-CCCCCACAACACAACCTACA-3′ and Reverse 5′-GTAGAGCCCATCTTGACCCG-3′.
Solutions
-
1.
Matrigel: Prepare 1X, 2X, and 4X aliquots as follows and store them at − 80 °C. See Step 1.1 for matrigel plating. (See Note 1).
-
1.
Thaw the matrigel on ice overnight at 4 °C.
-
2.
Swirl the bottle to ensure even mixing.
-
3.
To calculate the volume of matrigel required to make 1X, 2X, and 4X matrigel aliquots, divide the “Dilution Factor” listed in the certificate of analysis for the hESC-qualified matrigel by 4, 2, and 1, respectively. One 1X matrigel aliquot is enough to cover one 6-well plate.
-
4.
Dispense matrigel into appropriate aliquots using pre-cooled tubes.
-
5.
Immediately freeze aliquots at − 80 °C.
-
1.
-
2.
DKSFM complete media: Basal DKSFM media supplemented with 1% penicillin–streptomycin and growth supplement.
-
3.
RA: 10 mM stock solution reconstituted in DMSO. Aliquot and store at − 20 °C.
-
4.
BMP4: 100 μg/mL stock solution reconstituted in sterile 4 mM HCl. Aliquot and store at − 80 °C.
-
5.
Col IV: 2 mg/mL stock solution reconstituted in sterile 0.25% glacial acetic acid. Aliquot and store at − 20 °C (See Note 2).
-
6.
Y-27632: 10 mM stock solution reconstituted in sterile 1X PBS. Aliquot and store at − 20 °C.
-
7.
CnT07 complete media: Basal CnT07 media supplemented with 1% penicillin–streptomycin and supplements A, B, and C.
-
8.
DMEM complete media: Basal DMEM media supplemented with 10% FBS, 1% penicillin–streptomycin, and 0.5% Fungizone.
Methods
-
1.
Day -1:
-
1.
Prepare matrigel-coated plates
-
1.
Remove one 1X aliquot of matrigel from the − 80 °C freezer. Keep it on ice inside the hood until ready to be used (See Note 3).
-
2.
Transfer 6.25 mL of cold DMEM/F12 media to a 15 mL conical tube.
-
3.
Take 1 mL of cold DMEM/F12 media from Step 1.1.2 and add it to the vial with matrigel.
-
4.
Dissolve the matrigel by resuspending it gently in the media. Mix well.
-
5.
Transfer the matrigel suspension to the 15 mL conical tube from Step 1.1.2. Mix well by pipetting.
-
6.
Use 1 mL of matrigel solution per well to cover one 6-well plate. Shake the plate gently side to side, back and forth to spread the matrigel solution evenly over the surface.
-
7.
Incubate the plate for at least 1 h at room temperature inside the hood before plating PSCs (See Note 4).
-
1.
-
2.
Warm the dispase, DMEM/F12, and mTeSR1 media in the beads bath. (See Note 5).
-
3.
Inspect one well of PSCs (70%–80% confluent) (Fig. 1B) for spontaneous differentiation (white centers) using a dissecting microscope. Remove any white areas using a pulled glass pipette inside the laminar flow hood (See Note 6).
-
4.
Remove the media from the well and add 2 mL of DMEM/F12 media. Swirl the plate to wash the cells.
-
5.
Remove the media and add 1 mL of dispase. Incubate in the 37 °C incubator for 4 min.
-
6.
Following incubation, use an inverted microscope to confirm that the edges of the colonies are folded up. This indicates that the cells have started to lift from the plate and are ready for detachment (See Note 7).
-
7.
Gently remove the dispase and add 2 mL of DMEM/F12 media. Swirl the plate to wash the cells and gently remove the media (See Note 8).
-
8.
Repeat the washes two more times. Do not remove the media from the last wash.
-
9.
Gently scrape the colonies from the well and transfer the cell suspension to a 15 mL conical tube.
-
10.
Centrifuge the cells at 1200 rpm for 4 min.
-
11.
While waiting for the centrifugation, remove the matrigel from the 6-well plate coated in Step 1.1 and immediately add 1 mL of mTeSR1 media per well.
-
12.
After centrifugation, transfer the tube to the biological safety hood, remove the wash media, and gently resuspend the pellet in 10 mL of mTeSR1 (1:10 dilution) to break up any cell clumps (See Note 9).
-
13.
Add 1 mL of the cell suspension to each well. Discard or harvest the remaining cells for additional analyses.
-
14.
Check the size and density of the cells in the cultures using a dissecting microscope (See Note 10).
