Abstract
Globally, breast cancer is a serious concern that exhibits a persistent rise in its incidence and related mortality even after significant advancement in the field of cancer research. To find an alternative cure for the disease from natural resources we selected Bacopa monniera, a perennial ethnomedicinal plant popularly used for boosting memory and mental health. We isolated four different types of dammarane saponins, namely bacopasaponins C–F (1–4) from the plant and evaluated their toxic effects on two different types of human breast cancer cell lines—a hormone-responsive MCF7 and a triple-negative MDA-MB-231. Interestingly, MTT assay revealed a dose-dependent toxic effect of all four types of bacopasaponins on both of these cell lines, 4 being the most effective with 48 h-inhibitory concentration (IC50) of 32.44 and 30 µM in MCF7 and MDA-MB-231 respectively. Further, 4 caused significant alterations in normal cytomorphology and induction of apoptosis in both of these cell lines after 48 h of treatment. No caspase-8 activity was detected in these cell lines when exposed to 4 for 2, 24, and 48 h; instead, Western blotting analysis confirmed involvement of either caspase-9 (MCF7) or both caspase-9 and caspase-3 (MDA-MB-231) in the process of apoptosis indicating the occurrence of intrinsic mode. Additionally, at comparable effective doses to cancer, bacopasaponins showed much less toxicity in normal human peripheral blood lymphocytes (≥ 85% cell survival). Overall, the findings project bacopasaponin F, a natural constituent of Bacopa monniera, as an efficient and safer alternative for breast cancer therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global burden of cancer is growing rapidly and it is one of the major barriers to increasing life expectancy of people all over the world. According to the latest GLOBOCAN 2018 database, about 18.1 million new cases of cancer and 9.6 million deaths due to them were estimated in 2018 only [1]. Globally, in men, 1 in 5 develops cancer and 1 in 8 dies from the disease, whereas, in women, the development of cancer is 1 in 6, and death due to it is 1 in 11. Unlike the rest of the world, Asia and Africa share a higher proportion of cancer deaths (approximately 65%) as compared to their share in cancer incidence (approximately 54%) mainly due to limited access to timely diagnosis and treatment in several countries. Globally, cancers of the lung, female breast, and colorectum are having a major one-third share in the total number of cancer cases and demises. In women, breast cancer is the most frequently diagnosed cancer that causes maximum cancer death [2]. Common treatments for cancer are chemotherapy, surgery, and radiation therapy. All types of cancer treatment are having mild to severe side effects on the body. Chemotherapeutic drugs used for cancer treatment also harm normal cells like hematopoietic stem cells, hair follicles, neurons, the cells of the mouth, digestive tract, kidney, heart among many [3]. Several groups of researchers all over the world are involved to find out a better solution to fight cancer. Continued efforts should be there for the discovery of the most effective anticancer agents with fewer side effects and at the same time, will be easily accessible and less expensive.
Bacopa monniera (L.) Wettst, also known as ‘Brahmi’, is a creeping perennial plant that grows throughout the regions of Southern Asia. It is widely used as a ‘brain tonic’ for boosting memory and mental health. Indian traditional medicine also uses this herb as a cardiotonic and diuretic, and to treat several conditions like asthma, epilepsy, fever, and pain among many [4]. Saponins are the active constituents of the alcoholic extracts of the herb and several dammarane-type triterpenoid saponins have been reported [5,6,7,8]. Bacoside A, composed of jujubogenin isomer of bacopasaponin C, bacoside A3, and bacopaside II and −X, is responsible for inducing major neuropharmacological effects [9,10,11,12,13,14]. Alcoholic extracts of the herb have been reported to have various biological effects such as antileishmanial, antifungal, antimicrobial, and antioxidant activities along with its protective role in hepatic, cardiovascular, and renal functions [15,16,17,18,19]. Because of the strong positive impact of bacopasaponins in several ailments, its large-scale production is imperative and can be obtained using cell culture as a potential alternative to traditional agriculture [20,21,22,23].
