Abstract
Owing to the role of fractalkine in regulating cellular apoptosis/proliferation, we investigated fractalkine effects on apoptosis/proliferation signaling of granulosa cells in polycystic ovarian syndrome (PCOS) patients through in vitro and in vivo experiments. In vivo, granulosa cells were collected from 40 women undergoing oocyte retrieval (20 controls and 20 PCOS). The expression levels of fractalkine, BAX, Bcl2, Bcl2-XL, Bad, and TNF-α were assessed using RT-PCR. In vitro, we determined the effect of different doses of fractalkine on the expression of the above mentioned genes in GCs of both groups. We found that the expression levels of fractalkine and Bcl-2 were significantly lower in the GCs of PCOS patients compared to the control group (p < 0.05). In contrast, the expression levels of TNF-α and BAX were higher in the patient's group than in the control group. The results suggested that expression levels of fractalkine were negatively and positively correlated with the number of oocytes and fertilized oocytes respectively. Moreover, fractalkine could dose-dependently increase fractalkine and decrease BAD, BAX, Bcl-xl, and TNF-α expressions in the control GCs. In contrast, GCs collected from PCOS patients revealed an increase in expression of BAD, BAX, and Bcl-xl following fractalkine treatment. Our findings indicated that insufficient expression of fractalkine in PCOS patients is related with elevated apoptotic and inflammatory markers and reduced anti-apoptotic genes in the GCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most prevailing endocrine disorders with an incidence of 4% to 21% in women at reproductive age [1]. It is one of the main causes of infertility in women and accounts for almost 75% of anovulatory subfertilities [2]. It has been revealed that both inherited and environmental factors are involved in PCOS [3, 4]. Despite the numerous studies, the exact underlying cellular/molecular mechanisms of PCOS have not yet been understood.
Abnormal folliculogenesis is considered as one of the common features of PCOS which is associated with the failure in the maturation of antral follicles and extensive follicular atresia [5, 6]. It has been documented that an imbalance in the apoptosis of granulosa cells (GCs) may contribute to the abnormal folliculogenesis, as the oocyte maturation depends entirely on the surrounding GCs [6] [7]. In this regard, a high rate of apoptosis in the GCs of patients with PCOS has been previously reported [8].
It has been shown that the equilibrium between pro- and anti-apoptotic factors in GCs is crucial in the fate of a follicle [9]. Fractalkine (CX3CL1) is a chemokine that is involved in cell proliferation/apoptosis via modulating the expression of apoptotic genes and up-regulation of anti-apoptotic Bcl family proteins [10, 11]. Studies have shown that the levels of fractalkine grow during the preovulatory period and its expression is higher in luteinizing GCs than in the GCs of the follicular phase [12, 13]. Fractalkine plays a paracrine/autocrine role in preovulatory follicles; it has been demonstrated that in an in vitro condition, this cytokine could reverse the down-regulation of progesterone expression in GCs of PCOS patients [13, 14].
Owing to the pivotal role of fractalkine in regulating cellular apoptosis/proliferation signaling and also exist of an imbalance in apoptosis of GCs from PCOS, we investigated fractalkine effects on apoptosis/proliferation signaling of GCs in these patients through in vitro and in vivo experiments. In addition, as the GCs can affect the quality of oocytes and consequently ART outcomes, we evaluated the association between ART outcomes and fractalkine in GCs.
Materials and methods
Study population
We used PASS 11 software for estimation of sample size using 0.05 and 0.8 as alpha and beta values, respectively, as well as, mean expression levels of fractalkine in PCOS (0.642 ± 0.070) and control groups (1.610 ± 0.551) which were previously reported by Shuo, et al. [14]. The sample size was obtained five for each group; however, in order to increase the reliability of data and avoiding any bias, we considered 20 individuals for each group. So, twenty control women and 20 patients with PCOS were recruited in this study from who referred to the reproductive Center of Al-Zahra Hospital and Milad Fertility Clinic of Tabriz for intracytoplasmic sperm injection-embryo transfer (ICSI-ET) treatment. The control group aged 30.17 ± 4.92 years and had a regular menstrual cycle. These women selected from couples who had only male factor infertility. The PCOS patients had a mean age of 29.59 ± 5.81 years and were recruited if they had at least two of the Rotterdam-PCOS criteria (oligo/amenorrhea, clinical or biochemical hyperandrogenism, and polycystic ovaries on ultrasonography). We excluded women with endometriosis, immune system and inflammatory diseases, endocrine disorders, and history of smoking. All the procedures were conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Tabriz University of Medical Sciences (Approval cod: IR.TBZMED.REC.1396.181). Signed informed consent was also obtained from all participants.
