Abstract
Our previous study showed that soy milks could contain high levels of active soybean trypsin inhibitors (SBTI) if they were not properly processed. This study investigated the effects of consuming active SBTI on pancreatic weights, histology, trypsinogen production and expression of STAT3, receptors for androgen (AR) and estrogen (ER) in pancreas, liver and uterus of rats. Weanling Sprague–Dawley rats were randomly divided into 3 groups (8 females and 8 males/group) and fed diets containing either 20% casein protein (Casein) or 20% soy protein (SP) in the presence of high (1.42 BAEE unit/µg, SP + SBTI) or low (0.2 BAEE unit/µg, SP-SBTI) levels of active SBTI for 8 weeks. Ingestion of SP + SBTI diet markedly increased pancreatic weights and trypsinogen content (p < 0.01), and caused acinar cell hypertrophy, and reduced pancreatic STAT3, p-STAT3, AR and ERβ content, and increased uterine ERα and ERβ compared to the Casein or SP-SBTI diets (p < 0.05). The two SP-containing diets lowered hepatic STAT3, p-STAT3, and pancreatic ERα, and increased hepatic ERα and ERβ content in the female rats compared to the Casein diet (p < 0.05). This study demonstrated for the first time that consumption of high level of active SBTI not only increased pancreatic weights and acinar cell secretions, but also attenuated the expression of pancreatic STAT3, p-STAT3, AR, and ERβ proteins in both sexes and increased uterine ERα and ERβ content, and that dietary soy protein affected hepatic STAT3, p-STAT3, ERα and ERβ in a gender-dependent manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soy foods have become increasingly popular in Western diets in the last two decades. However, soybeans are one of the legumes containing the highest amount of trypsin inhibitors (TI), anti-nutritional factors naturally present in soybean seeds [1]. The major soybean TI (SBTI) are Kunitz trypsin inhibitor (KTI) and Bowman-Birk inhibitor (BBI), and both can strongly suppress the activity of pancreas-derived trypsin in the gastrointestinal tract thereby decreasing protein digestibility [2, 3]. KTI and BBI can be inactivated by thermal processes such as baking or boiling [3]. BBI is more heat stable than KTI because BBI has seven intra-chain disulfide bonds, and KTI only has 2 intra-chain disulfide bonds [2].
If soy foods/products are not heated long enough during processing, the active SBTI remaining in the soy foods can be very high. Our previous study showed that the active SBTI content in different brands of soy milks sold on the market were quite variable as their manufacturing processes, particularly the thermal durations and temperatures, have not been standardized, and that some commercial products contained up to 55.6% active SBTI of the raw soybean seeds [3]. However, the potential adverse effects of consuming high levels of active SBTI have not been fully understood.
Trypsin is one of the main protein digestive enzymes in the gastrointestinal tract, and is produced and released as trypsinogen, the inactive form of trypsin, from pancreas. Trypsinogen is activated after cleavage by enteropeptidase in duodenum. Acinar cells in the pancreas are responsible for synthesis, storage and secretion of trypsinogen [4], and synthesis of trypsinogen in the pancreas is regulated through a negative feedback mechanism. Once the enzymatic activity of trypsin in gastrointestinal tract is inhibited, the pancreas is stimulated to synthesize and secrete more enzyme from the endocrine glands. Continuing stimulation can overwork the pancreas [5]. It has been shown that consumption of active SBTI caused pancreatic hypertrophy, hyperplasia [6] and even tumor formation [7, 8]. However, the other effects of consumption of active SBTI such as on expression of genes for signal transducer and activator of transcription 3 (STAT3) and receptors for androgen (AR) and estrogen (ER) that are involved in the regulation of pancreatic functions have not been investigated.
STAT3 is expressed in various types of cells including pancreatic β cells. Inactive STAT3 is located in the cytoplasma and STAT3 activation is typically induced by phosphorylation. Once activated, phosphorylated STAT3 (p-STAT3) translocates into the nucleus and functions as a transcription factor for targeted genes. Pancreas-specific depletion of STAT3 reduced vascular endothelial growth factor A (VEGF-A) expression and microvascular density in the pancreas and caused glucose intolerance and impaired insulin secretion in mice [9]. AR in the pancreatic β-cells modulates β cell function in a gender-specific manner [10]. In the males, testosterone deficiency or AR depletion suppress glucose-stimulated insulin secretion whereas testosterone excess or AR activation in the females are associated with insulin resistance, hyperglycemia and β cell failure [11, 12].