In Traditional Chinese Medicine (TCM) clinic, some herbal medicines, enriched in dammarane-type triterpenoids (DTT) were used as complementary and alternative agents in cancer treatment, which are helpful for preventing tumor cell metastasis, relieving side effects of radiotherapy and chemotherapy and improving clinical care rate, such as Panax ginseng, Panax notoginseng, D. binecteriferum etc. Several literature reported that DTT showed cytotoxicity in many kinds of cancer cell lines [24]. The in vitro, analytical and in silico studies on the dichloromethane fraction of hydroalcoholic extract of Bacopa monniera have proved its great potential for development of anticancer phytochemicals [25]. The saponins of Bacopa monniera possess anxiolytic [26] and antioxidant effects [27, 28]. Both of these activities can prevent tumor formation by promoting psychological [29] and free radical balance [30] in the body, absence of which have been implicated in the genesis of tumor. The alcoholic extract of B. monniera has shown anticancer property in vitro for sarcoma-180 cells [31]. Bioassay-guided methods were used for screening of the effective extract fractions and active monomers of anti-tumor activity of the n-butanol soluble fraction of the methanol extract of B. monniera on the level of intact animal and molecular pharmacology. Anticancer activities of dammarane saponins, bacopaside E and bacopaside VII on human cancer cell lines MCF7, MDA-MB-231, SHG-44, HCT-8, A-549 and PC-3 M was reported [32]. Bacopasaponins C–F (1–4) were isolated from the purified saponins fractions of the n-butanol soluble fraction of the methanol extract of B. monniera [7, 9, 10].
Research on the cytotoxic activity of B. monniera in cancer cells is still in its infancy, and we are reporting, for the first time, a comparative analysis of the cytotoxic efficacy of four different bacopasaponins (C–F) (1–4) in two major types of human breast cancer cell lines MCF7 and MDA-MB-231. MCF7 is having both estrogen (ER) and progesterone receptors (PR) but lacks human epidermal growth factor receptor 2 (HER2); representing the breast cancer types curable by hormone therapy [33]. Conversely, MDA-MB-231 is a triple-negative breast cancer cell line (ER, PR, and HER2 negative); thus not cured by hormone or anti-HER2 therapy [34]. Mitomycin-C, a well-known anticancer chemotherapy drug, was used in this experiment for a comparative evaluation of bacopasaponins’ efficacy against these cell lines [35]. Besides, human peripheral blood lymphocytes (HPBLs) were used to understand their effect on normal cells.
Materials and methods
Materials
Roswell Park Memorial Institute (RPMI)-1640 and Dulbecco’s Modified Eagle’s Medium (DMEM) culture media, penicillin–streptomycin antibiotic solution, phytohaemagglutinin (PHA), fetal bovine serum (FBS), MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), radioimmunoprecipitation assay (RIPA) buffer, and skim milk were procured from Himedia Laboratories, India; BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium) and DMSO (dimethyl sulfoxide) were purchased from Merck, India; heparin and trypan blue were procured from SRL, India; histopaque, anti-human primary antibodies (Sigma-Aldrich, USA), ALP-linked goat anti-rabbit (Abcam, UK) and anti-mouse (Genei, India) secondary antibodies were used as received. Caspase-8 Assay Kit was procured from Sigma-Aldrich, USA. Cell-APOPercentage™ Apoptosis assay kit was purchased from Biocolor, UK. Double distilled water was used throughout.
