Abstract
Obesity is a complex disorder that is influenced by genetic and environmental factors. DNA methylation is an epigenetic mechanism that is involved in development of obesity and its metabolic complications. The aim of this study was to investigate the association between the RANKL and c-Fos gene methylation on obesity with body mass index (BMI), lipid parameters, homeostasis model assessment of insulin resistance (HOMA-IR), plasma leptin, adiponectin and resistin levels. The study included 68 obese and 46 non-obese subjects. Anthropometric parameters, including body weight, body mass index, waist circumference, and waist-hip ratio, were assessed. Serum glucose, triglycerides (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), plasma leptin, adiponectin and resistin levels were measured. Methylation status of RANKL and c-Fos gen were evaluated by MS-HRM. Statistically significant differences were observed between obese patients and the controls with respect to RANKL and c-Fos gene methylation status (p < 0.001). Also, statistically significant importance was observed RANKL gene methylation and increased level of leptin in obese subjects (p = 0.0081). At the same time, statistically significant association between methylation of c-Fos and increased level of adiponectin was observed in obese patients (p = 0.03) On the other hand, decreased level of resistin was observed where the c-Fos was unmetyladed in controls (p = 0.01). We conclude that methylation of RANKL and c-Fos genes have significant influences on obesity and adipokine levels. Based on literature this was the first study which shows the interactions between RANKL and c-Fos methylation and obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is one of the most important health problems with increasing in epidemic proportions in developed countries [1, 2]. It increases the risk of many associated comorbidities such as diabetes, heart disease, cognitive decline, infertility and certain cancers [3,4,5,6,7,8,9]. At the cellular level, inflammation and endoplasmic reticulum (ER) stress was induced by obesity [10] and triggers insulin/leptin resistance, hyperphagia, hyperglycemia and fatty liver disease [11]. Increased level of leptin or decreased level of adiponectin has an affect bone and bone resorption in obesity [12]. Leptin, is a small polypeptide hormone which is secreted by the adipocytes, and supports adipose as an energy storing organ also an active endocrine tissue [13].
The interaction between obesity and chronic inflammatory response, abnormal cytokine production, increased acute-phase reactants, and activation of inflammatory signalling pathways had been shown by the different experimental, epidemiological, and clinical studies [14]. Increased level of TNF-α, leptin and decreased level adiponectin were observed in obese patients and these triggers RANK/RANKL/OPG pathway activation [12, 15]. Researchers identified that increased level of cytokines promotes osteoclast activity by activating RANK/RANKL/osteoprotegerin pathway in obesity [12]. RANKL is a member of tumour necrosis factor (TNF) cytokine family and has an important role during the bone reconstruction [16, 17]. RANKL/RANK expressed in different tissues like bone bone marrow, lymphoid tissues [18], the hypothalamus and septal regions of the brain [18,19,20]. Also, RANKL expressed in the immune system which helps to regulation survival and function of the dendritic cells [21,22,23]. Obesity is characterized with chronic inflammation and the increased level of circulating and tissue proinflammatory cytokines which promotes osteoclast activity and bone resorption via receptor activator of NF-κB (RANK)/RANK ligand/osteoprotegerin pathway [12]. Thus, RANKL triggers different protein–protein interactions, enzyme–substrate reactions or protein translocation reactions. These reactions can be stimulated by directly RANKL/RANK system, or induced or enhanced in vivo [24]. Due to this direct or indirect catalytic and pleiotropic effects of RANKL in different tissue systems, takes attention of the researchers who works with bone or bone related diseases and obesity.
c-Fos gene encodes a transcription factor that involved in extracellular signal transduction and also important for external stimuli response of neurons. Acute stress decreased Fos- the glucagon-like peptide-1 receptor (GLP1R) expression in the lateral hypothalamic area [25]. Chagra et al. showed that food intake may be changed c-Fos expression and play a role in obesity [26].
Numerous studies have reported a significant association between DNA methylation with body weight regulation, adipogenesis and obesity. Many studies showed that DNA methylation at metabolic genes associated with obesity, such as HIF3A and SREBF1 [27, 28]. There is no published work showes the relationship between the RANKL and c-Fos genes methylation status and obesity. The aim of our study was to investigate the association between RANKL and c-Fos genes methylation status, BMI, lipid parameters, HOMA-IR, plasma leptin, adiponectin and resistin levels in obese subjects compared to non-obese subjects.