ICSI-ET cycle
All the participants were on their first ART cycle and underwent ovarian stimulation protocol; Based on the age and ovarian reserve of the patients, recombinant follicle-stimulating hormone (150–225 IU rFSH, Gonal-F; Merk Serono) was injected on day 2 of the menstrual cycle. Then, the growth of the follicles was monitored and HMG was administered (Menopur, Ferring) based on the size of the follicles. GnRH antagonist (0.25 mg/day, Cetrotide, Merk Serono) was injected when the diameter of the follicles reached 12–13 mm. Later, when follicles with a diameter of 17–18 mm were observed, 10,000 IU hCG for the control group and 5,000 for the PCOS group (Pregnyl, Merk Serono) were administered. The ovum pick-up was done 34–36 h after the hCG injection. All patients underwent intracytoplasmic sperm injection (ICSI) and received 100 mg progesterone (Progestone Depot-S; Fuji Pharmaceutical) until the embryo transfer. The fertilization rate was calculated by dividing the number of fertilized oocytes by the number of mature oocytes. All patients received 2–3 morphologically good-quality embryos and the biochemical pregnancy was evaluated 14 days after the transfer. Clinical pregnancy was approved by observing the intrauterine gestational sac, where the number of sacs per number of transferred embryos was considered as the implantation rate.
Isolation of the granulosa cells
Collected Cumulus–Oocyte Complexes (COCs) were used for isolation of the GCs, according to our previously described method [15]. Briefly, the GCs around the oocyte were isolated using a syringe needle and transferred into ISM1 medium. The cells were washed three times through centrifugation (1200 rpm for 10 min) using sterile phosphate-buffered saline (PBS). The isolation purity was previously confirmed by our group using flow cytometry against GC specific marker and CD45 (a surface marker specific for leukocytes) [15, 16]. The results indicated 95–98% purity of GCs isolation. For the in vitro experiment, the isolated GCs were cultured as described in the following section, while for in vivo, the cells were used for gene expression analysis. A schematic graph describing the experiments from GC isolation to molecular analysis is shown in Fig. 1.
Schematic graph describing the experiments of the study. ICSI Intracytoplasmic sperm injection, GCs granulosa cells, PCOS polycystic ovary syndrome, BAX BCL2 Associated X, BAD BCL2-antagonist of cell death, Bcl-2 B-cell lymphoma 2, Bcl-xl B-cell lymphoma-extra large, TNF-α tumor necrosis factor-alpha
Culture of GCs for the in vitro experiment
The isolated GCs were washed (three times) with Dulbecco’s modified Eagle’s medium/Ham’s F12 (1:1,) supplemented with 0.1 mg/mL streptomycin, 250 ng/mL amphotericin, and 100 U/mL penicillin. Then the cells were incubated in the same media (supplemented with 10% fetal calf serum) for two days in 95% humidity and 5% CO2 at 37˚C. This primary culture was applied to minimize the effect of gonadotrophin on the GCs as previously suggested by Rice et al. [17]. The GCs of each group (PCOS/Control) were divided into 3 plates (estimated cell density of 2 × 105 cells/mL); The cells in plate A were incubated for 48 h with DMEM F12 (Thermo Fisher Scientific, Waltham, MA), 10% FBS (Sigma-Aldrich, USA) and without fractalkine. The culture condition was similar in plates B and C; however, 100 and 150 ng/mL fractalkine was added in plate B and C, respectively. Each culture was independently conducted three times. After a 48 h incubation, the GCs were used for further analyses.
Gene expression analysis
The Trizol (Sigma-Aldrich, USA) reagent was applied for total RNA extraction using the manufacturer’s protocol. After the evaluation of RNA integrity (running on 2% agarose electrophoresis) and purity (260/280 nm absorbance ~ 2.0), the complementary DNA (cDNA) was synthesized by PrimeScript RT reagent Kit (Takara, JAPAN). The gene expression of Fractalkine, BAX, Bcl2, Bcl2-XL, Bad, and TNF-α was assayed using SYBR Green. In this regard, 7 μL SYBR Green PCR Master Mix (Takara, JAPAN), 0.7 μL of forward and reverse primers, and 0.7 μL cDNA were added into a sterile micro-tube and the solution volume was brought to 14 μL using DEPC Treated Water. The sequences of primers are summarized in Table 1. The following real time-PCR program was used for gene expression analysis: 5 min at 95 °C as an activation step, 40 cycles including 5 s denaturation at 95 °C, 30 s annealing at optimum temperature for each primer (listed in Table 1), and extension at 72 °C for 20 s. All the reactions were done in a Mic Real-Time PCR (BioMolecular Systems, Australia) detection system. Finally, the gene expression was normalized by a housekeeping gene (Glyceraldehyde 3-phosphate dehydrogenase) and the relative gene expression was obtained using the 2−∆∆Ct method. All reactions were performed in triplicate.