Isolation
The defatted extract of the air dried powdered whole plant of B.monniera (1.5 kg) were extracted in a percolator with methanol. The methanol extract was concentrated and partitioned between H2O and n-BnOH. The n-BuOH layer was washed with H2O and then removed under reduced pressure. The residue (52 g) thus obtained was dissolved in a minimum amount of MeOH, adsorbed on silica gel, dried and extracted successively with CHCl3, EtOAc, Me2CO and CHCl3-MeOH (4:1). The last three fractions were separately subjected to CC on silica gel (CHCl3–MeOH, 24:1, 20:1) as mobile phase and similar fractions so obtained were further purified by preparative TLC (CHCl3–MeOH–H2O, 35:13:2) and preparative HPLC on a µBondapak C18 column (10 µm) (7.8 × 300 mm) using solvent, methanol–water (65:35), flow rate 2 mL/min, detection at 210 nm and injection of 60 µL containing approximately 3 mg of the material followed by crystallization. Thus four bacopasaponins 1 (150 mg), 2 (35 mg), 3 (100 mg) and 4 (95 mg) were obtained. The four bacopasaponins C–F (1–4) were identified by IR, 1H & 13C NMR, 2D NMR and FABMS. The four polar saponins are bacopasaponin C (1) mp 222° [α]D −47.5° (MeOH, c 1.2), bacopasaponin D (2) mp 250° [α]D −42° (MeOH, c 0.14), bacopasaponin E (3) mp 266–270° [α]D −27.72° (MeOH, c 0.44) and bacopasaponin F (4) mp 260–264° [α]D −37.72° (MeOH, c 0.44). The four bacopasaponins C–F (1–4) (Fig. 1) were used in this experiment.
Cell culture
Breast cancer cell lines MCF7 and MDA-MB-231 were procured from the National Centre for Cell Science (Pune, India) and cultured in complete DMEM media (with 10% (v/v) FBS and 1% (v/v) penicillin–streptomycin). The culture was maintained at 37 °C temperature in the presence of 5% CO2 inside a humid chamber (Thermo Fisher Scientific, USA).
MTT assay in cancer cells
Cancer cells in a density of 1 × 104 cells were seeded into a 96 well-plate and kept until about 70% confluency is reached. After that, the cells were exposed to different concentrations of 1–4 for 48 h. Both negative (untreated cells) and positive controls (50 µM mitomycin-C-treated) were used along with the treated groups. At the end of the exposure, cells were further treated with 5 mg/mL MTT solution for 4 h, and the blue-purple formazan crystals formed were dissolved in DMSO after discarding cell culture supernatant and optical density (OD) was read on iMarkTM microplate absorbance reader (BIO-RAD, USA) at 595 nm. The values obtained was calculated as the percentage of viable cells compared to negative control following the formula:
MTT assay in normal human peripheral blood lymphocytes
Fresh blood was collected in heparinized tubes from five healthy non-smokers, non-alcoholic male donors (age group: 21–25 years) with written informed consent from them. The collection was made by pathologists at the university hospital. All experiments were done in full compliance with the “Ethical Guidelines for Biomedical Research on Human Subjects” formulated by the Indian Council of Medical Research, India. The work was also reviewed and approved by the Institutional Ethics Committee for Human Research of Visva-Bharati University. Human peripheral blood lymphocytes (HPBLs) were isolated following the protocol of Bøyum [36] with minor modifications [37]. Blood was gently layered over an equal volume of Histopaque followed by centrifugation at 1000×g for 30 min. The buffy layer formed after centrifugation was collected in 3–5 mL of PBS and further centrifugation was performed at 1000×g for 10 min. This washing step was repeated thrice. The pellet containing HPBLs was suspended in RPMI-1640 media at a density of 1 × 106 cells/mL and cell viability was found > 95% following the trypan blue dye exclusion method [38] HPBLs, following stimulation by PHA, cultured in RPMI-1640 media containing 10% (v/v) FBS and 1% (v/v) penicillin–streptomycin solution. After 24 h of culture, HPBLs were treated separately with IC50 concentrations of 1–4 determined in MCF7 and MDA-MB-231, whichever were higher, for another 48 h. Cell survival was checked by MTT assay as described in the previous section.
Cytomorphological evaluation of cancer cells
Cancer cells were seeded (5 × 104 cells/well) in a 24 well-plate and cytomorphological alterations were evaluated microscopically (Dewinter, Italy) after treatment with IC50 of bacopasaponin F for 48 h.