Materials and methods
Subjects
The study included a retrospective investigation of epigenetic alterations in obesity. Participants in this study were patients who attended the outpatient clinic of the Cengiz Topel Governmental Hospital, Yesilyurt. In the first group contained 68 obese patients who have a mean age of 42.43 ± 1 years and BMI of 35.42 ± 5.67 kg/m2. The second group, control group, included 46 non-obese subjects. The mean age of these subjects was 39.39 ± 1.196 years and their mean BMI was 22.58 ± 2.12 kg/m2.
We excluded adults with cancer, diabetes mellitus, hypertension, dyslipidemias, liver cirrhosis, kidney lithiasis, thyroid, cardiovascular, or any active inflammatory disease. None of the participants received any medications or applied any dietary or exercise program during the study. Medical history were questioned and written informed consent form obtained from all the subjects. The study protocol was approved by the Research Ethics Committee of the Near East University and performed in accordance with the Declaration of Helsinki (Project No: SAG-2016-2-012).
Anthropometric measurements
Weight (kg), height (m), hip circumference (cm) and waist circumference (cm) were measured at fasting state with light clothes from each subject. Hip circumference was measured by placing a measuring tape around fullest portion of the patient’s hips. Waist circumference was measured midway between the lowest rib (laterally) and the iliocristale landmark with flexible tape. BMI was estimated by dividing body weight (kg) by the square of height (m2). BMI ≥ 30 kg/m2 was accepted as an obese [29].
Biochemical parameters
Blood samples were obtained after an overnight fast. Circulating levels of serum glucose, triglycerides (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using an automated analyzer following an overnight fasting state (Abbott Architect C8000). Insulin concentrations were measured using an electrochemiluminescence assay (Ref. 12017547; Elecsys Corp., Lenexa, KS). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the formula: fasting insulin (µU/mL) × fasting glucose (mmol/L) divided by 22.5 [30].
Plasma leptin (ng/mL), adiponectin (µg/mL) and resistin (ng/mL) levels were determined by enzyme linked immunosorbent assay (ELISA) kits (DRG Intl., Inc., USA for leptin and Biovendor Laboratory, Inc., Brno, Czech Republic for resistin and adiponectin) according to the manufacturers’ protocols.
Determination of C-FOS and RANKL methylation status
Genomic DNA was extracted from whole blood samples according to the Qiagane AllPrep DNA/RNA/Protein isolation kit and NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific) was used to measure quantity of DNA. To determine the c-Fos and RANKL methylation, first bisulfite modification reaction was applied by using the EpiTect Bisulfite Kit according to the manufacturers’ protocol (Qiagen, Manchester, UK). Universal methylated and unmethylated DNA (EpiTect Control DNA Set, Cat No./ID: 59568) were used as methylated and unmethylated controls. We used QIAGEN Rotor Gene Q for MS-HRM to detect the methylation status of our samples (Qiagen, Manchester, UK). Primers were designed according to the EpiTect® HRM™ PCR Handbook (Table 1). The MS-HRM analysis was performed according to EpiTect® HRM™ PCR Handbook protocol. We used comparable amounts of template genomic DNA for all samples resulting in CT values below 30 and differing by no more than 3 CT values.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). Comparison between groups were analysed using Student’s t test and the χ2 test for continuous variables and categorical variables, respectively. Continuous variables in the two subgroups was performed by Mann–Whitney U test. A p value of < 0.05 was considered statistical significance. All statistical analyses were performed using the GraphPad Prism 7 software.
Results
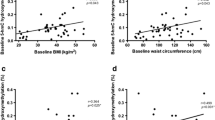
The anthropometric and metabolic characteristics of the patients were presented in Table 2. There is a no statistical significance observed between obese and non-obese subjects age. The plasma glucose, total cholesterol, triglycerides, LDL-cholesterol, HOMA-IR, leptin, and resistin levels were significantly higher in obese than non-obese subjects (p < 0.05). Additionally, the level of mean HDL-cholesterol and adiponectin were significantly decreased in obese subjects than non-obese subjects (p < 0.001).