ER has two subtypes, α and β. Both ERα and ERβ exist in pancreatic β-cells [13] and play important roles in the regulation of β-cell proliferation and insulin biosynthesis. It has been shown that ERβ selective agonist WAY200070 increased glucose-induced insulin release and pancreatic β-cell mass and improved glucose and insulin sensitivity in both normal and diabetic mice[14]. The mechanism involved is that activation of ERβ triggers the closure of ATP-sensitive K+ channel and enhance glucose-induced [Ca2+] oscillations and insulin release [15]. ERα mediates the stimulatory effects of 17β-estradiol on insulin content with possible involvement of ERK1/2 [13]. In addition, estrogen reduces β-cell loss and improves insulin resistance [16].
The objectives of this study were to examine the effects of dietary active SBTI at the levels similar to that contained in some of the commercially sold soy milks reported in our previous study [3] on pancreatic weights, histology, and trypsinogen content as well as expression of STAT3, p-STAT3, AR and ER in pancreas, liver and uterus of rats.
Materials and methods
Chemicals and reagents
Vitamin-free casein was purchased from Harlan Tekland (Madison, VI). Soy proteins (SP) containing high or low levels of active SBTI were provided by Dr. Stephen Gleddie at Agriculture and Agri-Food Canada (Ottawa, ON, Canada). The DC™ protein assay kit, precast polyacrylamide Criterion ™ TGX™ Stain-Free 12% gels, and horseradish peroxidase-conjugated goat anti-rabbit IgG antibodies were purchased from Bio-Rad Laboratories (Mississauga, ON, Canada). Rabbit polyclonal anti-STAT3 (C-20), anti-ERα (H-184), anti-ERβ (H-150), anti-AR (N-20), and mouse monoclonal anti-p-STAT3 (B-7) antibodies were purchased from Santa Cruz Biotechnology Inc. (Mississauga, ON, Canada). SuperSignal® West Dura Extended Duration Substrate for chemiluminescence ECL kit were from Thermo Scientific (Waltham, MA, USA). Western blot recycling kit was from Alpha Diagnostic International Inc. (San Antonio, TX, USA). SensoLyt™ red protease assay kits were obtained from AnaSpec Corporate (San Jose, USA). SBTI, bovine trypsin, hematoxylin and eosin were obtained from Sigma Chemical (Oakville, ON, Canada). Skim milk powder was purchased from Bulk Barn (Ottawa, ON, Canada). 17β-estradiol and testosterone ELISA kits were from Abcam Inc. (Toronto, ON, Canada). Glucose colorimetric assay kit was from Cayman Chemical Company (Ann Arbor, MI, USA) and rat ultrasensitive insulin ELISA kit was from ALPCO (Salem, NH, USA).
Preparation of soy proteins and measurement of SBTI activities
Soy proteins were prepared from the same soybean line, and their nutrient content including total protein, total fat, carbohydrate, total fiber and isoflavones were determined as described previously [17]. Activities of SBTI in soy proteins were measured according to the American Association of Cereal Chemmists’ Standard Method 22-40.01 [18].
Animals and diets
Animal experimental protocol was approved by the Health Canada Animal Care Committee, and all animal care and handling followed the guidelines of Canadian Council for Animal Care. Weanling Sprague–Dawley rats (Charles River, St Constant, Quebec, Canada) were housed individually in wire bottom cages in an environmentally controlled room maintained at 22 ± 1 °C and 60% relative humidity with a 12-h light/dark cycle. Rats were randomly divided into three groups (8 male and 8 female rats/group). After one week of acclimation, rats had ad libitum access to water and one of the 3 experimental diets for 8 weeks. The experimental diets contained either 20% casein protein (Casein), or 20% SP in the presence of high (SP + SBTI, containing 1.42 BAEE unit/µg or 46.7% of the activity contained in raw soybean) or low (SP-SBTI, containing 0.2 BAEE unit/µg or 6.5% of the activity contained in raw soybean) levels of active SBTI (Table 1). SP used in the preparation of SP + SBTI and SP-SBTI diets were isolated from the same soybean line, and were identical in all the compositions except for their content of active SBTI as a result of different durations of thermal processing. The levels of active SBTI in SP + SBTI or SP-SBTI diets were similar to the high or low levels of active SBTI contained in the commercially sold soy milks reported in our previous study [3].