Apoptosis assay
The assay was performed using the Cell-APOPercentage™ Apoptosis Assay kit (Biocolor, UK). Cancer cells at a density of 5 × 104 cells/well were seeded in a 24 well-plate and apoptosis induction was assessed after treatment with 1/3rd IC50 of 4 for 2 and 48 h. 30 min before completion of each experiment, the ‘APOPercentagedye’ was added (5% v/v) to the culture media, which, as mentioned in the kit’s guidelines, was selectively imported by cells undergoing apoptosis but not necrosis. For microscopic analysis, cells were washed carefully with PBS and studied under an inverted phase-contrast microscope (Dewinter, Italy). For the colorimetric assay, cells were trypsin-digested and exposed to ‘APOPercentage dye release reagent’ that caused the release of the dye from cells into the solution, and concentration of it was measured on BIO-RAD, USA at 550 nm.
Caspase-8 activity assay
To evaluate if 4 was able to induce cell death through extrinsic apoptosis pathway caspase-8 activity assay was performed following the manufacturer’s (Caspase-8 Assay Kit, Colorimetric, Sigma-Aldrich, USA) protocol. Briefly, both MCF7 and MDA-MB-231 were treated with respective 1/3rd IC50 of 4 for 2, 24, and 48 h. Cells were harvested and lysed in chilled RIPA buffer. Supernatants were collected following centrifugation at 12,000 rpm, 4 °C for 20 min, and further exposed to a substrate of caspase-8, Acetyl-Ile-Glu-Thr-Asp p-nitroaniline (Ac-IETD-pNA). Chromophore pNA was released into the solution after caspase-8 activity which was measured at 405 nm (BIO-RAD, USA).
Western blot analysis
For Western blotting, cancer cells were again treated with a 1/3rd IC50 concentration of 4 for 2, 24, and 48 h, and the process was performed as described by Bandyopadhyay et al. [39]. Both treated and untreated cells were lysed in chilled RIPA buffer following washing with ice-cold PBS. The cell lysate was centrifuged at 12,000 rpm, 4 °C for 20 min, and the amount of total protein in the supernatant was quantified following the Lowry method [40]. The same amount of protein content from control and treated groups were loaded onto the sodium dodecyl sulfate–polyacrylamide gel and run at a constant voltage (60 V) for 2–3 h (Bio-Rad, USA). Polyvinylidene fluoride (PVDF) membranes were used for electroblotting. The membranes were incubated with primary antibodies following blocking by skimmed milk. After careful washing by tris-buffered saline-tween 20 (TBST), ALP-linked goat anti-rabbit (for caspase-9, and caspase-3) or anti-mouse secondary antibodies (for β-actin) were added. The immunoreactive bands were detected using BCIP/NBT.
Statistical analysis
Tests were performed in triplicate and the data were expressed as a mean ± standard error of mean (SEM). Statistical analysis was done using one-way ANOVA following the Tukey method in Minitab 17 software and p < 0.05 were considered significant.
Results and discussion
Effect on cancer cell survival
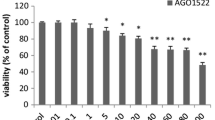
Both types of breast cancer cells were exposed to 1–4 for 48 h. In case of MCF7, a significant reduction in cell survival was evidenced after 25 µmol (µM) treatment of 1 and 4 whereas 2 and 3 showed significant effect only after the dose was doubled (Fig. 2a). Among these four bacopasaponins tested, the least effect was exerted by 3 with an inhibitory concentration (IC50) value of 169.67 µM whereas 1, 2, and 4 appeared with their respective IC50 values of 42.6 µM, 74.68 µM, and 32.44 µM. All bacopasaponins (1–4) induced significant cell death in MDA-MB-231 after 25 µM treatment for 48 h (Fig. 2b). Here 3 appeared as least effective with an IC50 value of 178.32 µM. IC50 values of other bacopasaponins are 40.02 µM (1), 75.12 µM (2), and 30 µM (4). The reason behind this differential effect of these bacopasaponins on cancer cells remains unknown through the present scope of our work and it demands thorough analysis to establish this structure-activity relationship further. However, in both types of breast cancer cell lines, 4 appeared as the most potent cytotoxic bacopasaponin with effective IC50 values lower than that of Mitomycin-C (50 µM) which prompted us to focus on this chemical only to elucidate the mode of bacopasaponin-mediated cancer cell death.
