Abstract
Malignant melanoma is a very aggressive and serious type of cutaneous cancer. Previous studies indicated the anti-cancer activity of aqueous extract of Moringa oleifera Lam. leaves (MOE) against a variety of cell lines. However, there has not been much research about the effect of MOE on melanoma. Therefore, this study was about to investigate the anti-proliferation mediated by apoptosis of MOE on human melanoma cell lines. Furthermore, the related molecular mechanisms of the apoptosis were also examined. An aqueous extract of Moringa oleifera leaves was prepared and the anti-proliferative activity on melanoma cells and normal cells was tested using WST-1 assay. The apoptotic hallmarks including DNA condensation and phosphatidylserine (PS) externalization were assessed. The expression of apoptosis-related genes and the depolarization of mitochondrial membrane potential were then examined to clarify the underlying molecular mechanisms. MOE inhibited cell growth of A375 cells and A2058 cells in a dose-dependent manner but had little effect on human normal fibroblasts. The cell growth inhibition was induced by apoptosis which was expressed via chromatin condensation and PS externalization. MOE decreased mitochondrial membrane potential. Additionally, MOE increased Bax/Bcl-2 ratio, activated Caspase-3/7, Caspase-9, PARP and AIF translocation, leading to apoptotic cell death. Our study indicated that MOE exerted significant anti-cancer effects on melanoma cells in vitro which involved mitochondria-mediated Caspase-dependent and Caspase-independent apoptosis pathways. These results provided a scientific approach for using Moringa oleifera leaves as an alternative therapy to treat skin cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma, the most serious type of skin cancer, usually derives from the malignant transformation of melanocytes causing dysfunction to normal cellular growth [1]. Current treatment options including surgery, radiation therapy, chemotherapy, have been used alone or in combination [2]. However, melanoma is highly aggressive, forming wide-spreading metastatic lesions, which causes resistance to most of the treatment therapies. Furthermore, many harmful side effects occur after the course of treatment [3]. Therefore, the identification of anti-cancer drugs with improved efficacy and fewer toxic side effects is required. Traditionally plant is a source of drugs with reputed efficiency against different types of cancer [4]. Concurrent administration of herbal medicines and anticancer drugs enabled to improve the efficiency while alleviating the side effects for patients [5, 6]. These activities were presumably due to synergistic activities, improving the immune system of patients and buffering the effects of toxicity [7]. However, most of the mechanisms involved have not been clearly understood. Therefore, in addition to the suggestion of new anti-cancer compounds, the finding of action mechanisms of herbal medicines has received much scientific attention.
Moringa oleifera Lam., cultivated in tropical and subtropical countries, is a nutritious plant that contains high amounts of proteins, vitamins, calcium and antioxidants. Moringa oleifera has been widely used in traditional medicine due to its high bioactivity, including anti-oxidative, anti-hyperglycemic, hepatoprotective, anti-inflammatory and anti-cancer functions [8, 9]. Recently, some studies have shown that the crude aqueous extract of Moringa oleifera leaves possessed high anti-cancer activity against various cancer cell lines. The majority of killing activity was supposed to undergo an apoptosis pathway. Particularly, Moringa oleifera aqueous extract reduced the proliferation of Panc-1 pancreatic cancer cells, and enhanced the killing efficiency of cisplatin on these cells. The anti-proliferation was presumably due to downregulating the expression of NF-kβ [10]. The study of Jung et al. showed that Moringa oleifera leaf soluble extract inhibited cell growth and induced apoptosis in HepG2 hepatocellular carcinoma cells. Orally administered extract reduced approximately 50% the growth of the HepG2 and A549 human lung cancer cells in vivo, suggesting to use soluble extracts of Moringa oleifera leaves as orally administered therapeutics to treat liver and lung cancers [11]. Some reports confirmed the apoptosis-inducing activity of Moringa oleifera leaves in A549 cells by stimulating oxidative stress, DNA fragmentation, upregulating the expression of pro-apoptotic proteins (p53, Smac/DIABLO, AIF), Caspases 3/7, downregulating the expression of anti-apoptotic protein (Nrf2) and enhancing the cleavage of PARP-1 [12, 13]. Similarly, Tiloke et al. demonstrated the aqueous extract of Moringa oleifera leaves inhibited HepG2 cells via arresting cell cycles of G1, S, G2/M. Corresponding to the administration of the extract, the expressions of c-myc, p-Bcl2 and Hsp70 were reduced while the expressions of Bax, Smac/DIABLO, Caspase-9, Caspase-3/7 and PARP-1 cleavage were significantly enhanced, leading to apoptosis-induced cell death [14].
There are not many studies mentioned about the effect of Moringa oleifera leaf aqueous extract on melanoma cells and the mechanism of action is still unclarified. In our study, MOE showed potential cytotoxicity against melanoma cells, while leaving no effect on normal cells. MOE induced apoptosis via mitochondria-mediated Caspase-dependent and Caspase-independent pathways. MOE activated the Caspase-3, Caspase-9, PARP and AIF translocation, resulting in the death of A375 melanoma cells. These findings provide an important fundament for the use of Moringa oleifera Lam. leaves in the development of alternative therapies for skin cancer treatment.
Materials and methods
Cell culture
A375 human malignant melanoma cells, A2058 human metastatic melanoma cells and WS1 normal human skin fibroblasts were supplied by the American Type Culture Collection (ATCC, Rockville, MD, USA). Primary human dermal fibroblasts (NHDF) isolated from neonatal foreskins were kindly gifted from Dr. Quan of University of Science, VNUHCM. A375 cells, A2058 cells, WS1 cells and NHDF cells were thawed and expanded in DMEM-F12, supplemented with 2 mM l-glutamine, 0.5% penicillin/streptomycin (pen/strep) and 10% FBS at 37 °C, 5% CO2 (DMEM-F12, l-glutamine, pen/strep and FBS were obtained from Thermo Fisher Scientific, Waltham, MA, USA). When the confluency reached approximately 80%, cells were sub-cultured for propagation. During culture, the mycoplasma PCR detection kit (iNtRON Biotechnology, Gyeonggi-do, Korea) was used to detect mycoplasma contamination.