DAPI and TUNEL staining
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was used to evaluate apoptosis status of GCs isolated from PCOS and control individuals and treated with fractalkine. For this purpose, the cells obtained from PCOS and control individuals were evaluated after 48 h culturing with and without (control) fractalkine at a dose of 150 ng/ml. We chose the highest dose of fractalkine as our gene expression analysis showed this dose could better affect apoptosis signaling. After removing the medium, the cells were washed and fixed with 4% paraformaldehyde. To perform TUNEL assay, ApopTag peroxidase in situ apoptosis detection kit (Oncor, Gaithersburg, MD, USA) was used according to the manufacturer’s protocol. Then the cells were counterstained with 2 μg/ml of DAPI (4′,6-diamidino-2-phenylindole, Sigma-Aldrich, USA) and incubated in the dark for 5 min at room temperature. Finally, the cells were evaluated under a fluorescent microscope (Olympus system, Japan). This experiment was performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS PC Statistics (version 23; SPSS Inc., Chicago, IL, USA). After confirming the normal distribution of the data by Kolmogorov–Smirnov test, we compared the data of the groups using t-test and one-way analysis of variance (ANOVA) followed by Tukey's test. The correlation between study variables was evaluated by the Pearson correlation test. Data have been presented as mean ± standard deviation (SD) and p-value < 0.05 was considered to be statistically significant.
Results
In vivo experiment
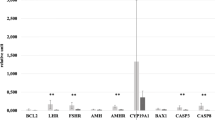
The characteristics of the study population are presented in Table 2. The data revealed significantly higher BMI in the patient group in comparison with the control group (p = 0.004). As shown in Fig. 2, we found that expression levels of fractalkine and Bcl-2 were significantly lower in the GCs of PCOS patients compared to the control group (p < 0.05). In contrast, the expression levels of TNF-α and BAX were higher in the patients than the control group (Fig. 2, p < 0.05). Correlation analysis between the expressions of genes revealed the existence of a positive correlation between expressions of BAD and BAX in all studied populations (rs = 0.429 and p = 0.020, Table 3). Further, there were positive and negative correlations between the expression of fractalkine with Bcl-xl and BAD in the PCOS group, respectively (rs = 0.653 and rs = -0.576, respectively).
Expression levels of the genes in granulosa cells (GCs) of polycystic ovary syndrome (PCOS) and control groups. BAX BCL2 associated X; BAD BCL2-antagonist of cell death, Bcl-2 B-cell lymphoma 2, Bcl-xl B-cell lymphoma-extra large, TNF-α tumor necrosis factor-alpha. *Represents a significant difference between the groups (p < 0.05)
Data about correlations between ART outcomes and expression of the genes are reported in Table 4. We found that expression levels of fractalkine were negatively and positively correlated with the number of oocytes and the number of fertilized oocytes, respectively. In the PCOS group, fractalkine had also a positive correlation with the fertilization rate. We also observed that TNF-α expression had a negative correlation with the fertilization rate in all populations and the control group. Moreover, Bcl-2 expression levels were significantly associated with the number of oocytes and the number of fertilized oocytes (rs = − 0.415 and rs = 0.412, respectively).
In vitro experiment
We evaluated the effect of fractalkine (at doses of 0, 100 and 150 ng/mL) on the expression of the genes in GCs collected from healthy and PCOS individuals and the results are presented in Fig. 3. We found that fractalkine could dose-dependently enhance the gene expression of fractalkine in the control GCs. Furthermore, in these cells, fractalkine at the doses of 100 and 150 ng/mL significantly decreased expression of BAD, BAX, Bcl-xl, and TNF-α genes. In contrast, GCs collected from PCOS patients revealed an increase in the expression of BAD, BAX, and Bcl-xl following fractalkine treatment at doses of 100 and 150 ng/mL (p < 0.05). We also found that the treatment of these GCs with 100 ng/mL fractalkine significantly decreased the expression of TNF-α (p < 0.05).