Assessment of cell survival (A-C) and cytomorphological alteration (D): cell survival (%) of human breast cancer cells MCF7 (A) and MDA-MB-231 (B), and normal human peripheral blood lymphocytes (HPBLs) (C) upon exposure to bacopasaponins C–F (1–4) for 48 h. Here, the control means untreated negative control; Mito-C means Mitomycin-C (50 µM)-treated positive control group. HPBLs were treated with respective inhibitory concentrations (IC50) of 1–4 in MCF7 and MDA-MB-231. Bars represent a mean ± standard error of mean. Means that do not share a letter are significantly different. Significance level α = 0.05. Cytomorphological alterations (D) of MCF7 and MDA-MB-231 were analyzed after treatment with respective IC50 of 4 for 48 h (microscopic magnification 200x)
Effect on survival of normal human peripheral blood lymphocytes
For a particular type of bacopasaponin, IC50 values were different for two different types of breast cancer cells and the higher one was selected to treat HPBLs such as 42.6 µM of 1, 75.12 µM of 2, 178.32 µM of 3, and 32.44 µM of 4 for 48 h, and cell survival were found to be 86.36%, 88.59%, 91.62%, and 84.24% respectively (Fig. 2c). This high percentage of cell survival, at those treatment concentrations which reduced cancer cell survival by 50%, clearly indicated that all bacopasaponins tested are less toxic to normal cells at equimolar treatment concentrations.
Effect on the normal cellular morphology of cancer cells
Both types of breast cancer cells, after exposure to respective 48 h IC50 of 4, exhibited significant alterations in normal cellular morphology (Fig. 2d) such as reduced lamellar expansion, and clumping of cells besides the loss of cellular adhesion. This finding, along with MTT data, indicated 4 as a potent cytotoxic agent for cancer cells that reduced their viability following significant cytomorphological alterations.
Apoptosis induction in cancer cells
As IC50 of 4 could reduce cancer cell population to half, a lower value—1/3rd IC50 concentration was chosen to study the mode of cell death. Apoptosis-committed cells and apoptotic bodies were found in both types of breast cancer cells after exposure to 1/3rd IC50 concentration of 4 for two different time points, i.e. 2 h and 48 h. Apoptosis induction is directly proportional qualitatively to the number of cells retaining the ‘APOPercentage dye’ (Fig. 3a) and quantitatively to the concentration of that dye (OD at 550 nm) released from cells after addition of ‘APOPercentage dye release reagent’ (Fig. 3b). The induction to apoptosis (but not necrosis) started just after 2 h of 4 treatment which ultimately increased manifold after 48 h. No significant difference was found between the effects of 4 in two different breast cancer cell lines. This finding is important because it is hard to treat triple-negative breast cancer cell lines, such as MDA-MB-231 compared to hormone receptor-positive breast cancer cell lines such as MCF7 [41]. Bacopasaponin F was found to be equally effective against both types of breast cancer cells, increasing its value as a potent cytotoxic agent in breast cancer therapeutics.
Evaluation of apoptosis-induction (a, b), and mode of apoptosis (c, d): apoptosis induction in human breast cancer cells MCF7 and MDA-MB-231 exposed to 1/3rd IC50 of bacopasaponin-F (4) for 48 h was studied microscopically (magnification 200x) (a), and by colorimetric analysis (b). Means that do not share a letter are significantly different. Expression patterns of cleaved caspase-9 in MCF7 (c), and cleaved caspase-9 and -3 in MDA-MB-231 (d) were analyzed by Western blot method and the intensity of the bands was estimated by densitometric analysis using ImageJ 1.52a software. The data were normalized taking β-actin as an internal control. Graphs represent mean ± standard error of mean. ⃰Significantly different from control. Significance level α = 0.05
Mode of apoptosis-induction in cancer cells
Various stimuli can induce apoptosis in cancer cells via intrinsic (mitochondrial stress-induced) and/or extrinsic (receptor-induced) pathways in which caspases (cysteine aspartate-specific proteases) play a central role [42]. Caspase-9 is an initiator caspase and the key player in the intrinsic apoptosis pathway [43]. Upon activation, it simultaneously activates caspase-3, an executioner caspase, which along with other executioner caspases (caspase-6, -7) cleaves different types of intracellular proteins ultimately killing the cell [44]. 4 at respective 1/3rd 48 h-IC50 in MCF7 and MDA-MB-231 caused a significant increase in active (cleaved) caspase-9 level after 24 h and 48 h of treatment (Fig. 3c, d). Interestingly, no caspase-8 activity was detected in this treatment condition. Cumulatively, these findings clearly indicated that 4 induced apoptosis in both types of breast cancer cells through the intrinsic apoptosis pathway. Caspase-3 is a downstream caspase for caspase-9 in MDA-MB-231 and its level was also found to be upregulated after 24 h and 48 h treatment of 4. MCF7 is a caspase-3-deficient cell line and the role of executioner caspase is played by caspase-6 or caspase-7 [45, 46]. Activity and expression pattern of initiator caspases, caspase-9, and caspase-8 respectively, are sufficient to state that 4 induced apoptosis in both types of breast cancer cell lines through the intrinsic pathway.