-
15.
Transfer the plate to the 37 °C incubator and shake it gently side to side, back and forth to distribute the cells evenly.
-
1.
-
2.
Day 0:
-
1.
Confirm that healthy, undifferentiated PSCs have attached to the plate.
-
2.
Prepare the Initiation Media
-
1.
Warm DKSFM complete in the 37 °C beads bath.
-
2.
Supplement DKSFM complete with 1 μM RA (stock 10 mM) and 25 ng/mL BMP4 (stock 100 μg/mL).
-
1.
-
3.
Remove the media from the wells and add 2 mL of Initiation Media to each well.
-
4.
Incubate the cells at 37 °C for 48 h.
-
1.
-
3.
Day 2: repeat steps 2.2–2.4 (See Note 11).
-
4.
Day 4–Day 8:
-
1.
Warm the CnT07 complete media in the 37 °C beads bath.
-
2.
Remove the media from the wells and add 2 mL of CnT07 complete media to each well.
-
3.
Incubate the cells at 37 °C.
-
4.
Add fresh media every other day (Steps 4.1–4.3)
-
1.
-
5.
Day 10–Day 16:
-
1.
Warm the CnT07 complete media in the 37 ºC beads bath and keep a Y-27362 aliquot (stock 10 mM) on ice.
-
2.
Remove the media from the wells and add 2 mL of CnT07 complete media supplemented with 10 μM Y-27632 (stock 10 mM) to each well (See Note 12).
-
3.
Incubate the cells at 37 °C.
-
4.
Add fresh media every other day (Steps 5.1–5.3)
-
1.
-
6.
Day 18:
-
1.
Monitor the confluency of the cells under an inverted microscope (10 × magnification). If the cells are > 80% confluent, proceed to Step 7 (See Note 13).
-
2.
Otherwise, repeat Steps 5.1–5.3 and passage the cells to Col IV-coated plates at day 20 following the instructions in Steps 7 and 8.
-
1.
-
7.
Prepare the Col IV-coated plates (See Note 14 and Note 15).
-
1.
Thaw the Col IV aliquot(s) (stock 2 mg/mL) on ice for 2–3 h at 4 °C.
-
2.
Dilute Col IV (stock 2 mg/mL) to a final concentration of 15 μg/mL in sterile 0.25% glacial acetic acid in PBS.
-
3.
Add 1 mL of the Col IV solution to each 60 mm plate and shake the plates to distribute the solution evenly (See Note 16).
-
4.
Incubate for 1 h at room temperature inside the biological safety hood. Keep the plates uncovered.
-
5.
After the incubation, remove the solution and wash the plates with sterile 1X PBS.
-
6.
Remove the PBS and wash the plates with sterile ddH2O.
-
7.
Remove the ddH2O and air-dry the plates inside the biological safety hood. Keep the plates uncovered.
-
8.
Once dried, use the plates immediately for passaging cells following Step 8 or seal the plates with parafilm, return them to their original packaging, and store at − 20 °C for up to 6 months.
-
1.
-
8.
Passaging cells to Col IV-coated plates
-
1.
Warm the CnT07 complete media, DMEM complete media, and 0.25% Trypsin–EDTA in the 37 °C beads bath. Keep a Y-27362 aliquot (stock 10 mM) on ice and the Col IV-coated plates from Step 7 at room temperature (See Note 17).
-
2.
Remove the media from the wells and add 2 mL of sterile 1X PBS to each well. Gently shake the plate to wash the cells.
-
3.
Remove the 1X PBS and add 0.75 mL of 0.25% Trypsin–EDTA to each well. Gently shake the plate to distribute it evenly.
-
4.
Incubate the plate in the 37 °C incubator for 8 min. Tap the plate and confirm that the cells are detaching from the plate using an inverted microscope.
-
5.
Collect and resuspend the cells from 6 wells in 8 mL of DMEM complete media and transfer the cell suspension to a 15 mL conical tube.
-
6.
Centrifuge cells at 1500 rpm for 5 min.
-
7.
Remove supernatant and wash the cells by adding 2 mL of CnT07 complete media to the pellet.
-
8.
Gently resuspend the cells up and down with a p1000 micropipette to obtain a single cell suspension.
-
9.
Centrifuge cells at 1500 rpm for 5 min.
-
10.
Prepare the Col IV-coated plates by adding 3 mL of CnT07 complete media supplemented with 10 μM of Y-27632.
-
11.
After the centrifugation, remove the supernatant and resuspend the pellet in 3 mL of CnT07 complete media supplemented with 10 μM of Y-27632 using a p1000 micropipette (1:3 dilution).
