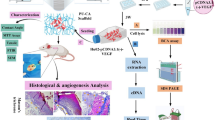
Abstract
Cell-based wound therapy is faced with some limiting factors that decrease the therapeutic efficacy of transplanted cells. In this study, we aimed to genetically modify fibroblast cells with anti-apoptotic Survivin gene (Birc5) before cell transplantation. In vitro, pIRES2-eGFP-Survivin plasmid was transfected into the fibroblast cells and the growth curve was evaluated for transfected and normal cells performing MTT assay. In vivo, two 6-diameter cutaneous wounds were created at mice dorsal skin. Fibrin clot was used as a delivery vehicle to transfer cells into the wound bed. The effects of four treatment groups including (a) Cell-SVV-Clot (b) Cell-GFP-Clot, (c) Normal cell-Clot and, (d) Clot alone were evaluated. After 1,2,3,7 and 14 days post-transplantation, the wounds were photographed for evaluating the wound closure rate and wound samples were obtained. Angiogenesis and formation of granulated tissue were assessed via H&E staining for wound samples. The expression levels of Survivin, VEGF, and bFGF genes were also determined using qRT-PCR. The MTT assay showed similar proliferation potential of transfected cells with normal cells verifying that Survivin had no detrimental effect. Compared to the Normal cell-Clot group, the Survivin overexpression was seen for 3 days in the Cell-SVV-Clot group verifying the cell survival during the early stage of wound healing. The Survivin further upregulated VEGF and bFGF expressions resulting in more angiogenesis and formation of granulated tissue by day 3 and 14. The treated wounds with Cell-SVV-Clot were regenerated with a higher wound closure rate by day 7 compared to Normal cell-Clot and Clot groups. Survivin enhanced wound healing through induction of VEGF and bFGF at particular times post-wounding that led to a more structured-epidermis with higher angiogenesis and granulation tissue formation rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin abnormalities in shape of different kinds of skin wounds; leave heavy economic costs and mental sufferings for affected individuals. The natural wound healing process is a cascade of organized events occurring in three inflammatory, proliferative and remodeling stages to recover and regenerate the damaged tissue [1]. After an injury, the chemo-attractants released by macrophages and platelets like PDGF (platelet-derived growth factor) and TNF-α (Tumor necrosis factor-alpha) recruit fibroblasts to the area [2]. After activation and differentiation of fibroblasts into myofibroblasts, they produce matrix metalloproteinases to infiltrate and destroy the fibrin clot and replace it with a new collagen-III rich extracellular matrix (ECM). At this point, the granulation tissue is formed and invaded by the newly formed vasculature [3]. Both fibroblasts and myofibroblasts close the wound through generating contractile forces. Therefore, the newly deposited ECM by fibroblasts and myofibroblasts provides the tensile strength of the healed tissue [4]. Currently, the application of different cells such as fibroblasts, keratinocytes, or stem cells for wound treatment is limited by the low survival rate of transplanted cells due to the inflammation, local hypoxia, and oxidative stress condition at the wound site [5]. Genetic modification helps researchers to acquire more desired characteristics in cells and tissues. The application of anti-apoptotic biomolecules may be able to protect cells against stress-induced cell death. The Birc5 gene (Baculoviral IAP repeat-containing 5) is located on the telomeric region of chromosome 17 which encodes Survivin. Survivin belongs to the inhibitors of apoptosis (IAP) protein family [6] which exerts anti-apoptotic function through inhibition of key caspases and also contributes to the cell division process and angiogenesis [7]. The high level of Survivin expression in cancer cells leads to cancer progression and resistance to ionizing irradiations and anticancer drugs which has made Survivin useful for both diagnosis and therapeutic applications [8]. On the contrary, it could be applied as a useful biomolecule to enhance cell viability and postpone their death [9]. It has also been demonstrated that Survivin expression is closely related to the secretion of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Both VEGF and bFGF are incredibly involved in the formation of new blood vessels [10]. One of the common scaffolds for efficient cell delivery in tissue engineering is plasma-based hydrogels [11]. Fibrin clot (also known as fibrin gel) is such a scaffold that self-assembles into a polymer network acting as a suitable cell carrier. Fibrin clot exerts biocompatibility and contains a complete set of cytokines and essential factors for cell growth, attachment, and differentiation [12]. In this study, Due to the oncogenic potential of Survivin overexpression, we transiently overexpressed its expression in fibroblast cells and delivered them to the wound site via fibrin clot. Finally, the wound-healing effects of all groups were assessed on the regeneration of mice cutaneous wounds.