Methylation status of RANKL and c-Fos genes
Compared statistical analysis of the RANKL gene methylation status in obese and non-obese subjects showed in Table 3. RANKL gene methylated in 4 of the 65 obese subjects (20%) and 16 of the 46 non-obese subjects (80%). There was significant difference between methylation and obese and non-obese subjects (p < 0.001).
c-Fos gene methylated in 53 of the 65 obese subjects (69.77%) and 23 of the 46 non-obese subjects (30.26%). There was significant difference between methylation and obese and non-obese subjects (p < 0.001) (Table 4).
Relationship between anthropometric and metabolic characteristics and RANKL gene methylation
There is a statistically significant association detected between methylated RANKL and leptin level in obese subjects (p < 0.001). The level of leptin were significantly higher in the RANKL methylated cases (p = 0.0081) (Table 5).
Relationship between anthropometric and metabolic characteristics and c-Fos gene methylation
Resistin level was significantly higher in unmethylated c-Fos in non-obese cases (p = 0.01). The level of the resistin is lower in c-Fos methylated non-obese cases (p = 0.01). Moreover, the level of the adiponectin were significantly higher in c-Fos methylated obese cases (Table 6).
Discussion
Genetic factors, unhealthy eating patterns, or a combination of these factors are the major inducers of an obesity [30]. Studies showed that obesity is the major cause of mortality and morbidity [31]. Several researchers show the effects of overweight on bone formation by decreasing apoptosis and effects on osteoporosis in humans [32,33,34,35].
To date, leptin (LEP), leptin receptor (LEPR), proopiomelanocortin (POMC), prohormone convertase 1 (PCSK1), melanocortin 4 receptor (MC4R), single-minded homologue 1 (SIM1), brain-derived neurotrophic factor (BDNF), and neurotrophic tyrosine kinase receptor type 2 (NTRK2) are well-established monogenic obesity related genes and mutations of these genes are related with early onset of obesity [36]. Until now, many genes identified which their expression is regulated with epigenetically and important for the obesity development, metabolic disorders, appetite control, insulin signaling, immunity, inflammation, growth, and circadian clock regulation [37,38,39,40]. Genome-wide DNA methylation analysis in leucocytes and adipose tissue shows abnormal methylation pattern in CLOCK (clock circadian regulator), BMAL1 (aryl hydrocarbon receptor nuclear translocator-like), PER2 (period circadian 2) genes which are known as circadian clock genes and UBASH3A (ubiquitin-associated and SH3 domain-containing protein A) and TRIM3 (tripartite motif containing 3) genes [41,42,43].
Until now, epigenetic regulation of LEP, ADIPOQ, PGC1α, IGF-2, IRS-1, LY86, MEST, PEG3, NNAT, PLAGL1, MEG3, NPY, IL6, TNF, TFAM and GLUT4 genes had been reported related with obesity or weight loss [44]. Also, obesity causes activation of the c-Jun N-terminal kinase (JNK) and nuclear factor-kappa B (NF-κB) signaling pathways [45]. While receptor activator of NF-κB (RANKL) binds to its receptor (RANK) and activates the NF-κB pathway and activation of the pathway triggers pro-inflammatory cytokines expression [46]. The expression of RANK and RANKL are related with glycemic control and obesity and these genes are expressed in human liver tissue and pancreatic β-cells [47]. Kiechl and colleagues showed that the concentration of soluble RANKL was associated with insulin resistance [47]. As we mentioned previously, activation of the transcription factor nuclear factor-κB (NF-κB) triggers the activation of inflammatory signaling pathways and related with insulin resistance and β-cell dysfunction [48, 49]. It activates T cells and endothelial cells or adipocytes [47] but there is a unknown interaction between skeleton and the immune systems which may contribute to hepatic insulin resistance. RANKL could be used to connect interaction between immune activation, bone resorption and obesity [47]. In our work, we identified statistically significant RANKL unmethylated in obese group (73.86%) and 80% of the non-obese group were methylated (p < 0.001). This confirms Kiechl and colleagues works [47] but due to the retrospective nature of our study we cannot confirm gene expression level and based on current literature this was the first study which shows the interaction epigenetic regulation of RANKL and obesity. This result should be led light to the further epigenetic studies.