Preparation of Moringa oleifera leaf extract
The leaves of Moringa oleifera Lam. were collected locally in March 2018 at the Institute of Tropical Biology. The plants were identified and authenticated by Dr. Son, Institute of Tropical Biology, Vietnam Academy of Science and Technology, Vietnam. The aqueous extraction of Moringa oleifera leaves was performed according to our previously described method. Briefly, the dried and powdered leaves of Moringa oleifera (200 g) were incubated with boiling water (95 °C, 7.500 mL) for 2 h and then the water was removed under vacuum. After filtering and lyophilization at − 40 °C, approximately 28.5 g of the aqueous extract (MOE) was obtained. MOE was stored at − 20 °C before use.
Cell viability assay
The effect of the extract on the cell viability was determined using WST-1 cell proliferation assay (Roche, Basel, Switzerland). Briefly, A375 cells, A2058 cells, WS1 cells and NHDF cells at a concentration of 3 × 103 cells per well were seeded into 96-well plates (Corning Inc., NY, USA) containing 100 μL of culture media. After 12 h incubation for adherence, cells were washed with PBS and exposed to various concentrations of MOE (0–200 µg/mL) for 24–72 h. After treatment, 10 μL WST-1 solution was added to each well, followed by incubation at 37 °C, 5% CO2 for 4 h. The absorbance was measured using Glomax Discover Microplate Readers (Promega, Madison, WI, USA) at 450 nm.
Clonogenic cell survival assay
The effect of the extract on cell survival was tested using clonogenic assay. A375 cells were plated into 6-well plates (Corning, NY, USA) at 1 × 105 cells per well in 2 mL medium. The cells were treated with different concentrations of MOE (50, 75 and 100 µg/mL) for 72 h. The cell density was reduced (approximately 500 cells/well), then the plate was incubated for an additional 10 days. Untreated cells were used as a control. Clones were fixed with 4% paraformaldehyde for 15 min at room temperature before staining with 0.1% Crystal Violet solution (Sigma-Aldrich, St. Louis, MO, USA) for 15 min. Stained clones with more than 50 cells were used for analysis.
4′,6-Diamidino-2-phenylindole (DAPI) nuclear staining assay
The morphological changes of the cells after MOE treatment were examined using DAPI staining assay. In the experiment, A375 cells were treated with 25, 50 and 75 µg/mL of MOE for 72 h. 0.1% DMSO and 50 µg/mL of Etoposide (Sigma-Aldrich, St. Louis, MO, USA) were used as a negative control and a positive control, respectively. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature. DAPI (Thermo Fisher Scientific, Waltham, MA, USA) was added to a final concentration of 1 μg/mL. The plate was incubated for 15 min at room temperature, followed by washing the unbound dye using PBS. The stained cells were observed using Cytell microscope (GE Healthcare, Illinois, United States).
Apoptosis assay
The effect of the extract on the cell apoptosis was determined using FITC Annexin V apoptosis detection assay (BD Biosciences, Franklin Lakes, NJ, USA) together with flow cytometry (FACSCalibur) (BD Biosciences, Franklin Lakes, NJ, USA). A375 cells were treated with different concentrations of MOE or 10 µg/mL Etoposide (as a positive control) or 0.1% DMSO (as a negative control) for 72 h. After washing with PBS, cells at the concentration of 5 × 104 cells/mL were supplemented with 5 μL Annexin V-FITC and 5 μL PI, gently mixed and incubated for 20 min at room temperature in darkness. Subsequently, the cells were added to 400 μL binding buffer (1×) before loading onto FACSCalibur. CellQuestPro software (BD Biosciences, Franklin Lakes, NJ, USA) was used for analysis. The controls were unstained cells or cells stained with Annexin V-FITC or PI alone.
Mitochondrial transmembrane potential assay
The effect of the extract on mitochondrial transmembrane potential (ΔΨm) was measured using DiOC6 (Thermo Fisher Scientific, Waltham, MA, USA), a fluorescent lipophilic cationic probe which is easy to penetrate and accumulate in mitochondria. Briefly, A375 cells were plated at a density of 5 × 105 cells/well in a 25 cm2 culture flask (Corning, NY, USA), following by treatment with different concentrations of MOE (50, 75 and 100 µg/mL). 0.1% DMSO and 50 µg/mL of Etoposide were used as a negative and a positive control, respectively. In another experiment, the cells were exposed to an antioxidant N-acetylcysteine for 1 h (NAC; 2 mM, Sigma-Aldrich, St. Louis, MO, USA) prior to treatment with 100 µg/mL of MOE, 0.1% DMSO solution and 50 µg/mL of Etoposide at 37 °C for 72 h. After washing by cold PBS, cells were resuspended in 40 nM DiOC6 and further incubated for 30 min in the dark at 37 °C, 5% CO2. The cells were subsequently washed and recovered in PBS before applying onto FACSCalibur. The results were expressed as a percentage of decrease in DiOC6 staining.
Real-time qRT-PCR assay
The expression of genes relating to the apoptosis pathway was analyzed using real-time qRT-PCR. The cells were treated with different concentrations of MOE for 72 h. Total cellular RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA, USA) following the instructions of the manufacturer. Biophotometer (Eppendorf, Hamburg, Germany) was used to measure the concentration of obtained RNA. qRT-PCR was performed using SYBR Green One-Step qRT-PCR kit (Thermo Fisher Scientific, Waltham, MA, USA) with Pikoreal Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The primers used in the experiments were shown in Table 1. The relative quantitation of gene expression was determined using the 2−ΔΔCt method [15]. All calculations were normalized using the β-actin gene as an internal control.