-
12.
Add 1 mL of the cell suspension to each 60 mm plate.
-
13.
Transfer the plates to the 37 °C incubator and shake them gently side to side, back and forth to distribute the cells evenly.
-
14.
Change media on the plates every other day.
-
15.
Once the ESPCs are ~ 85% confluent (Fig. 1C), repeat Steps 8.1–8.13 to passage cells to new Col IV-coated plates using a 1:4 or 1:5 dilution (See Note 18). Otherwise, PSC-derived ESPCs can be harvested for additional validation analyses or engineered into 3D PSC-EORs (Fig. 2) (See Note 19).
-
1.
Notes
-
Note 1: Matrigel polymerizes at room temperature. Keep matrigel on ice at all times and use pre-cooled materials.
-
Note 2: This step takes approximately 2–3 h. Seal the top of the glass vial with parafilm to prevent any contamination and dissolve the collagen powder by securely placing the vial in a nutating mixer at 2 °C–8 °C.
-
Note 3: One 1X matrigel aliquot is used to coat one 6-well plate. For more or bigger plates, adjust the volume of matrigel solution needed accordingly.
-
Note 4: Matrigel-coated plates can be prepared for immediate use or sealed with parafilm and stored at 4 °C for up to a week. If using stored matrigel-coated plates, warm the plate at room temperature for at least 30 min before plating the PSCs.
-
Note 5: A water bath can also be used to warm the reagents. We use beads baths to minimize the risk of contamination.
-
Note 6: Start the differentiation process with low passage, healthy, and undifferentiated PSCs. Undifferentiated PSCs grow as compact, flat, and multicellular colonies (Fig. 1B). These exhibit a high cytoplasmic to nuclear ratio and prominent nucleoli. With time, the center of the colonies become multilayered, dense, and differentiate, resulting in a bright appearance under phase contrast microscopy. These areas appear white when compared to the edges of the colonies and must be removed using a pulled glass pipette or a sterile micropipette tip. To make the pulled glass pipette, the narrow part of the glass pipette is heated with the flame of a burner and pulled away from the other end, stretching the glass. Then, the broken end of the glass is placed in the flame to generate a curve-shaped end that will be used to remove differentiated cells.
-
Note 7: If the edges are not folded, incubate the plate for an additional minute in the 37 °C incubator.
-
Note 8: It is recommended to add the media gently on the side of the well to prevent any cell detachment.
-
Note 9: Small colonies/clumps of cells need to be plated at low density. Cell dilution can vary depending on the initial confluency of the plate.
-
Note 10: If large clumps of cells are still visible, triturate these using a p1000 micropipette.
-
Note 11: Confirm morphological changes in the colonies. Colonies will no longer exhibit PSC features (rounded, defined edges, and undifferentiated colonies).
-
Note 12: Y-27632 is added to maintain keratinocyte lineage specification and prevent the substantial cell death observed in the cultures when using other keratinocyte differentiation protocols.
-
Note 13: Cells should not be overconfluent. Use of overconfluent, unhealthy cultures can result in growth defects and cell death.
-
Note 14: This step can be done on the same day as passaging the cells or days in advance and stored at − 20 °C for up to 6 months.
-
Note 15: Calculations vary depending on the total volume of Col IV solution needed. We suggest using 1 mL of the Col IV solution to cover a 60 mm plate.
-
Note 16: Do not discard the original packaging of the plates if storing them at − 20 °C for future use.
-
Note 17: If Col IV-coated plates were stored for future use, incubate the plates for at least 1 h at room temperature and apply ultraviolet light for 15 min inside the biological safety hood. Wash the plates with sterile 1X PBS and air-dry before proceeding to Step 8.2.
-
Note 18: In our hands, PSC-derived ESPCs (85% confluent) can be cultured for at least 4 passages using this differentiation protocol.
-
Note 19: In this study, 3D PSC-EOR cultures were generated using porcine Col I as an alternative to rat Col I [10]. Use of rat Col I may improve epidermal architecture and terminal differentiation. Furthermore, variability in PSC-EOR formation using this protocol suggests that, as observed for other PSC-derived tissues, generation of fully differentiated epidermis can vary depending on the donor PSC line used.