Effect of different doses of fractalkine (FKN) on the expression of the genes in GCs collected from healthy and polycystic ovary syndrome (PCOS) individuals. FNK fractalkine, BAX BCL2 Associated X, BAD BCL2-antagonist of cell death, Bcl-2 B-cell lymphoma 2, Bcl-xl B-cell lymphoma-extra large, TNF-α tumor necrosis factor-alpha. Significant difference in compared with aNormal, bNormal + 100 ng/ml FKN, cNormal + 150 ng/ml FKN, dPCOS, ePCOS + 100 ng/ml FKN. prime symbol (′) shows a significant difference at p < 0.001
Apoptosis assay showed that there was a high rate of apoptosis among GCs from PCOS patients compared to the cells obtained from healthy individuals after 48 h culture (Fig. 4). Moreover, culturing the GCs with fractalkine at a dose of 150 ng/mL increased and decreased apoptosis rate in the cells obtained from PCOS and healthy individuals, respectively.
Evaluating apoptosis rate of granulosa cells (GCs) after 48 h culturing with and without fractalkine (FKN) using TUNEL and DAPI staining. a GCs obtained from patients with polycystic ovarian syndrome (PCOS), b GCs obtained from PCOS patients at the presence of 150 ng/ml FKN, c GCs obtained from healthy women, d GCs obtained from healthy women at the presence of 150 ng/ml FKN
Discussion
In this study, we evaluated the effects of fractalkine on apoptosis/proliferation signaling of GCs in PCOS patients through in vitro and in vivo experiments. Since the PCOS patients received lower doses of hCG to avoid OHSS compared with the control group (5000 vs. 10,000 IU), the comparison of factors between the two groups could be affected by the hCG dose as a confounding factor. So, we conducted an in vitro study and found that different doses of hCG could not significantly affect the expression levels of the factors that were evaluated in the present study (data not shown).
Our results revealed that the expression levels of fractalkine in GCs of PCOS patients were significantly lower than those in the control group. In parallel with our findings, Huang et al. [14] reported lower levels of fractalkine in the follicular fluid and GCs of patients with PCOS compared to healthy women. However, there is not sufficient data to explain why GCs of PCOS patients had decreased expression levels of fractalkine. Nevertheless, it has been shown that the loss of fractalkine signaling could exacerbate obesity-induced adipose tissue inflammation and insulin resistance [18]. Further, Riopel et al. [19] reported the beneficial effects of chronic administration of fractalkine on glucose tolerance in mice. Since the insulin resistance in ovarian follicles and GCs is one of the main causes of PCOS [20, 21], it can be postulated that reduced expression of fractalkine in the GCs may contribute to the insulin resistance of these cells. Further studies are required to address the role of fractalkine in glucose tolerance of GCs and its association with the development of PCOS. It has been suggested that insulin-sensitizers, such as inositol isoforms, could be applied for PCOS treatment [22, 23]; So, future studies are required to investigate if the administration of insulin-sensitizers can improve PCOS complications partly through affecting fractalkine. Another role of fractalkine in PCOS can be related to its ability in the stimulation of progesterone production by GCs. In this regard, it has been reported that decreased levels of fractalkine could cause a dramatic reduction in progesterone secretion of GCs [14] and thereby paving the way for the development of PCOS. Furthermore, the role of fractalkine in anti-apoptosis signaling [10, 24,25,26] can be a mechanism by which fractalkine can be involved in PCOS pathogenesis (this mechanism has been further discussed in the present study).
Given the above-mentioned role of fractalkine in the GCs and follicles function, this factor can affect ovarian functions and ART outcomes. In this respect, we found that expression levels of fractalkine were positively correlated with the fertilization rate. To the best of our knowledge, there is no study regarding the association between fractalkine in ovaries and follicular fluid with ART outcomes. So, further studies are required to confirm our findings.
Our results revealed that the expression levels of pro-apoptotic (BAX) and anti-apoptotic (Bcl-2) genes were respectively higher and lower in GCs of PCOS patients in comparison with the control group, indicating a high activity of apoptosis signaling in the GCs of patients with PCOS. In supporting our results, a disruption in the apoptosis/anti-apoptosis balance of the GCs from PCOS patients has been reported [27,28,29,30,31]. Abnormal apoptosis/anti-apoptosis balance in GCs can affect the development of follicles and their atresia [32] and consequently cause follicular maturation failure, anovulation, and PCOS [8]. In this regard, imbalanced apoptosis/anti-apoptosis signaling has been considered as the major pathogenesis of PCOS [33]. Accordingly, we found positive and negative associations between anti-apoptotic (Bcl-2) and apoptotic (BAD) expression with the number of fertilized oocytes, respectively. Interestingly, fractalkine acts as a cell survival factor and has an anti-apoptotic effect on different cells [10, 25, 34]. It has been reported that fractalkine reduced the expression of apoptotic genes and upregulated anti-apoptotic proteins [11, 35]. We also found positive and negative correlations between the expressions of fractalkine with Bcl-xl and BAD in the GCs. So, it can be concluded that the high expression of apoptotic and low expression of anti-apoptotic genes in the GCs of PCOS patients could be due to diminished levels of fractalkine.