The diets were formulated following AIN-93G recommendations for rodents [19] except that casein was replaced by equal amounts of SP containing high or low levels of active SBTI in SP + SBTI and SP-SBTI diets, respectively. The diets were pelleted. Body weight and food intake were measured twice weekly. After 8 weeks of feeding, the rats were fasted overnight and euthanized via exsanguination from the abdominal aorta while under 3% isoflurane anesthesia. Liver, pancreas, and uterus were collected, weighed and frozen immediately in liquid nitrogen. Tissues were kept at −80 °C until analysis. One portion of the pancreas from the same area of each rat was fixed in 4% formaldehyde for histological analysis.
Hematoxylin and eosin staining
The fixed pancreatic samples were embedded in paraffin and sectioned. The sections were stained with hematoxylin and eosin. Briefly, the section slides were taken through brief changes of xylene, alcohol and water to hydrate the tissue. The slides were then stained with nuclear dye (hematoxylin) and rinsed, then stained in the counterstain (eosin). After rinses, the slides were dehydrated in water, alcohol and xylene, and then mounted with coverslips. Images were taken with a Zeiss 3 Axiocam microscope using the AxioVision 4.6 (Carl Zeiss, Toronto, ON) at 200 × magnification.
Tissue protein extraction and Western blotting
Frozen tissues were cut (~ 0.25 cm × 0.25 cm) and extracted in complete Frack’s buffer containing cOmplete™ mini protease inhibitor cocktail and Halt phosphatase inhibitor cocktail. The samples were then homogenized using the Polytron homogenizer and centrifuged for 20 min at 15,700 × g at 4 °C. The supernatant was collected and quantified for protein concentration using the Bio-Rad DC™ protein assay kit.
Eighty µg of total tissue protein were heat denatured and separated on a 12% Criterion™ TGX™ precast gel under SDS-PAGE conditions, and then transferred onto Immun-Blot PVDF membranes. Membranes were blocked for 1 h at room temperature with 5% skim milk in TBS-T (Tris-buffered saline with 0.5% Tween-20 solution) and then probed overnight with primary antibodies diluted (1:500 for AR, ERβ, 1:1000 for ERα, STAT3, and p-STAT3, 1:5000 for trypsinogen,1:10,000 for β-actin) in 5% skim milk with TBS-T at 4 °C. Membranes were then washed 2 × 7 min in TBS-T and then incubated with secondary antibodies at a dilution of 1:10,000 for 1 h at room temperature. After wash, the blots were incubated with SuperSignal™ West Dura Enhanced chemiluminescence ECL substrate as per manufacturer’s instructions, and then subjected to different exposure times using the BIO-RAD ChemiDoc™ MP Imaging System. To be reprobed with different antibody, membranes were stripped using the Western blot recycling kit. The specific protein bands were quantified using Scion Image Software. Abundance of the target proteins were normalised by their respective β-actin.
Serum 17β-estradiol, testosterone, insulin and glucose levels
Serum 17β-estradiol, testosterone and insulin concentrations were measured using the ELISA kits following manufacturers’instructions. Serum glucose levels were determined using the colorimetric assay kit.
Statistical analysis
The results are presented as means ± SEM. Data were analysed using one-way or two-way analysis of variance, and differences between means were assessed by Tukey HSD post-hoc test. A probability value of < 0.05 was considered to be statistically significant. Data analyses were conducted using Statistica Version 13.1 (StatSoft).