To summarize, dammarane-type triterpenoid saponins, major constituents of several reputed herbal medicines including B. monniera, are gaining attention for their widespread medicinal properties with fewer or no side effects [47,48,49,50]. In this study, bacopasaponin F, a jujubogenin bisdesmoside isolated from B. monniera showed the most pronounced cytotoxic effect among four different types of 7bacopasaponins (C–F) in two major types of human breast cancer cell lines MCF7 and MDA-MB-231 which, at the same time was found to be less toxic to normal HPBLs. Detailed investigation proved its toxic effect on cancer cells to be mediated through a caspase-9-dependent apoptosis pathway. These findings strongly suggest that bacopasaponin F can be considered as an effective phytochemical against both hormone-responsive as well as chemo-resistant triple-negative breast cancers.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 68:394–424
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA: Cancer J Clin 66:7–30
Oun R, Moussa YE, Wheate NJ (2018) The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans 47:6645–6653
Vishnupriya P, Padma VV (2017) A review on the antioxidant and therapeutic potential of Bacopa monnieri. React Oxyg Species 3:111–120
Jain P, Kulshreshtha DK (1993) Bacoside A1, a minor saponin from Bacopa monniera. Phytochemistry 33:449–451
Rastogi S, Pal R, Kulshreshtha DK (1994) Bacoside A3-A triterpenoid saponin from Bacopa monniera. Phytochemistry 36:133–137
Garai S, Mahato SB, Ohtani K, Yamasaki K (1996) Dammarane-type triterpenoid saponins from Bacopa monniera. Phytochemistry 42:815–820
Rastogi S, Kulshreshtha DK (1998) Bacoside A2-a triterpenoid saponin from Bacopa monniera. Indian J Chem 38:353–356
Garai S, Mahato SB, Ohtani K, Yamasaki K (1996) Bacopasaponin D-A pseudojujubogenin glycoside from Bacopa monniera. Phytochemistry 43:447–449
Mahato SB, Garai S, Chakravarty AK (2000) Bacopasaponins E and F: two jujubogenin bisdesmosides from Bacopa monniera. Phytochemistry 53:711–714
Srivastava P, Raut HN, Puntambekar HM, Desai AC (2012) Stability studies of crude plant material of Bacopa monnieri and quantitative determination of bacopaside I and bacoside A by HPLC. Phytochem Anal 23:502–507
Deepak M, Amit A (2013) ‘Bacoside B’—the need remains for establishing identity. Fitoterapia 87:7–10
Anand T, Prakash KB, Pandareesh MD, Khanum F (2014) Development of bacoside enriched date syrup juice and its evaluation for physical endurance. J Food Sci Technol 51:4026–4032
Singh R, Ramakrishna R, Bhateria M, Bhatta RS (2014) In vitro evaluation of Bacopa monniera extract and individual constituents on human recombinant monoamine oxidase enzymes. Phytother Res 28:1419–1422
Sinha J, Raay B, Das N, Medda S, Garai S, Mahato SB, Basu MK (2002) Bacopasaponin C: critical evaluation of anti-leishmanial properties in various delivery modes. Drug Deliv 9:55–62
Sabina EP, Peter SJ, Geetha A (2019) A comparison of hepatoprotective activity of bacoside to silymarin treatment against a combined Isoniazid and Rifampin-induced hepatotoxicity in female Wistar rats. J Histotechnol 42:128–136
Shahid M, Subhan F, Ullah I, Ali G, Alam J, Shah R (2016) Beneficial effects of Bacopa monnieri extract on opioid induced toxicity. Heliyon 2:1–30