Zhu and colleagues shows in vitro treatment in mice and lacked RANKL in their daily food. They identified changes in c-Fos expression during the response of peripheral RANKL in hypothalamus and they conclude that RANKL plays an important role as a food inhibitor and causes decreased body weight of mice [50]. Ostrowska et al. identified increased level of RANKL circulation in patients with anorexia nervosa, and then showed the RANKL level was depended on the severity of the anorexia nervosa [51, 52]. In in vitro studies shows injection of adenovirus vector harbouring murine soluble RANKL cDNA in mice triggers exhibit reduced food intake and body weight [53]. Zampetti and colleagues analysed OPG/RANKL ratio and they showed higher level of OPG/RANKL were associated with overweight/obese children and adolescents [54]. Studies showed that RANKL regulates hepatic insulin sensitivity and blockage of RANKL signalling proves insulin sensitivity and normalizes glucose concentrations in hepatocytes [55, 56]. On the other hand, Yeşilkaya and colleagues analysed OPG and RANKL levels in obese children but they did not find significant differences between obese and non-obese children [57]. In this work, we identified statistically significant differences of RANKL methylation between obese and non- obese cases. RANKL gene were unmethylated in obese group (73.86%) and 80% of the non-obese group were methylated (p < 0.001). Based on current literature this was the first study which shows the interaction epigenetic regulation of RANKL and obesity.
Within the hypothalamic regions, neurons expressed feeding-related markers. Researchers identified strong interaction between neuronal activation and c-Fos expression. It was triggers AgRP, MC4R and GLP1R expression in neurons [58]. Acute stress decreased Fos-GLP1R expression in the lateral hypothalamic area and increased orexigenic signaling in the brain [59]. Luna-Illades et al. showed that obesity diminished Fos expression in hypothalamic nuclei of obese N. Alstoni mice [60]. Considering our results which showed that c-Fos gene were methylated in 69.77% of the cases in obese group and in non-obese group 60.61% were unmethylated, one could then speculate that c-Fos gene methylation can be regarded as a susceptibility to obesity.
The role of estrogene on bone acts via RANKL and OPG and the deficiency of estrogen during the postmenopausal women causes increased RANKL level, and triggers osteoclastogenesis. Also, the bone protection of obese individuals who have a high level of leptin will be defective. Leptin plays an important role during the regulation of weight, energy expenditure, bone metabolism [61]. Leptin and receptors had been widely studied, and results show that leptin plays a role for function of metabolic functions, neuroendocrine function, immune function, reproduction, and bone metabolism [62,63,64,65]. Also, the concentration of leptin reflects energy storage in adipose tissue, and circulating leptin level was related with the amount of body fat [61]. Leptin is an adipocytokine produced in white adipose tissue and plays important roles in obesity, food intake, glucose homeostasis, and energy expenditure. It may participate in several mechanisms of obesity associated disease such as hypertension, metabolic syndrome, cardiovascular disease and bone diseases [46, 62]. Receptor activator of nuclear factor-kappaB ligand (RANKL) and its receptor (RANK) have been described for their roles in the regulation of bone resorption. Leptin induces synaptic activity that signals to promotes osteoblast proliferation and suppresses bone resorption effects of osteoclasts via RANKL synthesis [66]. Elefteriou et al. showed that leptin could inhibit the expression of RANKL in osteoblasts and therefore supressed osteoclast differentiation [67]. Moreover, Holloway et al. reported that leptin can osteoclast generation in vitro by decreasing the receptor activator of RANKL in stromal cells [68]. Consistent with these previous studies, we showed that RANKL methylated cases had significantly higher serum leptin levels than unmethylated RANKL gene cases in obese group. It can be concluded that leptin may regulate bone metabolism through RANKL synthesis.
Overall, our data support the conclusion that RANKL has a important role in the pathogenesis of obesity and provides a link between serum leptin level. These findings hold promise for the future development of new therapeutic and preventive approaches.
Adiponectin is one of the key adipokine which is secreted in adipocytes of adipose tissue and has an important role during the carbohydrate regulation and fat metabolism in insulin-sensitive tissues, and acts as an endogenous insulin-sensitizer [69]. Adiponectin level decrease in obese individual and inversely correlated with the presence of obesity-related complications [70,71,72]. On the other hand, resistin is another peptide hormone with biological properties opposite to adiponectin. It was found many tissues but it is expressed mainly in the adipose tissue [73]. In humans, obesity was found to be associated with high resistin serum levels [74, 75]. Hirai et al. discovered that resistin increased c-Fos transcription factor expression, however adiponectin suppressed resistin induced c-Fos expression in the intracellular signalling pathway [76]. These data suggest that adiponectin and resistin may show opposite effects on metabolism via different expression levels of transcription factors such as c-Fos. Considering our results, resistin level was significantly higher while the c-Fos was unmethylated in non-obese group. Furthermore, higher serum adiponectin level was observed when the c-Fos was methylated in obese group. Thus, our results may suggest that methylation status of c-Fos gene may be related with different levels and metabolic effects of resistin and adiponectin in obesity.