To confirm the role of fractalkine in the maintenance of apoptosis/anti-apoptosis balance in the GCs, we conducted an in vitro study. We found that fractalkine at the doses of 100 and 150 ng/mL could reduce the expression of BAD and BAX genes in the GCs of healthy women. Furthermore, fractalkine at a dose of 150 ng/mL could reduce apoptosis rate of the GCs. It has been approved that anti-apoptotic and proliferative effects of fractalkine are mediated by PI3K–Akt and MAPK/ERK signaling pathways [10, 11, 34]. In respect to the anti-apoptotic role of fractalkine, Akt activation causes phosphorylation of BAD and G3K3, and consequently apoptosis is inhibited [11, 34]. Furthermore, it has been shown that fractalkine contributed to pancreatic cancer cell survival by stimulating BCL‐2 and BCL‐xl expressions through Akt/NF‐κB/p65 signaling [25]. So, the results of the current study regarding stimulatory and inhibitory effects of fractalkine on the expression of respectively anti-apoptotic and apoptotic factors in normal GCs seem logical. Since apoptosis also occurs during the normal physiological condition and its dysregulation can be seen in different gynecological disorders such as endometriosis [36, 37], further studies can investigate the role of fractalkine in orchestrating apoptosis/proliferation signaling in these disorders.
Interestingly, the GCs from PCOS patients showed a different response to the fractalkine treatment, as in these cells fractalkine dose-dependently enhanced the expression of BAD, BAX, and Bcl-xl. Beside, fractalkine induce apoptosis in GCs from PCOS patients. Such a difference in the response to the fractalkine between GCs collected from PCOS patients and those from healthy women could be due to the impaired PI3K-AKT and MAPK/ERK signaling pathways in the ovary of PCOS women. Previous findings indicated an imbalance or deregulation in many of the PI3K-Akt intermediates in PCOS patients. For example, decreased levels of pGSK3α and pGSK3β (intermediated of PI3K-Akt signaling pathway) have been observed in patients with insulin resistance, which is one of the important issues of PCOS [38, 39]. Restuccia et al. [40] have also documented that eliminating Akt2 leads to the formation of ovarian cysts. Another study indicated a significant increase in p-Akt1 levels of GCs obtained from hyperandrogenic PCOS patients [39]. Given that fractalkine exerts its anti-apoptotic effect via mediators of PI3K-AKT and MAPK/ERK signaling pathways (e.g. Akt, GSK, and ERK) [11, 34] and these pathways are dysregulated in PCOS patients [39, 41, 42], our findings regarding the different response of GC from PCOS patients to fractalkine can be explained. Moreover, it has been demonstrated that when the anti-apoptotic signaling mediators are dephosphorylated, the signaling acts in favor of apoptosis [43, 44] as we observed in GCs of PCOS patients. Therefore, it can be postulated that PI3K-AKT signaling pathway may have a dual role and can mediate both follicular survival and apoptosis. However, in the PCOS patients, due to impaired signaling, the pathway induces expression of apoptotic factors rather than the anti-apoptotic factors. In confirming this hypothesis, we observed that the addition of fractalkine in culture media of GCs obtained from PCOS patients induced apoptosis as well as expression of apoptotic factors instead of anti-apoptotic genes. The underlying dual role of fractalkine and switching from anti-apoptotic into apoptotic function in GCs of PCOS patients should be investigated in future studies.