Methods
Plasmid preparation, expansion and, purification
The pIRES2-eGFP-Survivin plasmid was ordered and purchased (GeneCust, Dubelange, Luxembourg) (Sup Figs 1 and 2). The plasmid was expanded by CaCl2-mediated bacterial transformation [13]. Then plasmid extraction was performed by Maxi Prep kit (Favorgen Biotech, Taiwan, China) and the quantity and quality of extracted plasmid were measured by spectrophotometry at wavelength 260, 280, and 230 nm (Nanodrop 2000, Thermo Scientific, USA). The plasmid integrity was also confirmed by running of 1% agarose gel stained with Gel Red (Thermo Scientific, USA).
Cell culture
The RPMI-1640 medium (Gibco, Life Technologies, NY, USA) enriched with 10% fetal bovine serum (FBS) (Gibco) and 100 IU/mL-100 mg/mL penicillin–streptomycin (Gibco) was utilized for the culture of mouse L929 fibroblast cell line (ATCC® CCL-1™). A number of 1 × 106 fibroblast cells were cultured in a T25 culture flask and maintained in a humidified incubator with 5% CO2 at 37 °C. The medium changed every 2 days until they were confluent and detached with 0.25% trypsin- 0.04% EDTA (Gibco) for cell passages (Sup Fig. 3).
Gene modification
A number of 2.5 × 105 fibroblast cells in the third cell passage were detached and seeded in each well of a 6-well plate to reach 80% confluence the next day. Afterward, transfection was carried out in compliance with the lipofectamine 2000 (Invitrogen, Thermo Scientific, USA) instruction. In summary, the ratio of DNA: lipofectamine was set up as 1:2. For this, 1 µg pIRES2-eGFP-Survivin plasmid and 2 µL lipofectamine were diluted in 100 µL serum-antibiotic free medium separately. After 5 min, they gently mixed and maintained at room temperature for 20 min. In the meanwhile, the cells were washed twice with serum-antibiotic free medium to completely remove FBS. Then, 500 µL serum-antibiotic free medium and plasmid-lipofectamine mixture were added to the cells and after 5 h incubation in 37 °C, 1 mL complete medium was added. After 24 h, the cells were assessed under the fluorescent microscope (Nikon, AZ100, Japan). In this study, we used the GFP plasmid (sc-108083, Santa Cruz Biotechnology, CA, USA) both as a control to optimize and monitor transfection efficiency and as one of the treatment groups for wound healing assay. Transfection of GFP plasmid into fibroblast cells was carried out as before.
MTT assay
To evaluate the proliferation rate of Survivin-transfected cells, GFP-transfected cells and, Normal cells, MTT assay was performed at daily intervals for 5 days. Briefly, the initial number of 1 × 103 cells/well were seeded in a 96-well plate, and at each interval; cells were treated with 4 mg/mL MTT solution (Invitrogen, Thermo Scientific, USA) and maintained at 37 °C for 4 h. Then, 100 µL DMSO (Sigma-Aldrich, Germany) was added to solubilize the colored formazan crystals. Afterward, the absorbance was measured at wavelengths of 570–630 nm using Eliza reader instrument (Eliza Microplate Reader, STAT Fax 2100, USA) and the growth curve of each group was plotted.
Preparation of fibrin-cell construct
The fibrin-cell construct was basically prepared according to a method introduced by Eyrich et al. [14]. Briefly, 250 µL fibrinogen solution (100 mg/mL) (Sigma Aldrich, USA) per well was added into a 12 well-plate. Then a number of 3 × 105 fibroblast cells were resuspended in the fibrinogen and 250 µL thrombin (5 U/mL in 40 mM CaCl2) (Sigma-Aldrich, Germany) was added and kept at 37 °C for 20 min.
Assessment of cell presence in the fibrin-cell construct
Hoechst is a non-intercalating blue fluorescent dye that stains the DNA content of both fixed and living cells. To verify the cell presence in the fibrin clot, we used Hoechst 33258 (Sigma Aldrich, USA) dye and the procedure was performed based on the common protocols [15]. Briefly, paraffin-embedded blocks were prepared from fibrin-cell construct and cell-free fibrin clot as control. Then, 5 µm thickness slices were stained with 1:5000 diluted Hoechst dye and after PBS washing, the slides were assessed under the fluorescent microscope (Nikon, AZ100, Japan) the next day.
