Abstract
Matrix metalloproteinases (MMPs) are implicated in atherosclerosis evolution into a coronary artery disease (CAD). They could be used as biomarkers for a predictive approach when they are studied simultaneously. We aim in our study to demonstrate prospectively in patients with history of CAD that MMPs level is linked to clinical cardiovascular outcomes. Two hundred and eighteen patients diagnosed with CAD were followed prospectively for 5 years in the Cardiology Department of la Rabta Hospital University. Clinical cardiovascular outcomes during the period of the cohort were recorded. Measures were performed for biological and matrix markers at baseline. MMP-3, MMP-8, MMP-9, TIMP-1 and TIMP-2 were measured by ELISA in Sandwich assay. Fifty-nine cardiovascular outcomes occurred during the cohort period. By multivariate analysis, only MMP-3 persisted as a predictor for cardiovascular events even after adjustment. This metalloproteinase have been shown to be an independent predictor for cardiovascular outcomes (HR = 3.01; CI (1.3–6.95). The found cut-off value by receiver operating curve (ROC) was used for Kaplan–Meier analysis and revealed that patients with MMP-3 level higher than 9.3 ng/mL had a lower survival rate (p = 0.03). MMP-3 baseline level in patients with history of CAD is a potential predictor for cardiovascular outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis, a well-known pathophysiological phenomenon and the major cause for coronary artery disease (CAD), has been associated with matrix remodeling. The evolution and the rupture of atherosclerosis plaques are mediated by matrix metalloproteinases (MMPs), degrading enzymes that attack fibrous cap of these plaques [1]. Theses enzymes are produced by several types of cells including macrophages, smooth muscle and epithelial cells and have a wide range of substrates [2]. Extracellular matrix (ECM) equilibrium is regulated bya balance between MMPs and their tissue inhibitors of metalloproteinases (TIMPs) [3]. Atherosclerosis evolution into CAD is due to several factors including MMPs that could be used as biomarkers for a predictive approach when they are studied simultaneously to improve cardiovascular risk prediction. A vascular remodeling is established, in order to respond to major factors like hypertension, diabetes, lipid accumulation [4]. This process is an early reply to the probable establishment of an unstable angina, a heart failure, a stroke or an acute myocardial infarction (MI) [5]. Given that they are involved in ECM structure; abnormal MMPs activity has been related to the instability of plaque formation that is reflected in the complication of the disease in human and animal models [6, 7]. Consequently, several components of ECM increase by cardiac fibroblasts resulting in matrix breakdown [8,9,10]. One of the reasons for the progression of CAD is the imbalance between MMPs and TIMPs that leads to the partial obstruction and even a total occlusion of coronary artery lumen [11].
Few cohort studies have investigated the effect of MMPs on CAD evolution. One of them investigated the MMP-9 association with MI and stroke risk in elderly patients and found an univariate association of MMP-9 serum level and both end-points without independent risk marker significance [12].
Another study revealed the MMP-8 as an indicator of left ventricular remodeling in patients after acute MI [13] which has been associated to atherosclerosis development and was responsible for plaque collapse [14]. Furthermore, elevated TIMP-1 level predicted CAD in the Framingham study group [15]. In fact, only few MMPs circulating level were investigated for their impact in CAD evolution. Collagenase-2 (MMP-8) is capable of degrading type I of collagen [16] and gelatinase B (MMP-9) is capable of degrading gelatin [17]. Both of them are highly secreted in atherosclerosis lesions and are related to inflammation a major cause of the disease [18]. On the other hand, MMP-3 has a wide range of substrates including collagen types III, IV, IX and X, gelatin types I, III, IV and V. MMP-3 plays an activator role of other MMPs and is considered as a remodeling regulator [19]. TIMP-1 and TIMP-2 are specific tissue inhibitors of MMPs, interfering in the imbalance of degradation and secretion of matrix components [20]. The impact of all of these MMPs on a specific studied population may be revealed after a period of time and could highlight their effect on CAD patients. We aimed in our study to investigate prospectively the association between MMPs level and clinical cardiovascular outcomes and their predictive value for CVD complications in patients with history of CAD.
Materials and methods
Study population
We recruited in 2008 (T0), 428 patients with angiographically documented CAD. Coronary angiograms were clinically indicated and were performed in the Cardiology department of La Rabta Hospital University. Patients with a stenosis ≥ 50% in a major epicardial coronary artery were included. Excluded patients were those with history of hepatic, renal or cerebral disease. Exclusion criteria included also infections, autoimmune disease, valvular heart disease, peripheral atherosclerotic disease, recent MI < 72 h or any surgical procedure in the preceding 6 months. After considering exclusion and inclusion criteria, only 292 patients were considered for the study. Patients aged between 35 and 70 years and were from both genders. One hundred ninety-three healthy controls were also recruited: these controls were not suffering from any pathology and were normal in term of biological parameters, anthropometric measurements and matrix markers. Controls were used as a referent group for the different studied parameters. All participants gave their informed consent. The survey protocol was approved by the ethics committee for human research of La Rabta Hospital and conforms to the 1975 Helsinki declaration ethical guidelines.