As a consequence of the increased power of this study is that the first study which showed association of RANKL and c-Fos gene methylation with anthropometric parameters, lipid profile, HOMA-IR, leptin, adiponectin and resistin levels. Also, it was performed in well characterized individuals, with or without obesity. These results suggest that epigenetic studies are another perspective for the identification of the potential genes that play a role in obesity and weight regulation. Further insights into the underlying biological mechanisms and different pathways will be needed for the development effective treatment strategies and management of these traits.
In conclusion, our results suggest that the RANKL and c-Fos gene methylation status have association with obesity. Additionally, they have significant role on leptin, resistin and adiponectin levels. Further assessment of all other possible methylation status of different genes which might affect obesity and adipokine levels is required. Nowadays, the increased number of studies on obesity is even higher, because of increased prevalence worldwide, causes of many other pathologies like; alterations of reproductive capacity and epigenetic changes. The identification of epigenetic alterations of obesity is important for disease outcome and development of most effective therapy. The reversible nature of epigenetic modifications makes them important targets for a possible epigenetic therapy targets in obesity.
References
Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL et al (2016) American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 3:1–203
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C et al (2016) 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 37:2315–2381
Berger NA (2014) Obesity and cancer pathogenesis. Ann N Y Acad Sci 1311:57–76
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846
Ortega FB, Lavie CJ, Blair SN (2016) Obesity and cardiovascular disease. Circ Res 118:1752–1770
Procaccini C, Santopaolo M, Faicchia D, Colamatteo A, Formisano L et al (2016) Role of metabolism in neurodegenerative disorders. Metabolism 65:1376–1390
Broughton DE, Moley KH (2017) Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril 107:840–847
Fan W, Xu Y, Liu Y, Zhang Z, Lu L, Ding Z (2018) Obesity or overweight, a chronic inflammatory status in male reproductive System, leads to mice and human subfertility. Front Physiol 8:1117
Hohos NM, Skaznik-Wikiel ME (2017) High-fat diet and female fertility. Endocrinology 158:2407–2419
Wang S, Kaufman RJ (2012) The impact of the unfolded protein response on human disease. J Cell Biol 197:857–867
Pagliassotti MJ, Kim PY, Estrada AL, Stewart CM, Gentile CL (2016) Endoplasmic reticulum stress in obesity and obesity-related disorders: an expanded view. Metabolism 65:1238–1246
Cao JJ (2011) Effects of obesity on bone metabolism. J Orthop Surg Res 15(6):30
Mohamed-Ali V, Pinkney JH, Coppack SW (1998) Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord 22(12):1145–1158
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444(7121):860–867
Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Investig 95(5):2409–2415
Hanada R, Hanada T, Sigl V, Schramek D, Penninger JM (2011) RANKL/RANK-beyond bones. J Mol Med 89:647–656
Schoppet M, Preissner KT, Hofbauer LC (2002) RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol 22:549–553
Nakagawa T, Roth W, Wong P, Nelson A, Farr A et al (1998) Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science 280:450–453
Hanada R, Leibbrandt A, Hanada T, Kitaoka S, Furuyashiki T et al (2009) Central control of fever and female body temperature by RANKL/RANK. Nature 462:505–509
Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK et al (1999) Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone 25:525–534
Cremer I, Dieu-Nosjean MC, Maréchal S et al (2002) Long-lived immature dendritic cells mediated by TRANCE-RANK interaction. Blood 100:3646–3655
Wiethe C, Dittmar K, Doan T et al (2003) Enhanced effector an memory CTL responses generated by incorporation of receptor activator of NF-kappa B (RANK)/RANK ligand costimulatory molecules into dendritic cell immunogens expressing a human tumor-specific antigen. J. Immunol 171:4121–4130