References
Proksch E, Brandner JM, Jensen JM (2008) The skin: an indispensable barrier. Exp Dermatol 17(12):1063–1072. https://doi.org/10.1111/j.1600-0625.2008.00786.x
Metcalfe AD, Ferguson MW (2007) Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4(14):413–437. https://doi.org/10.1098/rsif.2006.0179
Belokhvostova D, Bernzanskyte I, Cujba AM, Jowett G, Marshall L, Prueller J, Watt FM (2018) Homeostasis, regeneration and tumour formation in the mammalian epidermis. Int J Dev Biol 62(6-7–8):571–582. https://doi.org/10.1387/ijdb.170341fw
Itoh M, Kiuru M, Cairo MS, Christiano AM (2011) Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci USA 108(21):8797–8802. https://doi.org/10.1073/pnas.1100332108
Schmook FP, Meingassner JG, Billich A (2001) Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int J Pharm 215(1–2):51–56. https://doi.org/10.1016/s0378-5173(00)00665-7
Zomer HD, Trentin AG (2018) Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci 90(1):3–12. https://doi.org/10.1016/j.jdermsci.2017.12.009
Charruyer A, Ghadially R (2009) Stem cells and tissue-engineered skin. Skin Pharmacol Physiol 22(2):55–62. https://doi.org/10.1159/000178864
Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, Scaglione D, Reichelt J, Klausegger A, Kneisz D, Romano O, Seconetti AS, Contin R, Enzo E, Jurman I, Carulli S, Jacobsen F, Luecke T, Lehnhardt M, Fischer M, Kueckelhaus M, Quaglino D, Morgante M, Bicciato S, Bondanza S, De Luca M (2017) Regeneration of the entire human epidermis using transgenic stem cells. Nature 551:327–332. https://doi.org/10.1038/nature24487
Uitto J, McGrath JA, Rodeck U, Bruckner-Tuderman L, Robinson EC (2010) Progress in epidermolysis bullosa research: toward treatment and cure. J Invest Dermatol 130(7):1778–1784. https://doi.org/10.1038/jid.2010.90
Ruiz-Torres S, Brusadelli MG, Witte DP, Wikenheiser-Brokamp KA, Sauter S, Nelson AS, Sertorio M, Chlon TM, Lane A, Mehta PA, Myers KC, Bedard MC, Pal B, Supp DM, Lambert PF, Komurov K, Butsch-Kovacic M, Davies SM, Wells SI (2021) Inherited DNA repair defects disrupt the structure and function of human skin. Cell Stem Cell 28(3):424-435.e6. https://doi.org/10.1016/j.stem.2020.10.012
Hoskins EE, Gunawardena RW, Habash KB, Wise-Draper TM, Jansen M, Knudsen ES, Wells SI (2008) Coordinate regulation of Fanconi anemia gene expression occurs through the Rb/E2F pathway. Oncogene 27(35):4798–4808. https://doi.org/10.1038/onc.2008.121
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. https://doi.org/10.1016/j.cell.2007.11.019
Kogut I, Roop DR, Bilousova G (2014) Differentiation of human induced pluripotent stem cells into a keratinocyte lineage. Methods Mol Biol 1195:1–12. https://doi.org/10.1007/7651_2013_64
Petrova A, Celli A, Jacquet L, Dafou D, Crumrine D, Hupe M, Arno M, Hobbs C, Cvoro A, Karagiannis P, Devito L, Sun R, Adame LC, Vaughan R, McGrath JA, Mauro TM, Ilic D (2014) 3D in vitro model of a functional epidermal permeability barrier from human embryonic stem cells and induced pluripotent stem cells. Stem Cell Reports 2(5):675–689. https://doi.org/10.1016/j.stemcr.2014.03.009
Lee J, Rabbani CC, Gao H, Steinhart MR, Woodruff BM, Pflum ZE, Kim A, Heller S, Liu Y, Shipchandler TZ, Koehler KR (2020) Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582(7812):399–404. https://doi.org/10.1038/s41586-020-2352-3
Stern CD (2005) Neural induction: old problem, new findings, yet more questions. Development 132(9):2007–2021. https://doi.org/10.1242/dev.01794
Chang C, Hemmati-Brivanlou A (1998) Cell fate determination in embryonic ectoderm. J Neurobiol 36(2):128–151
McMullan R, Lax S, Robertson VH, Radford DJ, Broad S, Watt FM, Rowles A, Croft DR, Olson MF, Hotchin NA (2003) Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol 13(24):2185–2189. https://doi.org/10.1016/j.cub.2003.11.050
An L, Ling P, Cui J, Wang J, Zhu X, Liu J, Dai Y, Liu Y, Yang L, Du F (2018) ROCK inhibitor Y-27632 maintains the propagation and characteristics of hair follicle stem cells. Am J Transl Res 10(11):3689–3700
Lock FE, Hotchin NA (2009) Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PLoS ONE 4(12):e8190. https://doi.org/10.1371/journal.pone.0008190