Our results indicated that TNF-α levels were elevated in GCs of women with PCOS in comparison with the control group. Previous studies have also revealed that women with PCOS have increased serum levels of TNF-α [45]. Elevated levels of TNF-α in the GCs of PCOS patients could be a result of excessive inflammation in these patients. Furthermore, we found a lower level of fractalkine in PCOS patients and previous studies have suggested that TNF-α is a positive regulator of fractalkine expression [46, 47]. So, it can be postulated that the elevation in TNF-α expression levels was a compensatory response to induce fractalkine expression and keep its concentration at physiological levels. On the other hand, fractalkine has an anti-inflammatory role [48,49,50] and can modulate TNF-α production through NF-κB [50,51,52]. Our in-vitro results were in parallel with previous reports regarding the negative effect of fractalkine on TNF-α production [21,22,23]. In this respect, we found that treatment of GCs with fractalkine could dose-dependently reduce the expression of TNF-α in GCs of both healthy and PCOS women. Moreover, we found that expression levels of TNF-α in the GCs were negatively associated with the fertilization rate of oocytes which is in good accordance with previous findings regarding the negative correlation between TNF-α levels in follicular or embryo culture fluids with fertilization rate [36, 37]. As the strength points of our study, it can be mentioned that this study for the first time reported the role of fractalkine in orchestrating apoptosis/proliferation signaling in GCs. Further, we evaluated fractalkine effects on apoptosis and anti-apoptosis factors both in vivo and in vitro. Also, we reported an association between fractalkine and ART outcomes. However, this study had a relatively small sample size and we did not evaluate the factors at the protein level. So, future studies are required to evaluate the factor at protein levels and use fractalkine signaling inhibitors to find the exact underlying mechanisms.
In conclusion, our findings suggested that insufficient expression of fractalkine in PCOS patients is related to elevated apoptotic and inflammatory markers (e.g. BAX and TNF-α) and reduced anti-apoptotic genes (e.g. Bcl-2) in the GCs and thus disturbed maturation of the oocyte. We also found that fractalkine in GCs was negatively and positively associated with the number of oocytes and the number of fertilized oocytes, respectively. Interestingly, our results showed that the effect of fractalkine on GCs collected from PCOS patients was totally different from what we found in healthy GCs.
References
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan R (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106(1):6–15
Diao F-Y, Xu M, Hu Y, Li J, Xu Z, Lin M, Wang L, Zhou Y, Zhou Z, Liu J (2004) The molecular characteristics of polycystic ovary syndrome (PCOS) ovary defined by human ovary cDNA microarray. J Mol Endocrinol 33(1):59–72
Rutkowska AZ, Diamanti-Kandarakis E (2016) Polycystic ovary syndrome and environmental toxins. Fertil Steril 106(4):948–958
Muralidhara KD, Adhikari PM, Muralidhara D (2015) A study on the pattern of genetic inheritance of polycystic ovarian syndrome. Br J Med Med Res 5(10):1230
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R (2011) Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 7(4):219
Asselin E, Xiao CW, Wang YF, Tsang BK (2000) Mammalian follicular development and atresia: role of apoptosis. Neurosignals 9(2):87–95
Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A (2008) Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab 93(3):881–887
Salehi E, Aflatoonian R, Moeini A, Yamini N, Asadi E, Khosravizadeh Z, Tarzjani MD, Abolhassani F (2017) Apoptotic biomarkers in cumulus cells in relation to embryo quality in polycystic ovary syndrome. Arch Gynecol Obstet 296(6):1219–1227
Tilly JL (2001) Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol 2(11):838
Boehme SA, Lio FM, Maciejewski-Lenoir D, Bacon KB, Conlon PJ (2000) The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. J Immunol 165(1):397–403
White GE, Tan TC, John AE, Whatling C, McPheat WL, Greaves DR (2009) Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovasc Res 85(4):825–835