Results
Food intake, body and organ weights
Both male and female rats fed SP + SBTI diet had significantly higher pancreatic weights than those fed Casein or SP-SBTI diets (p < 0.05), and their body weight gains (BWG) were not different among diets (p > 0.05, Table 2). The SP + SBTI diet increased food intake and lowered the food efficiency (ratio of BWG to food intake) in the male rats compared to the Casein diet (p < 0.05). The food intake and food efficiency in the female rats were not different among diets (p > 0.05). The weights of liver and uterus were not different among diets (p > 0.05, data not shown).
Serum 17β-estradiol, testosterone, insulin and glucose levels
Serum 17β-estradiol concentrations were significantly higher in the females than in the males (p < 0.01), and testosterone levels were not detectable in the females using the current assay kit. Serum glucose levsls were higher in the males than in the females (p < 0.001, Table 2) and insulin levels were not gender different (p > 0.05). Serum 17β-estradiol, testosterone and glucose concentrations were not different among diets (p > 0.05). Serum insulin levels were lower in both male and female rats fed SP + SBTI diet than in those fed SP-SBTI diet (p < 0.05). The ratios of serum insuln levels to pancreas weight were significantly lower in the rats fed SP + SBTI diet than in those fed Casein or SP-SBTI diets (p < 0.01).
Pancreatic histology and trypsinogen content
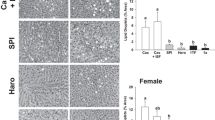
The pancreatic acinar cells in both male and female rats fed SP + SBTI diet were markedly enlarged compared to those fed Casein or SP-SBTI diets (Fig. 1A and B). The trypsinogen content in the pancreas of the rats fed SP + SBTI diet were significantly higher than those fed Casein or SP-SBTI diets (p < 0.01, Fig. 1C and D).
Hematoxylin & eosin stained images of the pancreatic sections (A, B) and trypsinogen content in the pancreas (C, D) of the rats fed diets containing either 20% casein protein (Casein) or 20% soy protein (SP) in the presence of high (SP + SBTI) or low (SP-SBTI) levels of active soybean trypsin inhibitors for 8 weeks. Acinar cells are in pink and nuclei are in blue (A, B). The images shown are representatives of 8 replicates of each diet at 200 × magnification. Values in (C) and (D) are mean ± SEM (n = 8) and means with different letters differ, p < 0.05
Pancreatic STAT3, p-STAT3, AR and ER protein content
Feeding of the SP + SBTI diet significantly reduced STAT3, p-STAT3, AR, and ERβ protein content in the pancreas of both male and female rats compared to the Casein or SP-SBTI diets (p < 0.01, Fig. 2A and B). Interestingly, the female rats fed either SP + SBTI or SP-SBTI diet had lower ERα protein content in the pancreas compared to those fed Casein diet, regardless of the content of active SBTI (p < 0.05, Fig. 2A), but this effect was not significant in the male rats (Fig. 2B).
Pancreatic STAT3, p-STAT3, androgen receptor (AR), estrogen receptor α (ERα) and β (ERβ) proteins in the female (A) and male (B) rats fed diets containing either 20% casein protein (Casein), or 20% soy protein (SP) in the presence of high (SP + SBTI) or low (SP-SBTI) levels of active soybean trypsin inhibitors for 8 weeks. Values are mean ± SEM (n = 8). Means with different letters differ, p < 0.05
Hepatic and uterine ER, AR, STAT3 and p-STAT3 content
Intake of the two SP-containing diets (SP + SBTI or SP-SBTI), regardless of the content of active SBTI, significantly lowered STAT3 and p-STAT3, and elevated ERα and ERβ protein content in the liver of the female rats compared to the Casein diet (p < 0.05, Fig. 3A), but these effects were not significant in the male rats (p > 0.05, data not shown). The rats fed SP + SBTI diet had higher uterine ERα and ERβ protein content than the rats fed Casein or SP-SBTI diets (p < 0.05, Fig. 3B). The SP-SBTI diet further increased hepatic ERβ, but lowered uterine AR abundance in the female rats compared to the SP + SBTI diet (p < 0.05).