Western blot assay
The expression of genes relating to the apoptosis pathway was analyzed using western blot. The cells were treated with different concentrations of MOE for 72 h. The treated cells or control cells were harvested, washed with ice-cold PBS 2 times and lysed in a cold lysis buffer. The protein concentration was determined using Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Samples were loaded onto 10% SDS-PAGE and then transferred to a sheet of specific polyvinylidene fluoride (PVDF) blotting membrane (Sigma-Aldrich, St. Louis, MO, USA) at 250 mA for 90 min. After blocking step using 1% BSA or 5% skim milk (Sigma-Aldrich, St. Louis, MO, USA) for overnight at 4 °C, the membranes were washed with PBS and incubated with indicated primary antibodies diluted 1:1000 at room temperature for 2 h. The primary antibodies used are Bax, Bcl-2, PARP, AIF and β-actin (Abcam, Cambridge, ENG, UK). The membranes were washed with PBS 3 times and incubated with goat anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase (HRP) diluted 1:5000 (Sigma-Aldrich, St. Louis, MO, USA) for 1 h. Bound antibodies were detected by enhanced chemiluminescence (ECL) (Sigma-Aldrich, St. Louis, MO, USA). The expression of β-actin was used as a normalizing control. The intensity of expressed proteins was analyzed using Image J software (NIH, Bethesda, MD, USA).
Caspase activity assay
Caspase activities were determined using the Caspase-Glo 3/7 and 9 assay kits (Promega, Madison, WI, USA). A375 cells with the density of 1 × 104 cells/well were seeded into a 96-well microtiter plate (Corning, NY, USA). Cells were exposed to various concentrations of MOE or 0.1% DMSO or 50 µg/mL of Etoposide for 72 h. After incubation, 100 µL of Caspase-Glo 3/7 and Caspase-Glo 9 reagents were added and the plates were incubated at room temperature in dark for 30 min. The luminescence was measured on Glomax Discover Microplate Readers (Promega, Madison, WI, USA). The data were expressed as relative light units (RLU) and fold change.
Cell death in the presence of Caspase inhibitors assay
A375 cells were incubated with or without 50 μM Caspase inhibitor Z-VAD-FMK (N-benzyloxycarbonyl-Val-Ala-Asp-fluoro-methylketone, RD Systems Inc., Minneapolis, USA) for 1 h, before treating with different concentrations of MOE or 0.1% DMSO (control) for 48 h. Cell viability, cell apoptosis and Caspase activity were investigated as previously described.
Immunofluorescence staining assay
For the AIF redistribution analysis, 5 × 104 cells/well were seeded onto 15-mm autoclaved cover glasses in 24-well culture plates. After washing with PBS, the cells were fixed using 3.7% formaldehyde and then permeabilized using 0.2% Triton X-100 in 10 min. The cells were immersed in Image-iT FX Signal Enhancer (Thermo Fisher Scientific, Waltham, MA, USA) for blocking, followed by incubating with appropriate primary anti-AIF antibodies (1:100) in 1% BSA for overnight at 4 °C. Cells were washed 3 times with PBS, then incubated with the Alexa® Fluor 488-labeled goat anti-mouse IgG with proper dilution (1:500) (Thermo Fisher Scientific, Waltham, MA, USA) for 2 h at 4 °C. The cells were washed 3 times with PBS and counterstained with DAPI before analysis. Fluorescence was visualized using Cytell microscope (GE Healthcare, Illinois, United States). Image J software (NIH, Bethesda, MD, USA) was used to merge pictures.
Statistical analysis
All the data were performed in three independent experiments (n = 3). Data were expressed as mean ± standard deviation (SD) of the mean. The Student’s t-test and ANOVA were used to analyze the significant difference. p-values < 0.05 were considered to be statistically significant.
Results
Effects of MOE on cell viability in human melanoma cells and normal cells
Human melanoma cell lines (A375 cells and A2058 cells) and normal human fibroblasts (WS1 cells and NHDF cells) were used to investigate the antiproliferative effects of the extract. Cells were treated with various concentrations (0 to 200 µg/mL) of MOE for 24–72 h. After treatment, the viability of cells was detected using WST-1 assay. As shown in Fig. 1, MOE enabled to inhibit the proliferation of both melanoma A375 cells and A2058 cells in a dose-dependent manner. After 72 h of MOE treatment, the viability of A375 cells was significantly decreased to 74.32 ± 4.96%, 63.35 ± 5.60% and 39.76 ± 3.72% at concentrations of 50, 75 and 100 μg/mL of MOE, respectively. At higher concentrations (150 and 200 μg/mL), MOE markedly decreased the cell proliferation to 19.08 ± 4.81% and 16.22 ± 3.08%, respectively when compared to control (p < 0.05 and p < 0.01, Fig. 1a). However, no obvious growth inhibitory effects of MOE were observed on A375 cells within 24 h or 48 h treatment (Fig. 1a). In addition, the IC50 values were 87.28 ± 2.75 μg/mL and 108.47 ± 3.12 μg/mL on A375 cells and A2058 cells, respectively. The results indicated that MOE was more cytotoxic on A375 cells than A2058 cells (Fig. 1b). Conversely, WS1 cells and NHDF cells were less susceptible to MOE-induced cytotoxicity. At concentrations of 150 μg/mL and 200 μg/mL, MOE slightly reduced the viability of the NHDF cells to approximately 73.67 ± 4.02% and 65.82 ± 5.12% compared to control, respectively (Fig. 1c). Similar results were observed in the WS1 cells (Fig. 1d).
Cytotoxicities of MOE on human melanoma cells and normal cells. The viability of A375 cells (a) and A2058 cells (b) compared to that of NHDF cells (c) and WS1 cells (d) after exposure to various concentrations of MOE for 24–72 h. Colony number of A375 cells (e) and A2058 cells (f) treated with various concentrations of MOE in colony-forming assay. Untreated cells were used as a negative control. Data were expressed as mean ± SD of three experiments. *p < 0.05 and **p < 0.01 compared to control
To verify the growth inhibition activity of MOE on melanoma cells, the colony formation of cells after treatment with various concentrations of MOE for 72 h was also examined. The data of Crystal Violet staining showed a significant reduction of the cloning efficiency in MOE-treated cells in comparison to control (p < 0.05 and p < 0.01, Fig. 1e, f). It was suggested that melanoma cells were damaged after MOE treatment. Since A375 cells were the most sensitive to MOE treatment, A375 cells incubated with the various concentrations of MOE (50, 75 and 100 µg/mL) for 72 h were used in the following studies.