Ben-Shlomo I, Rauch R, Avsian-Kretchmer O, Hsueh AJ (2007) Matching receptome genes with their ligands for surveying paracrine/autocrine signaling systems. Mol Endocrinol 21(8):2009–2014
Zhao P, De A, Hu Z, Li J, Mulders SM, Sollewijn Gelpke MD, Duan E-K, Hsueh AJ (2008) Gonadotropin stimulation of ovarian fractalkine expression and fractalkine augmentation of progesterone biosynthesis by luteinizing granulosa cells. Endocrinology 149(6):2782–2789
Huang S, Pang Y, Yan J, Lin S, Zhao Y, Lei L, Yan L, Li R, Ma C, Qiao J (2016) Fractalkine restores the decreased expression of StAR and progesterone in granulosa cells from patients with polycystic ovary syndrome. Sci Rep 6:26205
Yousefi S, Soleimanirad J, Hamdi K, Farzadi L, Ghasemzadeh A, Kazemi M, Mahdipour M, Rahbarghazi R, Nouri M (2018) Distinct effect of fetal bovine serum versus follicular fluid on multipotentiality of human granulosa cells in in vitro condition. Biologicals 52:44–48
Aghadavod E, Zarghami N, Farzadi L, Zare M, Barzegari A, Movassaghpour AA, Nouri M (2015) Isolation of granulosa cells from follicular fluid; applications in biomedical and molecular biology experiments. Adv Biomed Res 4:250
Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, Margara R, Hardy K, Franks S (2005) Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod 20(2):373–381
Nagashimada M, Ni Y, Ota T (2018) Loss of fractalkine-CX3CR1 signaling exacerbates obesity-induced adipose tissue inflammation and insulin resistance through M1 dominant shift in macrophages. Diabetes. https://doi.org/10.2337/db18-1989-P
Riopel M, Seo JB, Bandyopadhyay GK, Li P, Wollam J, Chung H, Jung S-R, Murphy A, Wilson M, de Jong R (2018) Chronic fractalkine administration improves glucose tolerance and pancreatic endocrine function. J Clin Invest. https://doi.org/10.1172/JCI94330
Zhu Q, Zuo R, He Y, Wang Y, Chen Z-J, Sun Y, Sun K (2016) Local regeneration of cortisol by 11β-HSD1 contributes to insulin resistance of the Granulosa cells in PCOS. J Clin Endocrinol Metab 101(5):2168–2177
Reyes-Muñoz E, Sathyapalan T, Rossetti P, Shah M, Long M, Buscema M, Valenti G, La Rosa VL, Cianci S, Vitale SG (2018) Polycystic ovary syndrome: implication for drug metabolism on assisted reproductive techniques—a literature review. Adv Ther 35(11):1805–1815
Laganà AS, Rossetti P, Sapia F, Chiofalo B, Buscema M, Valenti G, Rapisarda AMC, Vitale SG (2017) Evidence-based and patient-oriented inositol treatment in polycystic ovary syndrome: changing the perspective of the disease. Int J Endocrinol Metab 15(1):e43695
Laganà AS, Garzon S, Casarin J, Franchi M, Ghezzi F (2018) Inositol in polycystic ovary syndrome: restoring fertility through a pathophysiology-based approach. Trends Endocrinol Metab 29:768
Guo X, Pan Y, Xiao C, Wu Y, Cai D, Gu J (2012) Fractalkine stimulates cell growth and increases its expression via NF-κ B pathway in RA-FLS. Int J Rheum Dis 15(3):322–329
Wang H, Cai J, Du S, Guo Z, Xin B, Wang J, Wei W, Shen X (2017) Fractalkine/CX3CR1 induces apoptosis resistance and proliferation through the activation of the AKT/NF-κB cascade in pancreatic cancer cells. Cell Biochem Funct 35(6):315–326
Fogarty CE, Bergmann A (2015) The sound of silence: signaling by apoptotic cells. In: Current topics in developmental biology, vol 114. Elsevier, Amsterdam, pp 241–265
Sun C, Qiao J, Gao D (2006) The expression of apoptosis-related protein Bcl-2, Bax, P53 and PDCD5 in granulosa cells of the PCOS. Chin J Clin Obstetr Gynecol
Yeh J, Kim HH (1996) Polycystic ovary syndrome (PCOS): the possible roles of apoptosis in human granulosa cells. In: Polycystic ovary syndrome. Springer, New York pp. 51 70
Onalan G, Selam B, Baran Y, Cincik M, Onalan R, Gündüz U, Ural AU, Pabuccu R (2005) Serum and follicular fluid levels of soluble Fas, soluble Fas ligand and apoptosis of luteinized granulosa cells in PCOS patients undergoing IVF. Hum Reprod 20(9):2391–2395
Zheng Q, Li Y, Zhang D, Cui X, Dai K, Yang Y, Liu S, Tan J, Yan Q (2017) ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis 8(10):e3145
Ding L, Gao F, Zhang M, Yan W, Tang R, Zhang C, Chen Z-J (2016) Higher PDCD4 expression is associated with obesity, insulin resistance, lipid metabolism disorders, and granulosa cell apoptosis in polycystic ovary syndrome. Fertil Steril 105(5):1330–1337
Yu YS, Sui HS, Han ZB, Wei L, Luo MJ, Tan JH (2004) Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res 14(4):341