Hepatic (A) and uterine (B) STAT3, p-STAT3, androgen receptor (AR), estrogen receptor α (ERα) and β (ERβ) proteins in the female rats fed diets containing either 20% casein protein (Casein), or 20% soy protein (SP) in the presence of high (SP + SBTI) or low (SP-SBTI) levels of active soybean trypsin inhibitors for 8 weeks. Values are mean ± SEM (n = 8). Means with different letters differ, p < 0.05
Discussion
The present study has shown that dietary active SBTI at a level similar to the amount contained in some of the commercial soy milks reported in our previous study [3] significantly increased pancreatic weights and trypsinogen content, and caused acinar cell hypertrophy. This is in line with the study showing that feeding the diets containing increasing amounts of raw soybean flour for 36 weeks significantly elevated pancreatic weight, pancreatic nucleic acid and protein content of the rats in a dose-dependent manner, and induced pancreatic hyperplasia [20]. The food intake and BWG of the rats fed raw soybean diets were significantly lower [20, 21], and it was shown that these negative effects of raw soybean were attributed to dietary trypsin inhibitors [21]. In the present study, the BWG in both sexes and food intake in the female rats were not reduced by dietary active SBTI (SP + SBTI), however the food efficiency was reduced in the male rats fed the SP + SBTI diet compared to the Casein diet. These discrepancies among studies may be due to the differences in the length of feeding and levels of active SBTI used. Our study was relatively short and the amount of active SBTI contained in the diet was lower compared to the other studies using raw soybean flour or meal [20, 21].
The present study has also demonstrated that high level of dietary active SBTI attenuated pancreatic STAT3, p-STAT3, AR and ERβ protein abundances in both sexes. The SBTI-induced reductions in the abundance of these potent molecules may play a role in mediating the changes in physiological functions of pancreas. It has been shown that SBTI significantly reduced the size of islets of Langerhans in the pancreas and lowered its insulin content and insulin secretion following intravenous administration of glucose in rats [7]. STAT3 plays important role in the normal development and maintenance of islet microvascular network through direct regulation of VEGF-A expression as STAT3 has a putative binding site on the VEGF promoter [22]. Depletion of STAT3 in pancreas attenuated VEGF-A production and microvascular density in the pancreas and resulted in glucose intolerance and impaired insulin secretion in mice [9].
AR in the pancreas is important in regulating cell proliferation and apoptosis. It has been shown that testosterone deficiency in male rats increased apoptosis and reduced proliferation in the β cells and resulted in decrease in β cell mass [11, 23]. AR in β cells mediates the action of testosterone to enhance glucose-stimulated insulin secretion through potentiating the insulinotropic effect of glucagon-like peptide-1 derived from islet. AR depletion in pancreatic β-cell of the male mice changed the expression of many genes involved in β cell function [10], lowered glucose-stimulated insulin secretion and impared the ability of glucose clearance when challenged with glucose [11]. This suggests that AR is essential for β cell health and normal glucose-stimulated insulin secretion.
Both ERα and ERβ have insulinotropic effects. Stimulation of ERα and ERβ by estrogen agonist increased insulin secretion in rats [14]. Estradiol prevented β-cell failure in Zuckerman diabetic fatty rats [24]. Reduced pancreatic ERβ protein abundance by high level of SBTI could result in glucose intolerance and attenuation in glucose-induced insulin secretion. However, this needs to be further investigated using appropriate approaches and models.
SP-containing diets in this study significantly decreased STAT3 and p-STAT3, and increased ERα and ERβ protein contents in the liver of the female rats, regardless of the active SBTI content, compared to the Casein diet. However, these effects were not significant in the male rats. These gender-dependent effects of SP diets may be attributed to the difference in the endogenous estrogen levels, abundance of liver ER [25] and responsiveness to isoflavones between males and females. This is in line with the higher serum 17β-estradiol levels in the females than in the males in the present study. Isoflavones are the major phytoestrogens naturally present in soybeans and associated with soy proteins. Soy isoflavones have estrogenic and antiestrogenic properties and can bind both ERα and ERβ [26]. It has been shown that the female liver is more responsive to estrogen exposure than does male liver due to the more efficient nuclear uptake of cytosolic receptor-ligand complexes in females than in males [27]. Estrogen has been shown to suppress the activation of STAT3 due to direct physical interactions between STAT3 and ER [28]. The similar gender-specific effects were also observed in our previous studies [29, 30].