Effects of MOE on apoptosis induction in A375 cells
To determine whether MOE induce apoptosis in A375 cells, DAPI staining and FACS were performed. DAPI staining showed that there was the nucleus disorganization in MOE-treated cells or Etoposide-treated cells, including condensed chromatin (deeply dyed) and nuclear fragmentation compared to untreated cells (Fig. 2a). The results indicated that the cells were undergoing apoptosis. For further confirmation, double staining with Annexin V-FITC and PI was used to distinguish apoptotic cells using flow cytometry (Fig. 2b). As a result, MOE effectively induced apoptosis in A375 cells in a dose-dependent manner. After 72 h of treatment, the proportion of early and late apoptotic cells were 12.16 ± 2.85%, 26.12 ± 4.76% and 36.40 ± 5.86%, respectively in cells treated with 50, 75 and 100 µg/mL MOE in comparison with 3.28 ± 0.82% of control cells (p < 0.05 and p < 0.01, Fig. 2c). Etoposide-treated cells served as a positive control. After 72 h of treatment, the apoptotic rate in Etoposide-treated cells increased to approximately 45.32 ± 5.02%.
Induction of apoptosis in A375 cells treated with various concentrations of MOE for 72 h. Chromatin change in treated cells. The treated cells were stained with DAPI and visualized using fluorescent microscopy. MOE-treated cells showed significantly condensed chromatin compared to the intact genomic DNA of control cells. The arrows indicate condensed chromatin (a). Detection of apoptosis using Annexin V-FITC/PI double staining and FCM analysis. The survived cells (lower left), early apoptosis (lower right), late apoptosis (upper right) and necrosis (upper left) were represented (b). Representative graph of percentage of apoptotic cells (c). 0.1% DMSO and Etoposide were used as a negative control and a positive control, respectively in the experiments. Data were expressed as mean ± SD of three experiments. *p < 0.05 and **p < 0.01 compared to control
Effects of MOE on the expression of apoptosis-related genes in A375 cells
The expression of apoptosis-related genes, including Bax, Bcl-2, Caspase and PARP, was examined using Real-time qRT-PCR, Western blot and luminescent assays. As a result, Fig. 3 showed that the expression level of Bcl-2 was reduced to 0.68- and 0.53-fold, confirmed by qRT-PCR and Western blot respectively after treatment with 100 µg/mL of MOE for 72 h. In contrast, the expression of Bax increased 2.62-fold at the mRNA level and 1.85-fold at the protein level, leading to an increase in the pro-apoptotic/anti-apoptotic ratio of Bax/Bcl-2 when compared to those of the control (Fig. 3a, b). These results demonstrated that the apoptosis to cancer cells induced by MOE was mediated via targeting the pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2.
The expression of apoptotic regulatory proteins Bax, Bcl-2, Caspase-3/7, Caspase-9 and PARP in A375 cells. Cells were treated with MOE for 72 h. The expression of Bax, Bcl-2, Caspase-3, Caspase-9 and PARP were examined using qRT-PCR assay (a), Western blot assay (b, c) and luminescent assay (d). Densitometric analyses of these proteins were made relative to β-actin which was used as a housekeeping gene control (c). 0.1% DMSO and Etoposide were used as a negative control and a positive control, respectively. Data were expressed as mean ± SD of three experiments. *p < 0.05 and **p < 0.01 compared to control
The changes in the Bax/Bcl-2 ratio might initiate activation of the Caspase cascade. Therefore, to investigate whether Caspases are involved in apoptosis, the expressions of Caspase-3/7, Caspase-9 and PARP were examined. As a result, the expression of Caspase-3/7 and Caspase 9 showed an increase at the mRNA level but slight increase at the protein level (Fig. 3a, d). Particularly, 50 µg/mL MOE induced a slight increase of 1.08- and 1.02-fold in the activities of Caspase-3/7 and Caspase 9. At a higher concentration of MOE (100 µg/mL), the activation of Caspase-3/7 and Caspase-9 increased up to 1.75- and 1.42-fold respectively compared to control (p < 0.05, Fig. 3d). Furthermore, PARP is a preferred substrate processed by an executioner caspase and its cleavage is attributed to executioner caspase activation. MOE downregulated PARP expression at both mRNA and protein levels (Fig. 3a, c). Also, MOE enabled to cleave full-length PARP (116 kDa) into fragments detected by Western blot (Fig. 3b). A similar result of cleaved PARP was also observed in Etoposide-treated cells, a positive apoptotic control. The data indicate that MOE might induce Caspase-mediated apoptosis in A375 cells.
Effects of MOE on mitochondrial transmembrane potential in A375 cells
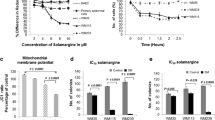
The change of apoptotic regulatory proteins could lead to a reduction of mitochondrial outer membrane permeabilization. The mitochondrial membrane potential (ΔΨm) of treated A375 cells was measured using fluorescent dye DiOC6, a specific probe for the disruption of ΔΨm. The results showed that 72 h treatment of MOE decreased ΔΨm in a dose-dependent manner (Fig. 4a). Particularly, there was a decrease of staining in a range from 3.56 ± 0.85% to 47.12 ± 5.28% in MOE-treated samples compared to controls, indicating the decrease of ΔΨm by MOE (p < 0.05 and p < 0.01, Fig. 4b). Besides, granular-like structures which were presumably derived from fragmentation of mitochondria were observed (Data not shown). Similar structures were also observed in Etoposide-treated samples which were used as a positive control. However, this formation was partially prevented when cells were pre-treated with NAC before treatment with MOE or Etoposide for 72 h (Fig. 4c). These results indicated that MOE induced apoptosis in A375 cells via decreased ΔΨm.