Wu X-Q, Wang Y-Q, Xu S-M, Liu J-F, Bi X-Y, Wang Z-Q, Zhang J-P (2017) The WNT/β-catenin signaling pathway may be involved in granulosa cell apoptosis from patients with PCOS in North China. J Gynecol Obstetr Hum Reprod 46(1):93–99
Chandrasekar B, Mummidi S, Perla RP, Bysani S, Dulin NO, Feng L, Melby PC (2003) Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem J 373(2):547–558
White GE, Greaves DR (2012) Fractalkine: a survivor's guide: chemokines as antiapoptotic mediators. Arterioscler Thromband Vasc Biol 32(3):589–594
Vetvicka V, Lagana AS, Salmeri FM, Triolo O, Palmara VI, Vitale SG, Sofo V, Králíčková M (2016) Regulation of apoptotic pathways during endometriosis: from the molecular basis to the future perspectives. Arch Gynecol Obstet 294(5):897–904
Laganà AS, Vitale SG, Salmeri FM, Triolo O, Frangež HB, Vrtačnik-Bokal E, Stojanovska L, Apostolopoulos V, Granese R, Sofo V (2017) Unus pro omnibus, omnes pro uno: a novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses 103:10–20
Long M, Zhou J, Li D, Zheng L, Xu Z, Zhou S (2015) Long-term over-expression of neuropeptide Y in hypothalamic paraventricular nucleus contributes to adipose tissue insulin resistance partly via the Y5 receptor. PLoS ONE 10(5):e0126714
Nekoonam S, Naji M, Nashtaei MS, Mortezaee K, Koruji M, Safdarian L, Amidi F (2017) Expression of AKT1 along with AKT2 in granulosa-lutein cells of hyperandrogenic PCOS patients. Arch Gynecol Obstet 295(4):1041–1050
Restuccia DF, Hynx D, Hemmings BA (2012) Loss of PKBβ/Akt2 predisposes mice to ovarian cyst formation and increases the severity of polycystic ovary formation in vivo. Dis Models Mech 5(3):403–411
Liu Q, Li Y, Feng Y, Liu C, Ma J, Li Y, Xiang H, Ji Y, Cao Y, Tong X (2016) Single-cell analysis of differences in transcriptomic profiles of oocytes and cumulus cells at GV, MI, MII stages from PCOS patients. Sci Rep 6:39638
Lan C-W, Chen M-J, Tai K-Y, Danny C, Yang Y-C, Jan P-S, Yang Y-S, Chen H-F, Ho H-N (2015) Functional microarray analysis of differentially expressed genes in granulosa cells from women with polycystic ovary syndrome related to MAPK/ERK signaling. Sci Rep 5:14994
Mikaeili S, Rashidi BH, Safa M, Najafi A, Sobhani A, Asadi E, Abbasi M (2016) Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch Gynecol Obstet 294(1):185–192
Accili D, Arden KC (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117(4):421–426
Delbes G, Hales BF, Robaire B (2009) Toxicants and human sperm chromatin integrity. MHR: Basic science of reproductive medicine 16 (1):14–22
Castilla J, Zamora S, Gonzalvo M, Del Castillo JL, Roldan-Nofuentes J, Clavero A, Björndahl L, Martínez L (2010) Sperm chromatin structure assay and classical semen parameters: systematic review. Reprod Biomed Online 20(1):114–124
Ward WS (2009) Function of sperm chromatin structural elements in fertilization and development. MHR 16(1):30–36
Zini A (2011) Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med 57(1–2):78–85
Bungum M, Bungum L, Giwercman A (2011) Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 13(1):69
Paoli D, Lombardo F, Lenzi A, Gandini L (2014) Sperm cryopreservation: effects on chromatin structure. In: Genetic damage in human spermatozoa. Springer, Berlin, pp 137–150
Björndahl L, Kvist U (2014) Structure of chromatin in spermatozoa. In: Genetic damage in human spermatozoa. Springer, New York, pp 1–11
Steger K, Balhorn R (2018) Sperm nuclear protamines: a checkpoint to control sperm chromatin quality. Anat Histol Embryol 47(4):273–279
Acknowledgments
We thank staff of Al-Zahra Hospital of Tabriz and Milad Fertility Center for providing the patients. Some of the data included are part of the M.Sc. thesis of Aydin Raei Sadigh.
Funding
This study was funded by the Stem Cell Research Center, Tabriz University of Medical Sciences [Grant Number: 57778].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Tabriz University of Medical (Approval cod: IR.TBZMED.REC.1396.181).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raei Sadigh, A., Darabi, M., Salmassi, A. et al. Fractalkine and apoptotic/anti-apoptotic markers in granulosa cells of women with polycystic ovarian syndrome. Mol Biol Rep 47, 3593–3603 (2020). https://doi.org/10.1007/s11033-020-05452-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05452-0