Wound healing model
A number of 24 female Balb/c mice (5–6 weeks old, weighing 25–30 gr) were purchased from Pasteur Institute of Iran and kept under a regular condition of 12:12 h light: dark cycle with free access to food and water. The intraperitoneal injection (IP) of ketamine (90 mg.kg of body weight) and xylazine (4.5 mg.kg of body weight) was utilized for anesthesia [16]. The mice's dorsal skin was shaved and two 6 mm-diameter cutaneous wounds were created by a biopsy punch (Fig. 1).
Preparation of a mouse model of cutaneous wound and treatment. The created initial 6 mm-diameter wounds by biopsy punch (a). Suturing the silicon rings, transplantation, and covering the treated wound with a thin plastic sheet to prevent the clot infiltration into the bandages (b). Covering wounds with sterile bandages (c)
Experimental groups and transplantation
Mice were divided into four treatment groups (each group included three mice): (a) Survivin-transfected cells delivered with fibrin clot (Cell-SVV-Clot), (b) GFP-transfected cells delivered with fibrin clot (Cell-GFP-Clot), (c) Normal cells delivered with fibrin clot (Normal cell-Clot) and, (d) fibrin clot alone (Clot). A 0.5 mm thickness silicon ring was sutured to every wound to preserve treatments longer at the wound site as well as preventing the fast wound contraction. Then, a thin plastic sheet was placed for inhibition of clot infiltration into the bandages. Finally, the wounds were covered by sterile bandages (Fig. 1).
Wound closure analysis
The wound sites were photographed on days (1, 2, 3, 7, and 14) post-transplantation. The initial wound size was 28.26 mm2 for all groups and the actual wound size (mm2) of all groups was measured at any interval using NIH ImageJ software. Then, the wound closure rate was calculated using the following formula.
Quantitative real-time RT-PCR
First, Survivin expression in normal and modified cells was evaluated by qRT-PCR. For this, the total RNA was extracted from transfected cells with pIRES2-eGFP-Survivin plasmid and normal cells using TRIzol (Yekta Tajhiz, IRI). RNA was reversed transcribed to cDNA according to the cDNA synthesis kit (Invitrogen, Thermo Scientific, USA) instructions. On the other hand, the expression rates of Survivin, VEGF, and bFGF genes at the wound site were assessed by qRT-PCR similarly. For this, the total RNA was extracted from wound tissues on days (1, 2, 3, 7, and 14) post-transplantation. The relative primers used for qRT-PCR are brought in Table 1. Samples were prepared using SYBR Green PCR Master Mix, (Master Mix SYBR, Yekta Tajhiz, IRI), and run in a qRT-PCR thermocycler (Rotor-gene 6000, Qiagen, Germany). Finally, the 2−∆∆CT method was used for the calculation of fold change expressions.
Histopathological assessment
The paraffin-embedded blocks were prepared from formalin-fixed wound samples. Then the H&E (Hematoxylin and Eosin) staining was performed for 3–4 µm thickness slices according to the usual protocols [17]. All slides were investigated by a pathologist who was blind for treatment groups under the light microscope. The wound regeneration parameters of angiogenesis and formation of granulated tissue were evaluated. For analysis of each parameter, three fields of each slide were monitored and the mean value of each parameter corresponding to each treatment group was determined. Finally, the coverage area (%) per high power field was reported as the desired parameter percentage and the statistical analysis was performed.
Statistical analysis
Animal experiments section was performed as duplicate. Statistical analysis was conducted by IBM SPSS, version 22 (SPSS Software, Chicago, USA) using two-way ANOVA. Data were reported as means ± standard deviation (SD) and the p-value less than 0.05 was considered as statistically significant data. Analytical graphs were also created by Graphpad Prism, version 8 (Graphpad Software, California, USA).
Results
Transfection efficiency of fibroblast cells
The transfection of pIRES2-eGFP-Survivin plasmid (Fig. 2a) and GFP plasmid (Fig. 2b) into the fibroblast cells was successfully performed using lipofectamine 2000 reagent. The transfection efficiency was evaluated by counting the transfected and non-transfected cells in several fields using Image J software and the following formula. The transfection efficiency of pIRES2-eGFP-Survivin and GFP plasmids was 40.6 ± 2.7 and 36 ± 2.1 respectively.