The loss of mitochondrial transmembrane potential (ΔΨm) in MOE treated A375 cells. Cells were treated with different concentrations of MOE for 72 h, stained with DiOC6, and then ΔΨm was analyzed using flow cytometry (a). ΔΨm was measured after the cells were incubated with NAC for 1 h, followed by exposing to 100 µg/mL MOE for 72 h (b, c). 0.1% DMSO and Etoposide were used as a negative control and a positive control, respectively. Data were expressed as mean ± SD of three experiments. *p < 0.05 and **p < 0.01 compared to control. #p < 0.05 compared to MOE or Etoposide-treated cells
Effects of MOE on A375 cell death in the presence of a pan-Caspase inhibitor
To clarify the role of the Caspases in MOE induced cell death, a Caspase inhibitor assay was performed. Cells were pre-treated with pan-Caspase inhibitor (Z-VAD-FMK) before being exposed to MOE. The effect of Z-VAD-FMK was detected at the cell viability, the rate of apoptosis and Caspase activation. The experimental results showed that Z-VAD-FMK successfully inhibited activation of Caspase-3 and Caspase-9 in response to MOE (p < 0.05, Fig. 5a). The cell viability of the controls was similar in the presence or absence of Z-VAD-FMK, indicating that the inhibitor was not toxic to the cells. In the Z-VAD-FMK-treated samples, MOE significantly reduced the cell viability compared to control. There was a statistically significant difference in the viability of the cells treated with MOE in the presence or absence of Z-VAD-FMK (p < 0.05, Fig. 5b). This likely indicates that MOE is able to induce cell death in the case of Caspase inhibition. Furthermore, FACS analysis also indicated that pre-treatment with Z-VAD-FMK resulted in considerable rescue of A375 cells from MOE-induced apoptosis. The apoptotic rates slightly decreased when cells were treated with MOE in the presence of Z-VAD-FMK compared to those treated with MOE alone (p < 0.05, Fig. 5c). These observations suggest that MOE also induce apoptosis following Caspase-independent pathway.
MOE induced-cell death in the presence of a pan-Caspase inhibitor in A375 cells. Cells were incubated with or without Z-VAD-FMK, following by exposure to various concentrations of MOE for 72 h. Z-VAD-FMK prevented Caspase activation in response to MOE. The expression levels of Caspase-3 and Caspase-9 were examined by luminescent assay (a). Z-VAD-FMK did not prevent the reduction of cell viability in response to MOE. The viability of cells was detected using WST-1 assay (b). Z-VAD-FMK partially suppressed apoptosis caused by MOE. The rates of apoptosis were determined using Annexin V-FITC/PI double staining and FACS analysis (c). 0.1% DMSO was used as a negative control. Data were expressed as mean ± SD of three experiments. *p < 0.05 and **p < 0.01 compared to control. #p < 0.05 compared to MOE-treated cells
Effects of MOE on AIF translocation in A375 cells
To investigate Caspase-independent apoptosis, the translocation of AIF into the nucleus was analyzed. The result showed that the expression of AIF in the total cell lysates was significantly increased when compared to control (p < 0.05, Fig. 6a, b). In addition, nuclear AIF localization was also observed after MOE treatment (Fig. 6c). Taken together, these data revealed that MOE-induced apoptosis in A375 cells was mediated through both Caspase-dependent manner and Caspase-independent manner. The Caspase-dependent pathway is relating to Caspase-3/7 and Caspase-9 while Caspase-independent pathway is following upregulation and nuclear translocation of AIF.
The expression and nuclear translocation of AIF in MOE treated A375 cells. The expression of AIF was examined by Western blot (a). Densitometric analyses of AIF were made relative to β-actin which was used as a housekeeping gene control (b). The translocation of AIF from mitochondria to the nucleus in A375 cells was analyzed by immunofluorescence (c). Untreated cells were used as a negative control. Data were expressed as mean ± SD of three experiments. **p < 0.05 compared to control
Discussion
In this study, a crude aqueous extract of Moringa oleifera Lam. leaves inhibited the growth of human melanoma cells in a dose-dependent manner, but had little effect on human normal cells. The mechanism of cell death was involved in enhancing the ratio of Bax/Bcl-2 along with reducing mitochondrial membrane potential. The apoptotic pathway is considered dependent and independent with Caspases.
Some previous studies revealed the anti-proliferative activity of crude extract of Moringa oleifera Lam. leaves or fruits on B16F10 murine melanoma cells [16, 17] and A2058 human melanoma cells [18]. However, the mechanisms of action have not been clarified yet. In our study, the inhibitory activity of MOE on A375 cells and A2058 cells is dose-dependent (Fig. 1a, b). MOE inhibited the growth of A375 cells and A2058 cells with IC50 of 87.28 ± 2.75 μg/mL and 108.47 ± 3.12 μg/mL, respectively. According to the data of American National Cancer Institute (NCI) and the study of Geran et al. [19], our MOE showed moderate anti-melanoma activity. The killing activity was supposed to be relating to the morphological changes of cells including cytoplasmic shrinkage and vacuolization after treatment with MOE (data not shown). These results were also confirmed by clonogenic assays (Fig. 1e, f). Among two cell lines, A375 cells were more sensitive to the extract than A2058 cells, thus, this cell line was selected for further experiments. In contrast, there was no apparent cell death observed in case of fibroblasts at equivalent doses, suggesting that cancerous cells are more susceptible to MOE (Fig. 1c, d). Similar result of aqueous extract of Moringa oleifera leaves which induced cytotoxicity toward HepG2 cells with minimal toxicity to normal HEK293 cells and peripheral blood mononuclear cells (PBMCs) was reported [14]. In another study, crude ethanol extract of Moringa oleifera leaves specifically reduced the growth of A2058 human melanoma cells, while there was no effect on HaCaT human keratinocyte cells [18]. There is no critical explanation for the difference between two cell types in response to the extract, presumably due to the more sensitive of cancer cells to the harm. Additionally, these effects are supposed to be caused by some biocompounds in the extracts which selectively target cancer cells with little effect on normal cells. Particularly, d-allose, a rare monosaccharide found in Moringa oleifera leaves, induces the expression of thioredoxin interacting protein (TXNIP), which functions as tumor suppressor and metastasis suppressor. d-allose inhibits the growth of cancer cells at G1 phase without exerting appreciable effects on normal cells [20]. In addition, benzylthiocyanate and niazimicin are bioactive compounds found in Moringa oleifera leaves, exhibited anti-cancer activity [21]. Benzylthiocyanate inhibits growth, promotes apoptosis and prevents metastasis of cancer cells via up-regulating the expression of apoptosis-related proteins, such as Caspase-3, Caspase-8 and PARP-1 and down-regulating the expression of metastasis-related proteins, such as survivin, MMP-2, MMP-9 and CXCR4 [22]. Niazimicin interacts with glycosyltransferase which was highly expressed in cancer cells. The inhibition of glycosyltransferase activity results in suppression of cell growth [23]. Compared to normal cells, cancer cells grow rapidly and migrate from place to place. Therefore, anti-cancer agents are required to target their mode of action. Overall, these data suggest that MOE possessed an anti-proliferative activity against melanoma.