The rats fed high level of SBTI had higher uterine ERα and ERβ proteins than those fed Casein or SP-SBTI diet. This may be due to decreased proteolysis of ER proteins as a result of inhibition of uterine proteases by SBTI. It has been shown that endogenous proteases are present in the cytosol of the uterine tissues in both humans and rats [31, 32]. Those proteases were similar to trypsin and could hydrolyze the substrates of trypsin and uterine ER proteins [31]. SBTI, particularly BBI, could be absorbed and widely distributed in the body including the bloodstream after oral intake [33]. Administration of estradiol stimulated uptake of SBTI into uterus and increased uterine trypsin inhibitory capacity in mice [34]. Although the serum 17β-estradiol levels in the rats fed high level of active SBTI were not different from those fed other two diets in the present study, the estrogenic isoflavones contained in the SP can bind both ERα and ERβ and may play a role in stimulating uterine uptake of SBTI, thereby enhancing the abundances of uterine ER proteins through inhibiting the hydrolytic actions of proteases on ERα and ERβ.
Consumption of soy foods is becoming increasingly popular in Western countries because of their potential health benefits such as reducing risk for coronary heart disease, Type 2 diabetes and certain types of tumors. However, if the soy foods are not properly processed (at an adequate temperature or duration of heating), the high levels of active SBTI could decrease protein digestibility and cause pancreatic hypertrophy or hyperplasia as having been shown in the rodents [6, 8]. SBTI have similar inhibitory effects on the enzymatic activity of both bovine and human trypsins as shown by us [3] and others [35, 36], suggesting that consumption of inadequately processed soy foods/products containing high levels of active SBTI may have similar impacts in humans.
The heat required for inactivation of SBTI in soy foods leads to the denaturation of lipoxygenases and major storage proteins such as glycinins [5] as a result of interchanges of disulfide linkages within the proteins. A complete inactivation of SBTI activity could cause overheating which could destroy lysine, tryptophan and cysteine, and result in decreased nutritional quality of the total protein. It is believed that 4–10% residual trypsin inhibitory activity is necessary to ensure the highest nutritional value for processed soy foods [5, 37]. Our present study showed that 6.5% residual SBTI activity in the diet did not significantly change most of the parameters measured compared to the Casein diet, suggesting that soy foods containing low levels of active SBTI are safe to be consumed.
Conclusion
In summary, this study demonstrated for the first time that consumption of high level of active SBTI not only caused pancreatic enlargement and increased its enzymatic production, but also reduced the abundance of pancreatic STAT3, p-STAT3, AR, and ERβ proteins in both sexes, and enhanced the expression of uterine ERα and ERβ compared to the Casein, or low level of active SBTI group. Consumption of soy protein attenuated both STAT3 and p-STAT3, however enhanced ERα and ERβ expression in the livers of the female rats. Further studies should assess the impacts of consumption of high levels of active SBTI on the physiological functions mediated through these potent molecules such as glucose-stimulated insulin secretion and glucose tolerance in appropriate models using AR and ER specific agonists.