Haque SM, Chakraborty A, Dey D, Mukherjee S, Nayak S, Ghosh B (2017) Improved micropropagation of Bacopa monnieri (L.) Wettst. (Plantaginaceae) and antimicrobial activity of in vitro and ex vitro raised plants against multidrug-resistant clinical isolates of urinary tract infecting (UTI) and respiratory tract infecting (RTI) bacteria. Clin Phytosci 3:1–10
Jain P, Sharma HP, Basri F, Priya K, Singh P (2017) Phytochemical analysis of Bacopa monnieri (L.) Wettst. and their anti-fungal activities. Indian J Tradit Knowl 16:310–318
Leonard J, Seth B, Sahu BB, Singh VR, Patra N (2018) Statistical optimization for enhanced bacoside a production in plant cell cultures of Bacopa monnieri. Plant Cell Tissue Organ Cult 133:203–214
Bansal M, Reddy MS, Kumar A (2017) Optimization of cell growth and bacoside-A production in suspension cultures of Bacopa monnieri (L.) Wettst. using response surface methodology. Vitro Cell Dev Biol Plant 53:527–537
Majumdar S, Garai S, Jha S (2011) Genetic transformation of Bacopa monnieri by wild type strains of Agrobacterium rhizogenes stimulates production of bacopa saponins in transformed calli and plants. Plant Cell Rep 30:941–954
Majumdar S, Garai S, Jha S (2012) Use of the cryptogein gene to stimulate the accumulation of bacopa saponins in transgenic Bacopa monnieri plants. Plant Cell Rep 31:1899–1909
Ruan J, Zheng C, Qu L, Liu Y, Han L, Yu H, Zhang Y, Wang T (2016) Plant resources, 13C-NMR spectral characteristic and pharmacological activities of dammarane-type triterpenoids. Molecules 21:1047. https://doi.org/10.3390/molecules21081047
Mallick MN, Akhtar MS, Najm MZ, Tamboli ET, Ahmad S, Husain SA (2015) Evaluation of anticancer potential of Bacopa monnieri L. against MCF-7 and MDA-MB 231 cell line. J Pharm Bioallied Sci 7:325–328
Bhattacharya SK, Bhattacharya A, Kumar A (2000) Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res 14:174–179
Tripathi YB, Chaurasia S, Tripathi E, Upadhyay A, Dubey GP (1996) Bacopa monniera Linn. as an antioxidant mechanism of action. Indian J Exp Biol 34:523–526
Bhattacharya SK, Ghosal S (1998) Anxiolytic activity of a standardized extract of Bacopa monniera: an experimental study. Phytomedicine 5:77–82
Anderson BL, Kiecoli-Glaser JK, Glaser R (1994) A biobehavioral model of cancer stress and disease course. Am Psychol 49:389–404
Pawar R, Gopalakrishnan C, Bhutani KK (2001) Dammarane triterpene saponin from Bacopa monniera as the superoxide inhibitor in polymorphonuclear cells. Planta Med 67:752–754
Elangovan V, Govindasamy S, Ramamoorthy N, Balasubramanian K (1995) In vitro studies on the anticancer activity of Bacopa monnieara. Fitotherapia 6:211–215
Peng L, Zhou Y, Kong DY, Zhang WD (2010) Antitumor activities of dammarane triterpene saponins from Bacopa monniera. Phytother Res 24:864–868
Comşa Ş, Cimpean AM, Raica M (2015) The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res 35:3147–3154
Amaral I, Silva C, Correia-Branco A, Martel F (2018) Effect of metformin on estrogen and progesterone receptor-positive (MCF-7) and triple-negative (MDA-MB-231) breast cancer cells. Biomed Pharmacother 102:94–101
Al-Otaibi WA, Alkhatib MH, Wali AN (2018) Cytotoxicity and apoptosis enhancement in breast and cervical cancer cells upon coadministration of mitomycin C and essential oils in nanoemulsion formulations. Biomed Pharmacother 106:946–955
Bøyum A (1976) Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol 5:9–15