Apoptosis is a process of cell killing that helps to balance the proliferation of cells in the body. Therefore, apoptosis induction to kill cells is considered as a valuable strategy to control cancer cells. Apoptosis is generally characterized by typical structural changes, including cell shrinkage, DNA fragmentation, chromatin condensation, externalization of PS and activation of apoptosis signals [24, 25]. Our study showed that MOE induced apoptosis in A375 cells determined by chromatin condensation and the PS externalization, main features of apoptotic cell death (Fig. 2). The apoptosis was initiated by activation of Caspase 9, Caspase 3/7, cleavage of PARP and the translocation of AIF into the nucleus (Figs. 3, 6). Studies demonstrated that the crude extract of Moringa oleifera. leaves contained bioactive compounds which are majorly natural phenolic compounds, including flavonoids and phenolic acids [26,27,28]. The potency of Moringa oleifera leaves to inhibit cell growth and induce apoptosis in cancer cells mainly depends on these compounds. The study of Sreelatha et al. [29] showed that aqueous extract of Moringa oleifera leaves containing flavonoids, such as quercetin and kaempferol triggered apoptosis in KB human tumor cells by increasing ROS levels in the cell, fragmenting cellular DNA and condensing chromatin. Another study has demonstrated fractions derived from methanol extract of Moringa oleifera leaves had stronger anti-proliferative activity on HCT116 human colorectal carcinoma cells by presenting chromatin condensation and apoptosis bodies when compared to kaempferol, a flavonoid found in Moringa oleifera leaves [30]. Similarly, astragalin and isoquercetin were identified as the main cytotoxic components in Moringa oleifera leaves against HCT116 cells through decreasing ERK1/2 phosphorylation [31].
The intrinsic apoptosis pathway caused by UV radiation, stress or chemotherapeutics is mediated through permeabilization of mitochondria membrane, leading to the release of Cytochrome c or small molecules into the cytosol, activating subsequent Caspases and resulting in cell death [25, 32]. The intrinsic apoptotic pathway is mainly triggered by Bcl-2 family members, including both anti-apoptotic proteins (Bcl-2, Bcl-xL, Mcl-1) and pro-apoptotic proteins (Bax, Bak, t-Bid) [33, 34]. Among them, Bcl-2 and Bax are two typical members of Bcl-2 family which involve in the regulation of cellular apoptosis. The change of pro-apoptotic/anti-apoptotic proteins stimulates the release of Cytochrome c and other molecules from mitochondria to the cytosol, triggering the activation of Caspase cascade and leading to cell death [33]. In previous studies, the ratio of Bax/Bcl-2 enables to use as an indicator for apoptosis initiation [35,36,37,38]. Our study indicated that MOE induced mitochondria-mediated apoptosis on A375 cells via upregulating the expression of Bax while suppressing the expression of Bcl-2 simultaneously, leading to increase Bax/Bcl-2 ratio (Fig. 3). The study of Mansour et al. [38] also demonstrated that Moringa oleifera leaf extract induced apoptosis by upregulation of Bax while downregulation of Bcl-2 in HepG2 cells. The changes in the Bax/Bcl-2 ratio could lead to a reduction of mitochondrial outer membrane permeabilization (MOMP), which is an important event in apoptosis. ΔΨm, an indicator for disruption of mitochondrial integrity, is a hallmark of intrinsic apoptosis. Irreversible mitochondrial depolarization decreased ΔΨm, leading to the release of small molecules into the cytosol [39]. Our experiment showed that MOE significantly decreased ΔΨm in A375 cells, leading to MOMP. This result was partially inhibited when cells had been pre-treated with antioxidant (NAC) (Fig. 4). The downstream of MOMP involved in both Caspase-dependent and Caspase-independent pathways. The Caspase-dependent pathway involves in initiator Caspase such as Caspase 9 which cleaves and activates executioner Caspase such as Caspase-3/7, catalyzing the cleavage of many key cellular proteins such as PARP, an apoptosis-specific marker, leading to cell death [34]. In the present study, MOE enabled to increase the cleavage of PARP and expression of Caspase-3, Caspase-9 (Fig. 3).