MTT results
Related growth curves showed that normal cells have a relatively more proliferation rate rather than modified cells (Fig. 3a). However, the doubling time was calculated as 29.3 ± 0.2 h for normal cells, 31 ± 1 h for Survivin-transfected cells and, 32 ± 3 h for GFP-transfected cells. The doubling times were not statistically significant (p value = 0.42) (Fig. 3b). Therefore, results showed that lipofectamine-mediated genetic modification has left no detrimental effect on the normal cell cycle.
Cell presence confirmation in the fibrin-cell construct
Hoechst 33,258 staining showed the embedded cells in the fibrin-cell construct compared to the cell-free fibrin clot. The embedded cells are shown in blue color due to their stained DNA content (Fig. 4).
Wound closure assessment
The macroscopic results of wound healing showed that the clot alone group closed the wound faster than other groups within the first 24 h post-transplantation (p < 0.05). But after 3 days, the Cell-SVV-Clot group closed the wound with a higher rate compared to the clot group (p < 0.01). Interestingly after 7 days, the high wound closure rate was seen for Cell-SVV-Clot compared to Normal cell-Clot and Clot (p < 0.01) which demonstrates the beneficial effect of Survivin at the wound site. Finally, after 14 days, all groups closed the wound beneficially and no significant difference was detected for wound closure rates however, the minimum scar formation was seen for Cell-SVV-Clot and Normal Cell-Clot groups (Fig. 5a).
Macroscopic results of wound healing for Cell-SVV-Clot, Normal Cell-Clot, Cell-GFP-Clot and, Clot on days (1, 2, 3, 7, and 14) post-transplantation. The higher wound healing has occurred for Cell-SVV-Clot and Normal Cell-Clot groups with a minimal scar formation on day 14. Scale bars in mm (a). The wound closure rate: although the Clot alone increased the wound closure within 24 h compared to other groups (p < 0.05), the higher wound closure rate was detected for Cell-SVV-Clot group on day 3 compared to clot (p < 0.01) and on day 14 compared to Normal cell-Clot and Clot groups (p < 0.001) (b). Error bars refer to SD. *p < 0.05, **p < 0.01 and ***p < 0.001
Angiogenesis assessment
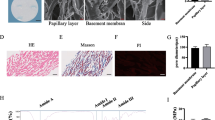
Based on H&E results of wound samples, the formation of new endothelial tubes was considered as angiogenesis (Fig. 6b, shown by yellow arrows) in which demonstrated no particular differences between groups. However statistical analysis of mean values of endothelial tubes showed the higher angiogenesis rate for Cell-SVV-Clot compared to Cell-GFP-Clot (p < 0.0001), Normal cell-Clot (p < 0.05) and Clot (p < 0.001) groups after 3 days. The Normal cell-Clot also showed more angiogenesis rate compared to Cell-GFP-Clot by day 3 (p < 0.01) (Fig. 7a).
Histopathology assessments for wound samples of Cell-SVV-Clot, Normal cell-Clot, Cell-GFP-Clot and, Clot groups on days (1, 2, 3, 7, and 14) post-transplantation (a). Scale bar = 100 µm. Granulation tissue formation and angiogenesis parameters of wound samples after 14 days are shown in white and yellow arrows respectively. The Cell-SVV-Clot showed a better-structured epidermis with higher granulated tissue and angiogenesis rate (b). Scale bar = 100 µm
Analysis of wound repair parameters based on H&E staining results for Cell-SVV-Clot, Cell-GFP-Clot, Normal cell-Clot and, Clot groups on days (1, 2, 3, 7 and 14) post-transplantation. Angiogenesis rate: the higher angiogenesis rate was seen for the Cell-SVV-Clot group compared to Cell-GFP-Clot (p < 0.0001), Normal cell-Clot (p < 0.05) and Clot (p < 0.01) groups on day 3 (a). The granulation tissue formation rate: more granulated tissue was formed for Cell-SVV-Clot compared to Cell-GFP-Clot on day 1 (p < 0.05), 7 (p < 0.001), and 14 (p < 0.0001). After 14 days, the Cell-SVV-Clot showed more granulation tissue formation compared to Normal cell-Clot (p < 0.05), Cell-GFP-Clot (p < 0.0001), and Clot (p < 0.0001) groups (b). Error bars refer to SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Granulation tissue formation assessment
Based on H&E results, the granulation tissue formation was almost similar for all groups (Fig. 6a). But the width of granulated tissue was more detectable for Cell-SVV-Clot group compared to other groups over 14 days post-transplantation (Fig. 6b, shown by white arrows). Statistical analysis of granulation tissue formation also demonstrated the significant difference in formation and maturation of granulated tissue for Cell-SVV-Clot compared to Cell-GFP-Clot group on day 7 (p < 0.001) and day 14 compared to other groups (p < 0.05), suggesting the probable longer cell survival at the wound site due to the Survivin overexpression (Fig. 7b).