Yu Q, Gu JX, Kovacs C et al (2003) Cooperation of TNF family members CD40 ligand, receptor activator of NF-kappa B ligand, and TNF-alpha in the activation of dendritic cells and the expansion of viral specific CD8 + T cell memory responses in HIV-1-infected and HIV-1-uninfected individuals. J. Immunol. 170:1797–1805
Raju R, Balakrishnan L, Nanjappa V, Bhattacharjee M, Getnet D, Muthusamy B, Kurian Thomas J, Sharma J, Rahiman BA et al (2011) A comprehensive manually curated reaction map of RANKL/RANK-signaling pathway. Database (Oxford). https://doi.org/10.1093/database/bar021
Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD (1999) Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24:155–163
Chagra SL, Zavala JK, Hall MV, Gosselink KL (2011) Acute and repeated restraint differentially activate orexigenic pathways in the rat hypothalamus. Regul Pept 167(1):70–78
Dick KJ, Nelson CP, Tsaprouni L, Sandling JK, Aïssi D et al (2014) DNA methylation and body-mass index: a genome-wide analysis. Lancet 383:1990–1998
Chambers JC, Loh M, Lehne B et al (2015) Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case–control study. Lancet Diabetes Endocrinol 3:526–534
World Health Organization (1995) Physical status: the use and interpretation anthropometry. Report of a WHO Expert Committee, vol 854. WHO Technical Report Series. WHO, Geneva, pp 321–344
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasismodel assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia 28:412–419
Williams LM (2012) Hypothalamic dysfunction in obesity. Proc Nutr Soc 71:521–533
Powell K (2007) Obesity: the two faces of fat. Nature 447(7144):525–527
Ehrlich PJ, Lanyon LE (2002) Mechanical strain and bone cell function: a review. Osteoporos Int 13(9):688–700
Núñez NP, Carpenter CL, Perkins SN, Berrigan D, Jaque SV et al (2007) Extreme obesity reduces bone mineral density: complementary evidence from mice and women. Obesity 15(8):1980–1987
Rosen CJ, Bouxsein ML (2006) Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2(1):35–43
Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B et al (2008) Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 23(1):17–29
D’Angelo CS, Koiffmann CP (2012) Copy number variants in obesity-related syndromes: review and perspectives on novel molecular approaches. J Obes. https://doi.org/10.1155/2012/845480
Okamura M, Inagaki T, Tanaka T, Sakai J (2010) Role of histone methylation and demethylation in adipogenesis and obesity. Organogenesis 6(1):24–32
Pinnick KE, Karpe F (2011) DNA methylation of genes in adipose tissue. Proc Nutr Soc 70(1):57–63
Drummond EM, Gibney ER (2013) Epigenetic regulation in obesity. Curr Opin Clin Nutr Metab Care 16(4):392–397
Van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS, Members of EpiSCOPE (2015) Epigenetics and human obesity. Int J Obes 39(1):85–97
Carless MA, Kulkarni H, Kos MZ, Charlesworth J, Peralta JM et al (2013) Genetic effects on DNA methylation and its potential relevance for obesity in Mexican Americans. PLoS ONE 8(9):e73950
Xu X et al (2013) A genome-wide methylation study on obesity: differential variability and differential methylation. Epigenetics 8(5):522–533
Bouchard L, Rabasa-Lhoret R, Faraj M, Lavoie ME, Mill J et al (2010) Differential epigenomic and transcriptomic responses in subcutaneous adipose tissue between low and high responders to caloric restriction. Am J Clin Nutr 91(2):309–320
Lopomo A, Burgio E, Migliore L (2016) Epigenetics of obesity. Prog Mol Biol Transl Sci 140:151–184
Sharma M, Vikram NK, Misra A, Bhatt S, Tarique M et al (2013) Assessment of 11-β hydroxysteroid dehydrogenase (11-βHSD1) 4478T>G and tumor necrosis factor-α (TNF-α)-308G>A polymorphisms with obesity and insulin resistance in Asian Indians in North India. Mol Biol Rep 40(11):6261–6270
Chen XX, Yang T (2015) Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metab 33(5):474–485
Kiechl S, Wittmann J, Giaccari A, Knoflach M, Willeit P et al (2013) Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med 19(3):358–363
Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132:2169–2180
Donath MY et al (2005) Mechanisms of β-cell death in type 2 diabetes. Diabetes 54(suppl. 2):S108–S113
Zhu P, Zhang Z, Huang X, Liang S et al (2018) RANKL reduces body weight and food intake via the modulation of hypothalamic NPY/CART expression. Int J Med Sci 15(10):969–977