Abbreviations
- AR:
-
Androgen receptor
- BBI:
-
Bowman-Birk inhibitors
- ER:
-
Estrogen receptor
- KTI:
-
Kunitz trypsin inhibitor
- SBTI:
-
Soybean trypsin inhibitor
- SP:
-
Soy protein
- STAT3:
-
Signal transducer and activator of transcription 3
- p-STAT3:
-
Phosphorylated STAT3
References
Bacon JR, Wanigatunga SCDR, An J, Fenwick GR (1995) A microassay for the analysis of trypsin inhibitor activity in peas. Food Chem 52:77–80
Xu Z, Chen Y, Zhang C, Kong X, Hua Y (2012) The heat-induced protein aggregate correlated with trypsin inhibitor inactivation in soymilk processing. J Agric Food Chem 60:8012–8019
Xiao CW, Wood CM, Robertson P, Gilani GS (2012) Protease inhibitor activities and isoflavone content in commercial soymilks and soy-based infant formulas sold in Ottawa, Canada. J Food Comp Anal 25:130–136
Logsdon CD, Ji B (2013) The role of protein synthesis and digestive enzymes in acinar cell injury. Nat Rev Gastroenterol Hepatol 10:362–370
Yuan S, Chang SK, Liu Z, Xu B (2008) Elimination of trypsin inhibitor activity and beany flavor in soy milk by consecutive blanching and ultrahigh-temperature (UHT) processing. J Agric Food Chem 56:7957–7963
Temler RS, Dormond CA, Simon E, Morel B (1984) The effect of feeding soybean trypsin inhibitor and repeated injections of cholecystokinin on rat pancreas. J Nutr 114:1083–1091
McGuinness EE, Morgan RG, Wormsley KG (1984) Effects of soybean flour on the pancreas of rats. Environ Health Perspect 56:205–212
Cheftel JC, Cuq JL, Lorient D (1985) Amino acids, peptides, and proteins. In: Fennema OR (ed) Food chemistry. Marcel Dekker Inc, New York
Kostromina E, Gustavsson N, Wang X, Lim CY, Radda GK, Li C, Han W (2010) Glucose intolerance and impaired insulin secretion in pancreas-specific signal transducer and activator of transcription-3 knockout mice are associated with microvascular alterations in the pancreas. Endocrinology 151:2050–2059
Xu W, Morford J, Mauvais-Jarvis F (2019) Emerging role of testosterone in pancreatic ß-cell function and insulin secretion. J Endocrinol. https://doi.org/10.1530/JOE-18-0573
Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, De GK, Kim SH, Wu H, Zhang H, Verhoeven G, Katzenellenbogen JA, Mauvais-Jarvis F (2016) Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab 23:837–851
Mishra JS, More AS, Kumar S (2018) Elevated androgen levels induce hyperinsulinemia through increase in Ins1 transcription in pancreatic beta cells in female rats. Biol Reprod 98:520–531
Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquie M, Gauthier BR, Nef S, Stefani E, Nadal A (2008) Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One 3:e2069
Alonso-Magdalena P, Ropero AB, Garcia-Arevalo M, Soriano S, Quesada I, Muhammed SJ, Salehi A, Gustafsson JA, Nadal A (2013) Antidiabetic actions of an estrogen receptor beta selective agonist. Diabetes 62:2015–2025
Soriano S, Ropero AB, Alonso-Magdalena P, Ripoll C, Quesada I, Gassner B, Kuhn M, Gustafsson JA, Nadal A (2009) Rapid regulation of K(ATP) channel activity by 17ß-estradiol in pancreatic ß-cells involves the estrogen receptor ß and the atrial natriuretic peptide receptor. Mol Endocrinol 23:1973–1982
Vogel H, Mirhashemi F, Liehl B, Taugner F, Kluth O, Kluge R, Joost HG, Schurmann A (2013) Estrogen deficiency aggravates insulin resistance and induces beta-cell loss and diabetes in female New Zealand obese mice. Horm Metab Res 45:430–435
Chen Q, Wood C, Gagnon C, Cober ER, Frégeau-Reid JA, Gleddie S, Xiao CW (2014) The alpha’ subunit of beta-conglycinin and the A1–5 subunits of glycinin are not essential for many hypolipidemic actions of dietary soy proteins in rats. Eur J Nutr 53:1195–1207
AACC (1999) AACC International Method 22-40.01: Measurement of trypsin inhibitor activity of soy products-spectrophotometric method. Approved Methods of Analysis, 11th ed
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Crass RA, Morgan RG (1982) The effect of long-term feeding of soya-bean flour diets on pancreatic growth in the rat. Br J Nutr 47:119–129
Nitsan Z, Nir I, Liener IE (1983) Accentuated response to raw soya-bean meal by meal feeding. Arch Toxicol Suppl 6:177–181
Cheranov SY, Karpurapu M, Wang D, Zhang B, Venema RC, Rao GN (2008) An essential role for SRC-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood 111:5581–5591