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa SI, Muguruma K, Sasai Y (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25:681–686. https://doi.org/10.1038/nbt1310
Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM (2003) Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci USA 100(19):10788–10793. https://doi.org/10.1073/pnas.1834401100
Heasman SJ, Ridley AJ (2008) Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9(9):690–701. https://doi.org/10.1038/nrm2476
Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S (2000) Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 57(5):976–983
Dabelsteen S, Hercule P, Barron P, Rice M, Dorsainville G, Rheinwald JG (2009) Epithelial cells derived from human embryonic stem cells display p16INK4A senescence, hypermotility, and differentiation properties shared by many p63+ somatic cell types. Stem Cells 27(6):1388–1399. https://doi.org/10.1002/stem.64
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F (2015) Tissue-based map of the human proteome. Science 347(6220):1260419. https://doi.org/10.1126/science.1260419
The Human Protein Atlas, The Skin-specific Proteome <https://www.proteinatlas.org/humanproteome/tissue/skin>
Chermnykh ES, Alpeeva EV, Vorotelyak EA (2020) Transglutaminase 3: the involvement in epithelial differentiation and cancer. Cells 9(9):1996. https://doi.org/10.3390/cells9091996
Schrode N, Saiz N, Di Talia S, Hadjantonakis AK (2014) GATA6 levels modulate primitive endoderm cell gate choice and timing in the mouse blastocyst. Dev Cell 29(4):454–467. https://doi.org/10.1016/j.devcel.2014.04.011
Fisher JB, Pulakanti K, Rao S, Duncan SA (2017) GATA6 is essential for endoderm formation from human pluripotent stem cells. Biol Open 6(7):1084–1095. https://doi.org/10.1242/bio.026120
Lee D, Norby K, Hayes M, Chiu YF, Sugden B, Lambert PF (2016) Using organotypic epithelial tissue culture to study the human papillomavirus life cycle. Curr Protoc Microbiol. 41:14B.8.1-14B.8.19. https://doi.org/10.1002/cpmc.4
Johnen C, Chinnici C, Triolo F, Plettig J, Bräutigam K, Amico G, Young M, Over P, Esteban-Vives R, Schmelzer E, Conaldi PG, Turner M, Thompson R, Zeilinger K, Rubin P, Vizzini G, Gridelli B, Gerlach JC (2013) Phenotypical characterization of 6–21-week gestational age human dermis and epidermal cell isolation methods for in vitro studies on epidermal progenitors. Burns 39(2):300–310. https://doi.org/10.1016/j.burns.2012.05.025
Bhat PJ (2008) Galactose regulon of yeast: from genetics to systems biology, 1st edn. Springer, Berlin, pp 26–27. https://doi.org/10.1007/978-3-540-74015-5
Roth, V. (2006). Doubling time computing. <http://www.doubling-time.com/compute.php>
Hoskins EE, Morreale RJ, Werner SP, Higginbotham JM, Laimins LA, Lambert PF, Brown DR, Gillison ML, Nuovo GJ, Witte DP, Kim M, Davies SM, Mehta PA, Butsch Kovacic M, Wikenheiser-Brokamp KA, Wells SI (2012) The Fanconi anemia pathway limits human papillomavirus replication. J Virol 86(15):8131–8138. https://doi.org/10.1128/JVI.00408-12
Chen J, Jacox LA, Saldanha F, Sive H (2017) Mouth development. Wiley Interdiscip Rev Dev Biol 6(5):e275. https://doi.org/10.1002/wdev.275
Piboonniyom S, Duensing S, Swilling NW, Hasskarl J, Hinds PW, Münger K (2003) Abrogation of the retinoblastoma tumor suppressor checkpoint during keratinocyte immortalization is not sufficient for induction of centrosome-mediated genomic instability. Cancer Res 63(2):476–483
Acknowledgements
We thank Dr. James Wells, Dr. Christopher Mayhew, and Amy Pitstick from the Pluripotent Stem Cell Core for reagents. This study was supported by NIH R01 grants CA223790 and CA228113, and by a grant from the Fanconi Anemia Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ruiz-Torres, S., Lambert, P.F., Wikenheiser-Brokamp, K.A. et al. Directed differentiation of human pluripotent stem cells into epidermal stem and progenitor cells. Mol Biol Rep 48, 6213–6222 (2021). https://doi.org/10.1007/s11033-021-06588-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06588-3