Most anti-cancer compounds induce cell death following Caspase-dependent apoptosis. However, evidence showed that some compounds induce both Caspase-dependent and Caspase-independent pathways. To reveal the apoptosis pathway of MOE, the irreversible pan-Caspase inhibitor Z-VAD-FMK was used. A375 cells were pre-treated with Z-VAD-FMK before exposure to MOE. Figure 5 demonstrated that treatment with Z-VAD-FMK not only successfully inhibited the activation of Caspase but also partially suppressed apoptosis compared to controls (Fig. 5). The result showed that MOE might cause apoptosis to cancer cells via Caspase-independent pathway after mitochondrial damage. Similarly, previous studies have reported that Z-VAD-FMK prevented partially apoptosis induction in Centipedegrass leaf extract-treated SK-MEL-5 human melanoma cells [40], Ginkgo biloba extract-treated Mel-AT human melanoma cells [41] and Houttuynia cordata Thunb leaf extract-treated A375 cells [42]. The Caspase-independent pathway is related to the release of AIF or Endonuclease G (EndoG) from the inter and inner membrane of mitochondria. The translocation of AIF into the nucleus leads to the condensation of chromatin and fragmentation of DNA [25, 32]. Our results revealed that MOE induced the upregulation and translocation of AIF into the nucleus (Fig. 6). Similar AIF-mediated Caspase-independent pathways have been reported in response to Ginkgo biloba extract in Mel-RM cells and Mel-AT cells [41], Sutherlandia frutescens extract in A375 cells [43] and Quisqualis indica extract in B16F10 cells [44]. Consequently, MOE caused cell death in A375 cells via both Caspase-dependent and Caspase-independent apoptosis pathways. This result is corroborated by the previous finding that Moringa oleifera aqueous leaf extract triggered apoptosis in A549 cells through affecting mitochondrial viability, activating Caspase and increasing the expression of apoptotic inducible factors including Cytochrome c, Smac/DIABLO and AIF [13].
Conclusions
Our study showed that MOE from the leaves of Moringa oleifera Lam. inhibited cell proliferation and induced apoptosis in A375 human melanoma cells. The apoptosis was triggered by increasing in Bax/Bcl-2 ratio, leading to the loss of mitochondrial membrane integrity. The mechanism of cell death is involved in Caspase-dependent pathway via activating Caspase 3/7, Caspase 9, PARP cleavage and Caspase-independent pathway via AIF translocation. These results prompt us to use Moringa oleifera leaves as alternative therapeutics for skin cancer treatment. However, further studies are required to provide the information about the bioactive ingredients as well as establish a comprehensive screening for evaluating their efficacy while comparing to the original extract using in vitro and in vivo models.
Abbreviations
- AIF:
-
Apoptosis induced factor
- BSA:
-
Bovine serum albumin
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DMSO:
-
Dimethyl sulfoxide
- EDTA:
-
Ethylenediaminetetraacetic acid
- EtBr:
-
Ethidium bromide
- FACS:
-
Fluorescence-activated cell sorting
- FITC:
-
Fluorescein isothiocyanate
- HRP:
-
Horseradish peroxidase
- MOE:
-
Moringa oleifera Lam. extraction
- MPT:
-
Mitochondrial permeability transition
- MOMP:
-
Mitochondrial outer membrane permeabilization
- mRNA:
-
Messenger RNA
- NAC:
-
N-acetylcysteine
- PAGE:
-
Polyacrylamide gel electrophoresis
- PI:
-
Propidium iodide
- PS:
-
Phosphatidylserine
- PVDF:
-
Polyvinylidene fluoride
- qRT-PCR:
-
Real-time quantitative reverse transcription PCR
- SDS:
-
Sodium dodecyl sulfate
- RLU:
-
Relative light unit
- Z-VAD-FMK:
-
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone
References
Singh S, Zafar A, Khan S, Naseem I (2017) Towards therapeutic advances in melanoma management: an overview. Life Sci 1(174):50–58
Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, Valladares M, González-Fernández A (2011) Assessment of the evolution of cancer treatment therapies. Cancers 3(3):3279–3330
Batus M, Waheed S, Ruby C, Petersen L, Bines SD, Kaufman HL (2013) Optimal management of metastatic melanoma: current strategies and future directions. Am J Clin Dermatol 14(3):179–194
Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, Vachon CM, Schild SE, McWilliams RR, Hand JL et al (2007) Melanoma melanoma in the 21st century, part 2: staging, prognosis, and treatment. Mayo Clin Proc 82(4):490–513
Vickers A (2002) Botanical medicines for the treament of cancer: rationale, overview of current data, and methodological considerations for phase I and II trials. Cancer Invest 20(7–8):1069–1079
Yildirım I, Kutlu T (2015) Anticancer agents: saponin and tannin. Int J Biol Chem 9:332–340
Zhou X, Seto SW, Chang D, Kiat H, Razmovski-Naumovski V, Chan K, Bensoussan A (2016) Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front Pharmacol 7:201
Anwar F, Latif S, Ashraf M, Gilani AH (2007) Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res 21:17–25
Vergara-Jimenez M, Almatrafi MM, Fernandez ML (2017) Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 6(4):E91
Berkovich L, Earon G, Ron I, Rimmon A, Vexler A, Lev-Ari S (2013) Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med 13:212
Jung IL, Lee JH, Kang SC (2015) A potential oral anticancer drug candidate, Moringa oleifera leaf extract, induces the apoptosis of human hepatocellular carcinoma cells. Oncol Lett 10(3):1597–1604
Tiloke C, Phulukdaree A, Chuturgoon AA (2013) The antiproliferative effect of Moringa oleifera crude aqueous leaf extract on cancerous human alveolar epithelial cells. BMC Complement Altern Med 13:226
Madi N, Dany M, Abdoun S, Usta J (2016) Moringa oleifera’s nutritious aqueous leaf extract has anticancerous effects by compromising mitochondrial viability in an ROS-dependent manner. J Am Coll Nutr 35(7):604–613
Tiloke C, Phulukdaree A, Gengan RM, Chuturgoon AA (2019) Moringa oleifera aqueous leaf extract induces cell-cycle arrest and apoptosis in human liver hepatocellular carcinoma cells. Nutr Cancer 4:1–10