Gene expressions
The Survivin was highly overexpressed in Survivin-transfected cells than normal cells (p < 0.01) (Fig. 8d) indicating the effective transfection rate of fibroblast cells and successful expression of this gene within cells. At the wound site, Survivin overexpression was observed for Cell-SVV-Clot compared to Normal cell-Clot for the first three days post-transplantation (p < 0.0001) which is due to the transient expression of pIRES2-eGFP-Survivin plasmid within cells (Fig. 8a). Therefore, we can verify the cell presence at the wound site at least for three days post-transplantation. On the other hand, in the presence of Survivin-modified fibroblast cells, the pick expression of VEGF occurred by day 3 (p < 0.01) (Fig. 8b) and the peak expression of bFGF was seen by day 14 post-transplantation (p < 0.001) (Fig. 8c).
Expression rate of Survivin (Birc5) gene (a), VEGF gene (b) and, bFGF gene (c) for Cell-SVV-Clot compared to Normal cell-Clot on days (1, 2, 3, 7 and 14) post-transplantation. The Survivin overexpression at the wound site was detected for 3 days (p < 0.0001). The VEGF peak expression was seen by day 3 (p < 0.01) and bFGF peak expression was detected by day 14 (p < 0.0001). Expression rate of Survivin (Birc5) gene in Survivin-transfected cells compared to normal cells (d). The high fold change of Survivin gene (p < 0.01) demonstrates the effective transfection efficiency and successful expression of pIRES2-eGFP-Survivin plasmid within cells. Error bars refer to SD. **p < 0.01, ***p < 0.001, ****p < 0.0001
Discussion
Survivin has recently attracted attention for enhancing the survival rate of cells and tissues in transplantation due to its anti-apoptotic function [9]. The essential role of Survivin in tissue repair has been determined using Survivin knockout [18] or Survivin deficient mice [19] after inducing injury. In cell-based wound therapy, the use of fibroblasts and/or keratinocytes alone [20] or in combination with skin grafts and membranes [21, 22] promotes wound healing. For instance, Şakrak et al. applied fibroblast cells for treatment of a full-thickness wound concurrently with a skin graft. It increased the neovascularization, collagen synthesis, and fibroblast density while the wound contraction had been decreased [23]. However, the beneficial effects of cell therapy are appeared to be limited due to the harsh environment of injured areas such as local ischemia, inflammation, and oxidative stress condition [24, 25]. Therefore, we hypothesized that Survivin overexpression may be able to improve the efficiency of transplantation by protecting cells against the forces during cell delivery and the harsh environment of the wound bed. Of note, due to the oncogenic potential of Survivin, there are considerations for Survivin overexpression especially for clinical translation [9].
Here, we transiently overexpressed Survivin in the fibroblast cells that last for 3 days post-transplantation and further upregulated VEGF and bFGF expression. Based on the literature, stable Survivin overexpression was done in Mesenchymal stem cells (MSCs) for treatment of the myocardial infarction. Similarly, Survivin upregulated the VEGF expression rate leading to enhanced capillary density and reduced infarct size [26]. Liu et al. also transplanted Survivin-overexpressed MSCs for the treatment of brain stroke in a rat model. They reported that Survivin increases the VEGF and bFGF secretion both in the supernatant of cell culture and ischemic tissue resulting in a reduced infarct volume by day 4 after stroke [27]. Here, we assigned fibroblast cells for transplantation due to their unique potential in the production of ECM [3]. Besides, the similar therapeutic effects of Adipose-derived stem cells and fibroblast cells showed by Steinberg et al. encouraged us to investigate the Survivin overexpression effects in fibroblast cells. Steinberg et al. reported that both Adipose-derived stem cells and fibroblast cells contribute similarly to the formation of granulated tissue which is mainly related to their similarity in terms of signaling and differentiation potential [28].