Harada N, Yoda Y, Yotsumoto Y, Masuda T, Takahashi Y, Katsuki T, Kai K, Shiraki N, Inui H, Yamaji R (2018) Androgen signaling expands beta-cell mass in male rats and beta-cell androgen receptor is degraded under high-glucose conditions. Am J Physiol Endocrinol Metab 314:E274–E286
Tiano JP, Delghingaro-Augusto V, Le MC, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, Prentki M, Mauvais-Jarvis F (2011) Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Invest 121:3331–3342
Coldham NG, Sauer MJ (2000) Pharmacokinetics of [(14)C]Genistein in the rat: gender-related differences, potential mechanisms of biological action, and implications for human health. Toxicol Appl Pharmacol 164:206–215
Xiao CW (2008) Health effects of soy protein and isoflavones in humans. J Nutr 138:1244S-1249S
Thompson C, Lucier GW (1983) Hepatic estrogen responsiveness. Possible mechanisms for sexual dimorphism. Mol Pharmacol 24:69–76
Yamamoto T, Matsuda T, Junicho A, Kishi H, Saatcioglu F, Muraguchi A (2000) Cross-talk between signal transducer and activator of transcription 3 and estrogen receptor signaling. FEBS Lett 486:143–148
Xiao CW, Wood CM, Weber D, Aziz SA, Mehta R, Griffin P, Cockell KA (2014) Dietary supplementation with soy isoflavones or replacement with soy proteins prevents hepatic lipid droplet accumulation and alters expression of genes involved in lipid metabolism in rats. Genes Nutr 9:373
Chatterjee C, Liu J, Wood C, Gagnon C, Cober ER, Fregeau-Reid JA, Gleddie S, Xiao CW (2018) The alpha’ subunit of beta-conglycinin and various glycinin subunits of soy are not required to modulate hepatic lipid metabolism in rats. Eur J Nutr 57:1157–1168
Notides AC, Hamilton DE, Rudolph JH (1973) The action of a human uterine protease on the estrogen receptor. Endocrinology 93:210–216
Tilzer LL, McFarland RT, Plapp FV, Evans JP, Chiga M (1981) Different ionic forms of estrogen receptor in rat uterus and human breast carcinoma. Cancer Res 41:1058–1063
Billings PC, St Clair WH, Maki PA, Kennedy AR (1992) Distribution of the Bowman Birk protease inhibitor in mice following oral administration. Cancer Lett 62:191–197
Finlay TH, Katz J, Rasums A, Seiler S, Levitz M (1981) Estrogen-stimulated uptake of alpha 1-protease inhibitor and other plasma proteins by the mouse uterus. Endocrinology 108:2129–2136
Figarella C, Negri GA, Guy O (1974) Studies on inhibition of the two human trypsins. In: Fritz H, Tschesche H, Greene LJ (eds) Proteinase inhibitors. Proceedings of the 2nd International Research Conference. Springer, Berlin/Heidelberg/New York
Mallory PA, Travis J (1975) Inhibition spectra of the human pancreatic endopeptidases. Am J Clin Nutr 28:823–830
Guerrero-Beltran JA, Estrada-Giron Y, Swanson BG, Barbosa-Canovas GV (2009) Pressure and temperature combination for inactivation of soymilk trypsin inhibitors. Food Chem 116:676–679
Acknowledgements
The authors would like to thank Dr. Stephen Gleddie at Agriculture and Agri-Food Canada for providing soy proteins. We thank the technicians in the Scientific Service Division, Food Directorate, Health Canada for their assistance during the animal experimentation phase.
Funding
This research was supported by Health Canada.
Author information
Authors and Affiliations
Contributions
CWX designed the animal study and conducted data analysis and prepared the manuscript. CW coordidated the animal study and conducted sample analysis. LAC, ML, and MR analysed the samples and participated in manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
All authors in this paper have read the final manuscript and approved for publication.
Animal rights
The animal experimental protocol was approved by the Health Canada-Ottawa Animal Care Committee, and all animal handling and care followed the guidelines of the Canadian Council for Animal Care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, CW., Wood, C., Cunningham, L.A. et al. Effects of dietary active soybean trypsin inhibitors on pancreatic weights, histology and expression of STAT3 and receptors for androgen and estrogen in different tissues of rats. Mol Biol Rep 48, 4591–4600 (2021). https://doi.org/10.1007/s11033-021-06491-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06491-x