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Purwal L, Pathak AK, Jain UK (2010) In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma. Pharmacologyonline 1:655–665
Gismondi A, Canuti L, Impei S, Di Marco G, Kenzo M, Colizzi V, Canini A (2013) Antioxidant extracts of African medicinal plants induce cell cycle arrest and differentiation in B16F10 melanoma cells. Int J Oncol 43(3):956–964
Guon TE, Chung HS (2017) Moringa oleifera fruit induce apoptosis via reactive oxygen species-dependent activation of mitogen-activated protein kinases in human melanoma A2058 cells. Oncol Lett 14(2):1703–1710
Geran RI, Greenberg NH, Macdonald MM, Shumacher AM, Abbott BJ (1972) Protocols for screening chemical agents and natural products against animal tumors and other biological systems (3rd edition). Cancer Chemother Rep 3:1–91
Al-Asmari AK, Albalawi SM, Athar MT, Khan AQ, Al-Shahrani H, Islam M (2015) Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS ONE 10(8):e0135814
Tiloke C, Anand K, Gengan RM, Chuturgoon AA (2018) Moringa oleifera and their phytonanoparticles: potential antiproliferative agents against cancer. Biomed Pharmacother 108:457–466
Zhu M, Li W, Dong X, Chen Y, Lu Y, Lin B, Guo J, Li M (2017) Benzyl-isothiocyanate induces apoptosis and inhibits migration and invasion of hepatocellular carcinoma cells in vitro. J Cancer 8(2):240–248
Pangastuti A, Amin IF, Amin AZ, Amin M (2016) Natural bioactive compound from Moringa oleifera against cancer based on in silico screening. J Teknol 78:315–318
Johnson VL, Ko SC, Holmstrom TH, Eriksson JE, Chow SC (2000) Effector caspases are dispensable for the early nuclear morphological changes during chemical-induced apoptosis. J Cell Sci 113:2941–2953
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, Upadhyay G, Singh HB (2009) Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol 47(6):1109–1116
Oboh G, Ademiluyi AO, Ademosun AO, Olasehinde TA, Oyeleye SI, Boligon AA, Athayde ML (2015) Phenolic extract form Moringa oleifera leaves inhibits key enzymes linked to Erectile dysfunction and oxidative stress in Rat’s penile tissue. Biochem Res Int 2015:175950
Valdez-Solana MA, Mejía-García VY, Téllez-Valencia A, García-Arenas G, Salas-Pacheco J, Alba-Romero JJ (2015) Nutritional content and elemental and phytochemical analyses of Moringa oleifera grown in Mexico. J Chem 2015:9
Sreelatha S, Jeyachitra A, Padma P (2011) Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol 49(6):1270–1275
Tragulpakseerojn J, Yuki R, Honda T, Yamaguchi N (2014) Apoptotic activities of the extract from Moringa oleifera leaves on human HCT116 colon cancer cells. Fundam Toxicol Sci 1(4):143–149
Tragulpakseerojn J, Yamaguchi N, Pamonsinlapatham P, Wetwitayaklung P, Yoneyama T, Ishikawa N, Ishibashi M, Apirakaramwong A (2017) Anti-proliferative effect of Moringa oleifera Lam (Moringaceae) leaf extract on human colon cancer HCT116 cell line. Trop J Pharm Res 16(2):371–378
Lorenzo HK, Susin SA (2004) Mitochondrial effectors in caspase-independent cell death. FEBS Lett 557(1–3):14–20
Tsujimoto Y (1998) Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 3(11):697–707
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441–446
Zhang Y, Xu X, He P (2011) Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol Med Rep 4(1):25–29
Aliparasti MR, Alipour MR, Almasi S, Feizi H (2015) Ghrelin administration increases the Bax/Bcl-2 gene expression ratio in the heart of chronic hypoxic rats. Adv Pharm Bull 5(2):195–199
Al-Qathama A, Gibbons S, Prieto JM (2017) Differential modulation of Bax/Bcl-2 ratio and onset of caspase-3/7 activation induced by derivatives of Justicidin B in human melanoma cells A375. Oncotarget 8(56):95999–96012
Mansour M, Mohamed MF, Elhalwagi A, El-Itriby HA, Shawki HH, Abdelhamid IA (2019) Moringa peregri na leaves extracts induce apoptosis and cell cycle arrest of hepatocellular carcinoma. Biomed Res Int 2019:2698570
Ly JD, Grubb DR, Lawen A (2003) The mitochondrial membrane potential in apoptosis; an update. Apoptosis 8(2):115–128
Badaboina S, Bai HW, Park CH, Jang DM, Choi BY, Chung BY (2013) Molecular mechanism of apoptosis induction in skin cancer cells by the centipedegrass extract. BMC Complement Altern Med 13:350
Wang Y, Lv J, Cheng Y, Du J, Chen D, Li C, Zhang J (2015) Apoptosis induced by Ginkgo biloba (EGb761) in melanoma cells is Mcl-1-dependent. PLoS ONE 10(4):e0124812
Yanarojana M, Nararatwanchai T, Thairat S, Tancharoen S (2017) Antiproliferative activity and induction of apoptosis in human melanoma cells by Houttuynia cordata Thunb extract. Anticancer Res 37(12):6619–6628
van der Walt NB, Zakeri Z, Cronjé MJ (2016) The Induction of Apoptosis in A375 Malignant Melanoma Cells by Sutherlandia frutescens. Evid Based Complement Altern Med 2016:4921067
Mukhopadhyay R, Kazi J, Debnath MC (2018) Synthesis and characterization of copper nanoparticles stabilized with Quisqualis indica extract: evaluation of its cytotoxicity and apoptosis in B16F10 melanoma cells. Biomed Pharmacother 97:1373–1385
Acknowledgements
This research is funded by Graduate University of Science and Technology, Vietnam Academy of Science and Technology, Ha Noi, Vietnam under Grant number: GUST.STS.ĐT2018-SH01.
Author information
Authors and Affiliations
Contributions
BHD, CCD, NQCH, TPTN, TLL carried out the experiments. BHD and CCD designed, coordinated the study and wrote manuscript. CCD and NSH supervised the studies and revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Do, B.H., Nguyen, T.P.T., Ho, N.Q.C. et al. Mitochondria-mediated Caspase-dependent and Caspase-independent apoptosis induced by aqueous extract from Moringa oleifera leaves in human melanoma cells. Mol Biol Rep 47, 3675–3689 (2020). https://doi.org/10.1007/s11033-020-05462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05462-y