Based on our results, we suggest that Survivin induces VEGF and bFGF secretion in fibroblast cells which is consistent with Fan et al. [26] and Liu et al. [27] findings in Mesenchymal stem cells explained above. Here, we detected the peak expression of VEGF by day 3 and peak expression of bFGF by day 14. Interestingly, the histopathology results of wound samples showed more angiogenesis rate in Cell-SVV-Clot by day 3 and also more granulation tissue formation by day 14 post-transplantation in comparison to other groups. This confirms the relation of bFGF and VEGF with the improvement of angiogenesis and formation of granulated tissue. These results are consistent with previous studies in utilizing of VEGF and bFGF growth factors for wound therapy. For instance, a synthesized scaffold comprising PLGA (poly lactic-co-glycolic acid) nanoparticles loaded with VEGF and bFGF showed a higher wound closure rate, granulation tissue formation, and re-epithelialization rate [29]. In another study by Lohmeyer et al., the artificial skin was loaded with genetically modified keratinocytes and fibroblast cells expressing PDGF and VEGF/bFGF respectively. The nude mice wounds were treated with genetically modified artificial skin which led to a higher epithelialization and vascularization rate [30]. Eggers et al. also used the VEGF-expressing fibroblasts for treatment of ischemic hind leg of rat model which significantly increased the CD-31+ blood vessels on day 14 and the vessel growth on day 5 compared to the non-transfected cells and saline groups [31].
However, it has been demonstrated that VEGF could act as a double-edged sword in wound healing and its consistent expression leads to formation of hemangioma [32], abnormalities in maturation of epidermis along with its ability for attracting the opportunist pathogens [33]. Here, we detected the transient VEGF upregulation in Cell-SVV-Clot group which increased the wound closure rate by supplying more blood vessels (Fig. 5b). This is consistent with one study that took advantage of temporary VEGF expression in wound therapy [34].
On the other side, bFGF has been identified for its roles in stimulation of fibroblast proliferation and migration. Indeed, bFGF increases the human dermal fibroblast proliferation through ERK1/2 and JNK pathways in a time and dose-dependent manner [35], and fibroblast migration rate through the PI3-Kinase-Rac1-JNK pathway [36]. In particular, bFGF promotes both angiogenesis and granulation tissue formation in a dose-dependent manner [37, 38].
Our results showed that Survivin has further upregulated bFGF at the wound site. The H&E results of wound samples showed a higher width of granulated tissue across the wound bed in the Cell-SVV-Clot group compared to other groups after 14 days post-transplantation (Figs. 6b and 7b). Akasaka et al. claimed that long-term bFGF treatment induces apoptosis in fibroblast cells and suppresses the granulation tissue formation [39]. However, we are not able to anticipate the effects of long-term bFGF expression after 14 days based on our study design.
In conclusion, by a single gene modification, we transiently overexpressed Survivin in L929 fibroblast cells which upregulated the VEGF and bFGF expressions at particular times at the wound site. Survivin-overexpression enhanced the efficiency of fibroblast cell-based wound therapy through increasing angiogenesis, granulation tissue formation, and wound closure rate at the early stage of wound healing (Fig. 9). However, more investigation in genetic modification of cells before transplantation would be required in the future. As a future perspective, the Survivin-expressing fibroblasts and/or stem cells for treatment of different types of wounds would be promising.
The schematic pattern of study design and wound healing mechanisms. The mouse fibroblast cells were transfected with pIRES2-eGFP-Survivin plasmid (shown as pIRES2-eGFP-SVV plasmid in figure) and GFP plasmid separately. The non-transfected cells remained as normal cells. After preparation of fibrin clot, the three treatment groups were prepared as Cell-SVV-Clot, Cell-GFP-Clot, and Normal cell-Clot. The cell-free clot was also considered as the fourth group (Clot). After animal modeling and transplantation, the effects of each group were assessed on days 1, 2, 3, 7, and 14 post-transplantation. The Survivin overexpression upregulated the VEGF and bFGF expression at particular times post-transplantation led to higher wound closure, angiogenesis, and formation of granulated tissue
Footnotes
1-bFGF (basic fibroblast growth factor), 2-Birc5 (Baculoviral IAP repeat-containing 5), 3-ECM (Extracellular matrix), 4-FBS (Fetal bovine serum), 5-H&E (Hematoxylin &Eosine), 6-IAP (Inhibitor of apoptosis family), 7-PDGF (Platelet-derived growth factor), 8-TNF-α (Tumor necrosis factor- α), 9-VEGF (Vascular endothelial growth factor).