Ostrowska Z, Ziora K, Oswiecimska J, Swietochowska E, Szapska B et al (2012) RANKL/RANK/OPG system and bone status in females with anorexia nervosa. Bone 50:156–160
Ostrowska Z, Ziora K, Oswiecimska J, Swietochowska E, Wolkowska-Pokrywa K (2012) Dehydroepiandrosterone sulfate, osteoprotegerin and its soluble ligand sRANKL and bone metabolism in girls with anorexia nervosa. Postepy Hig Med Dosw 66:655–662
Enomoto T, Furuya Y, Tomimori Y, Mori K, Miyazaki J, Yasuda H (2011) Establishment of a new murine model of hypercalcemia with anorexia by overexpression of soluble receptor activator of NF-kappaB ligand using an adenovirus vector. J Bone Miner Metab 29:414–421
Zampetti S, Lucantoni F, Pacifico L, Campagna G, Versacci P et al (2019) Association of OPG-RANKL ratio with left ventricular hypertrophy and geometric remodeling in male overweight/obese youths. J Endocrinol Invest 42(4):427–434
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Investig 117(1):175–184
Goto H, Hozumi A, Osaki M, Fukushima T et al (2011) Primary human bone marrow adipocytes support TNF-alpha-induced osteoclast differentiation and function through RANKL expression. Cytokine 56(3):662–668
Yeşilkaya E, Bideci A, Çamurdan O, Boyraz M, Vurucu S, Cinaz P (2010) Association of osteoprotegerin and RANKL levels with insulin resistance in pubertal obese children. Cent Eur J Med 5(2):261–267
Swanson LW, Sawchenko PE (1983) Hypothalamic Integration: organization of the paraventricular and supraoptic nuclei. Ann Rev Neurosci 6:269–324
Luna-Illades C, Morales T, Miranda-Anaya M (2017) Decreased food anticipatory activity of obese mice relates to hypothalamic C-FOS expression. Physiol Behav 179:9–15
Legiran S, Brandi ML (2012) Bone mass regulation of leptin and postmenopausal osteoporosis with obesity. Clin Cases Miner Bone Metab 9(3):145–149
Stofkova A (2009) Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul 43(4):157–168
Farooqi IS, O’Rahilly S (2009) Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr 89(3):980–984
Reid IR (2009) Adipokine effects on bone. Clin Rev Bone Miner Metab 7:240–248
Schwartz MW, Seeley RJ, Tschöp MH et al (2013) Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503:59–66
Karsenty G (2006) Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 4:341–348
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X et al (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520
Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM et al (2002) Leptin inhibits osteoclast generation. J Bone Miner Res 17:200–209
Fasshauer M, Blüher M (2015) Adipokines in health and disease. Trends Pharmacol Sci 36:461–470
Yatagi T, Nagasaka S, Taniguchi A, Fukushima M, Nakamura T (2003) Hypoadiponectinemia is associated with visceral FAT accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism 52:1274–1278
Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S (2003) Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23:85–89
Li S, Shin HJ, Ding EL, van Dam RM (2009) Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302:179–188
Nogueiras R, Gallego R, Gualillo O, Caminos JE, Garcia-Caballero T et al (2003) Resistin is expressed in different rat tissues and is regulated in a tissue- and gender-specific manner. FEBS Lett 548:21–27
Huang X, Yang Z (2016) Resistin’s, obesity and insulin resistance: the continuing disconnect between rodents and humans. J Endocrinol Investig 39:607–615
Jamaluddin MS, Yan S, Lü J, Liang Z, Yao Q, Chen C (2013) Resistin increases monolayer permeability of human coronary artery endothelial cells. PLoS ONE 8:84576
Hirai H, Satoh H, Kudoh A, Watanabe T (2012) Interaction between resistin and adiponectin in the proliferation of rat vascular smooth muscle cells. Mol Cell Endocrinol 366(1):108–116
Funding
This study was supported by Near East University Scientific Research Project Unit—Grant Number: SAG-2016-2-012. We would like to thank all the participants involved in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalkan, R., Becer, E. RANK/RANKL/OPG pathway is an important for the epigenetic regulation of obesity. Mol Biol Rep 46, 5425–5432 (2019). https://doi.org/10.1007/s11033-019-04997-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04997-z