Bandyopadhyay A, Banerjee PP, Shaw P, Mondal MK, Das VK, Chowdhury P, Karak N, Bhattacharya S, Chattopadhyay A (2017) Cytotoxic and mutagenic effects of Thuja occidentalis mediated silver nanoparticles on human peripheral blood lymphocytes. Mater Focus 6:290–296
Tennant JR (1964) Evaluation of the trypan blue technique for determination of cell viability. Transplantation 2:685–694
Bandyopadhyay A, Roy B, Shaw P, Mondal P, Mondal MK, Chowdhury P, Bhattacharya S, Chattopadhyay A (2019) Cytotoxic effect of green synthesized silver nanoparticles in MCF7 and MDA-MB-231 human breast cancer cells in vitro. Nucleus 63:191–202
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
O’Reilly EA, Gubbins L, Sharma S, Tully R, Guang MH, Weiner-Gorzel K, McCaffrey J, Harrison M, Furlong F, Kell M, McCann A (2015) The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clin 3:257–275
Tummers B, Green DR (2017) Caspase-8: regulating life and death. Immunol Rev 277:76–89
Li P, Zhou L, Zhao T, Liu X, Zhang P, Liu Y, Zheng X, Li Q (2017) Caspase-9: structure, mechanisms and clinical application. Oncotarget 8:23996–24008
Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES (2017) Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8:1–14
Jänicke RU (2009) MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res Treat 117:219–221
Finco FDBA, Kloss L, Graeve L (2016) Bacaba (Oenocarpus bacaba) phenolic extract induces apoptosis in the MCF-7 breast cancer cell line via the mitochondria-dependent pathway. NFS J 5:5–15
Zhang XS, Cao JQ, Zhao C, Wu XJ, Zhao YQ (2015) Novel dammarane-type triterpenes isolated from hydrolyzate of total Gynostemma pentaphyllum saponins. Bioorgan Med Chem Lett 25:3095–3099
Fu Q, Yuan HM, Chen J, Shi JY (2016) Dammarane-type saponins from Ziziphus jujube and their inhibitory effects against TNF-α release in LPS-induced RAW 246.7 macrophages. Phytochem Lett 16:169–173
Xu W, Zhang JH, Liu X, Jia AL, Liu XL, Wang XW, Qiu ZD (2017) Two new dammarane-type triterpenoid saponins from ginseng medicinal fungal substance. Nat Prod Res 31:1107–1112
Shi G, Wang X, Zhang H, Zhang X, Zhao Y (2018) New dammarane-type triterpene saponins from Gynostemma pentaphyllum and their anti-hepatic fibrosis activities in vitro. J Funct Foods 45:10–14
Acknowledgements
AB gratefully acknowledges Senior Research Fellowship (09/202(0057)/2016-EMR-I) from the Council of Scientific & Industrial Research (CSIR), New Delhi, India. PPB is grateful to UGC (New Delhi, India) for the Meritorious Fellowship. Financial support from CSIR, New Delhi, India is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The work was also reviewed and approved by the Institutional Ethics Committee for Human Research of Visva-Bharati University.
Informed consent
Human blood samples were collected with written informed consent from them.
Research involving human participants and/or animals
All experiments were done in full compliance with the “Ethical Guidelines for Biomedical Research on Human Subjects” formulated by the Indian Council of Medical Research, India.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bandyopadhyay, A., Garai, S., Banerjee, P.P. et al. Bacopasaponins with cytotoxic activity against human breast cancer cells in vitro. Mol Biol Rep 48, 2497–2505 (2021). https://doi.org/10.1007/s11033-021-06284-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06284-2