References
Mori Y, Nakagami G, Kitamura A et al (2019) Effectiveness of biofilm-based wound care system on wound healing in chronic wounds. Wound Repair Regen 27(5):540–547
Kim WJ, Mohan RR, Mohan RR, Wilson SE (1999) Effect of PDGF, IL-1alpha, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest Ophthalmol Vis Sci 40(7):1364–1372
Li B, Wang JHC (2011) Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J Tissue Viability 20(4):108–120
Yates CC, Rodrigues M, Nuschke A et al (2017) Multipotent stromal cells / mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res Ther 8(1):193
Xue X, Liu Y, Zhang J, Liu T, Yang Z, Wang H (2015) Bcl-xL genetic modification enhanced the therapeutic efficacy of mesenchymal stem cell transplantation in the treatment of heart infarction. Stem Cells Int 2015:176409
McKenzie JA, Grossman D (2012) Role of the apoptotic and mitotic regulator survivin in melanoma. Anticancer Res 32(2):397–404
Jha K, Shukla M, Pandey M (2012) Survivin expression and targeting in breast cancer. Surg Oncol 21(2):125–131
Shojaei F, Yazdani-Nafchi F, Banitalebi-Dehkordi M, Chehelgerdi M, Khorramian-Ghahfarokhi M (2018) Trace of survivin in cancer. Eur J Cancer Prev 28(4):365–372
Assadiasl S, Mousavi MJ, Amirzargar A (2018) Antiapoptotic molecule survivin in transplantation: helpful or harmful? J Transplant 2018:1–6
Przybylski M (2009) A review of the current research on the role of bFGF and VEGF in angiogenesis. J Wound Care 18(12):516–519
Miller ED, Song F, Smith JD et al (2018) Plasma-based biomaterials for the treatment of cutaneous radiation injury. Wound Repair Regen 27(2):139–149
Clover AJP, Kumar AHS, Isakson M et al (2015) Allogeneic mesenchymal stem cells, but not culture modified monocytes, improve burn wound healing. Burns 41(3):548–557
Chang A, Chau V, Landas J (2017) Preparation of calcium competent Escherichia coli and heat-shock transformation. JEMI methods 1(June):22–25
Eyrich D, Brandl F, Appel B et al (2007) Long-term stable fibrin gels for cartilage engineering. Biomaterials 28(1):55–65
Buceviˇ J, Lukinaviˇ G (2018) The use of hoechst dyes for dna staining and beyond. Chemosensors. https://doi.org/10.3390/chemosensors6020018
Clouthier S, Luther T, Wicha M. Ketamine/Xylazine Containing Anesthesia for Mouse Surgery Preparation. Available from: https://www.med.umich.edu/wicha-lab/SOP/SOP 6.3- Ketamine Xylazine Anesthesia 4–2–2015.pdf.
Fischer AH, Jacobson KA, Rose J, Zeller R. (2008) Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008(5), pdb.prot4986.
Chen J, Chen J-K, Conway EM, Harris RC (2013) Survivin mediates renal proximal tubule recovery from AKI. J Am Soc Nephrol 24(12):2023–2033
Kindt N, Menzebach A, Van de Wouwer M, Betz I, De Vriese A, Conway EM (2007) Protective role of the inhibitor of apoptosis protein, survivin, in toxin-induced acute renal failure. FASEB J 22(2):510–521
Velander P, Theopold C, Bleiziffer O et al (2009) cell suspensions of autologous keratinocytes or autologous fibroblasts accelerate the healing of full thickness skin wounds in a diabetic porcine wound healing model. J Surg Res 157(1):14–20
Kouhbananinejad SM, Derakhshani A, Vahidi R et al (2019) A fibrinous and allogeneic fibroblast-enriched membrane as a biocompatible material can improve diabetic wound healing. Biomater Sci 7(5):1949–1961
Cengiz C, Soyocak A, Karabag Y (2011) The effects of combined application of autogenous fibroblast cell culture and full-tissue skin graft (FTSG) on wound healing and contraction in full-thickness tissue defects e Aydan Ko. Burns 8:2–8
Şakrak T, Köse AA, Kivanç Ö et al (2012) The effects of combined application of autogenous fibroblast cell culture and full-tissue skin graft (FTSG) on wound healing and contraction in full-thickness tissue defects. Burns 38(2):225–231
Havasi A, Borkan SC (2011) Apoptosis and acute kidney injury. Kidney Int 80(1):29–40
Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI (2005) Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol 46(7):1339–1350
Fan L, Lin C, Zhuo S et al (2009) Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail 11(11):1023–1030
Liu N, Zhang Y, Fan L et al (2011) Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J. Transl. Med. https://doi.org/10.1186/1479-5876-9-105
Steinberg JP, Hong SJ, Matthew R, Galiano RD, Mustoe TA (2012) Equivalent effects of topically-delivered adipose-derived stem cells and dermal fibroblasts in the ischemic rabbit ear model for chronic wounds. Aesthet Surg J 32(4):504–519
Losi P, Briganti E, Errico C et al (2013) Acta biomaterialia fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater 9(8):7814–7821
Lohmeyer JA, Liu F, Krüger S, Lindenmaier W, Siemers F, MacHens HG (2011) Use of gene-modified keratinocytes and fibroblasts to enhance regeneration in a full skin defect. Langenbeck’s Archives of Surgery 396:543–550
Eggers C, Mu J (2015) VEGF transfer based on gene- modified fibroblasts using a hypoxia-induced vector to modulate neoangiogenesis in ischaemic regions of myocutaneous transplants. Int J Oral Maxillofac Surg. https://doi.org/10.1016/j.ijom.2014.06.018
Chang J, Most D, Bresnick S et al (1999) Proliferative hemangiomas: analysis of cytokine gene expression and angiogenesis. Plast Reconstr Surg 103(1):1–10
Birkenhauer E, Neethirajan S (2015) A double-edged sword: the role of VEGF in wound repair and chemoattraction of opportunist pathogens. Int J Mol Sci 16(4):7159–7172
Hirsch T, von Peter S, Dubin G et al (2006) Adenoviral gene delivery to primary human cutaneous cells and burn wounds. Mol Med 12(9–10):199–207
Makino T, Jinnin M, Muchemwa FC et al (2010) Basic fibroblast growth factor stimulates the proliferation of human dermal fibroblasts via the ERK 1⁄2 and JNK pathways. Br J Dermatol 162(4):717–723
Kanazawa S, Fujiwara T, Matsuzaki S, Shingaki K, Taniguchi M (2010) bFGF regulates PI3-kinase-rac1-JNK pathway and promotes fibroblast migration in wound healing. PLoS ONE 5(8):1–12
Okumura M, Okuda T, Okamoto T, Nakamura T, Yajima M (1996) Enhanced angiogenesis and granulation tissue formation by basic fibroblast growth factor in healing-impaired animals. Arzneimittelforschung 46(10):1021–1026
Okumuram OT, Nakamura T, Yajima M (2011) Acceleration of wound healing in diabetic mice by basic fibroblast growth factor. Biol Pharm Bull 19(4):530–535
Akasaka Y, Ono I, Yamashita T, Jimbow K, Ishii T (2004) Basic fibroblast growth factor promotes apoptosis and suppresses granulation tissue formation in acute incisional wounds. J Pathol 203(2):710–720
Acknowledgements
The authors thank the Cellular and Molecular Research Center of Shahrekord University of Medical Sciences for their supports.
Funding
This research was funded by Shahrekord University of Medical Sciences (Grant Number:1396-08-74-516).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that they have no conflict of interest.
Research involving animals
All animal intervention in this study was carried out according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, Revised 1985). The ethics code was also received from Ethics Committee of Shahrekord University of Medical Sciences for animal experiments in this research. (Ethics code: IR.SKUMS.RE.1396.203).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shojaei-Ghahrizjani, F., Rahmati, S., Mirzaei, S.A. et al. Does survivin overexpression enhance the efficiency of fibroblast cell-based wound therapy?. Mol Biol Rep 47, 5851–5864 (2020). https://doi.org/10.1007/s11033-020-05656-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05656-4