Clinical follow up and endpoint of the study
The study endpoint combined fatal and non-fatal cardiovascular outcomes according to Cutlip et al. recommendations [21]. The sample size calculation was performed before starting the study with a 95% statistical power with 5 years as a follow-up period and 2.4 hazard ratio capable of detecting inter-group difference. The required sample was estimated to be at least 89. Thereby, with 292 cases available for the cohort study we fulfilled the minimal need of patient number. Maintained patients were followed for 5 years, recorded death was classified in two sections: cardiovascular mortality and non-cardiovascular mortality. Cardiovascular mortality was recorded according to the death certificate in patients file present in the department of Cardiology. Non-cardiovascular mortality was obtained by contacting the patient family. At the end of follow-up all of the occurring cardiovascular outcomes were noted. All of these events were recorded and have been considered for further analyses.
Sample collection procedure and biochemical analyses
Blood samples were gathered in the Cardiology department of La Rabta Hospital University. Patients’ peripheral blood was collected into heparin, citrate, and sodium-fluoride-containing tubes after fasting overnight and centrifuged for 15 min at 4 °C (3000 × g). Plasma samples were used for matrix markers analysis and have been stored at −80 °C. Measurements were performed after gathering the samples. Other biochemical analyses were performed in a period of 2 h. Glycaemia was determined by the glucose-oxidase method, cholesterol and triglycerides by colorimetric enzymatic methods. After precipitation with magnesium chloride phosphotungstate, HDL-cholesterol was measured. Immunoturbidimetric method was used to determine C-reactive protein (CRP) level. All of the biochemical analyses were performed using a Hitachi 912 analyzer (Roche Diagnostics, Mannheim, Germany).
Estimated Glomular filtration rate (eGFR) mL/min per 1.73 m2 has been calculated according to the abbreviated Modification of Diet in Renal Disease (MDRD) study equation: GFR = 186 × (serum creatinine)−1.154 × (age)−0.203 × 1.21 (in African subjects) and × 0.742 (in women), as mentioned in K/DOQI Clinical Practice Guidelines [22,23,24]. Immune cells like White blood cell (WBC), monocytes, neutrophils and lymphocytes were determined by complete blood count (CBC). ABX Micros 60 automaton (Horiba Medical, Kyoto, Japan) was used to determine Hematological parameters.
Matrix markers measurements
Plasmatic levels of MMP-3 (DY513), MMP-8 (DY908), MMP-9 (DY911), TIMP-1 (DY970) and TIMP-2 (DY 971) were measured in citrate plasma using ELISA in Sandwich method (Duoset, R&D Systems, Lille, France). All samples were measured in duplicates. The inter-assay and intra-assay coefficients of variation were less than 6%.
Statistical analysis
Statistical analysis was performed using the SPSS software for Windows (version 18.0). The Kolmogorov–Smirnov test was used to verify the normality of the distribution of continuous variables. Normally distributed variables were compared using parametric tests (Student t test). In case of non-normal variable distribution which was the case of the studied MMPs and CRP, they were transformed into natural logarithm to improve normal distribution approximation and were also calculated by the same t test for parametric variables. Unpaired t test was used to compare independent series. Quantitative variables were shown as mean values ± standard deviation. Categorical variables were calculated by Chi square (χ2) Pearson test. Pearson correlation method was used for overall patients in order to study the different correlations between continuous parameters.
Cardiovascular occurring outcomes during the cohort were combined and associated to MMPs baseline levels using the Cox proportional hazard model. Regression models were used to assess the natural logarithm transformed levels of MMPs to clinical outcomes. Three models were used: the first one included age and gender. The other models included major risk factors and treatment (statins and β blockers). Since MMP-3 persisted in the three models, we used an additive effect for hazard ratios through progressive adjustment by adding each time the measured level of MMPs in the study.
The sensitivity, the specificity and the cut-off value of MMP-3 were determined by the receiver operating curve (ROC) analysis. The Kaplan–Meier method was used to estimate cumulative survival, and the differences in survival curves were compared with the log rank test. Statistical significance was accepted when p < 0.05.
Results
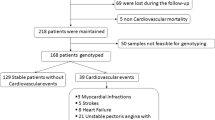
Figure 1 showed the followed recruitment process of the studied population and the major occurring events. Of the 292 recruited patients, sixty-nine were lost at follow-up due to the loss of contact like the change of phone number and the rejection of a second thought of an exam, fourteen died: cardiovascular reason (n = 9), and five for non-cardiovascular mortality including cancer (n = 2), accident (n = 2), unknown (n = 1). Only 218 were tracked in the cardiology department in 2013 and have been available for the cohort study. Among these patients, fifty-nine cardiovascular outcomes occurred in this period of time including: cardiovascular mortality (n = 9), MI (n = 5), strokes (n = 8), hospitalization for heart failure (n = 11), unstable angina pectoris with a repeat target for revascularization (n = 22), and renal dysfunction onset (n = 4) (Fig. 1).
Summarizing Table 1 showed that both groups of patients were more likely to be older, male, hypertensive and to have diabetes at baseline (Table 1). Recruited patients with and without fatal cardiovascular outcomes were older than controls and presented a higher profile of biological parameters when comparing the recruited patients to the controls. Patients had an increased level for major biological parameters, except for HDL and eGFR that have been higher in controls than patients.
The comparison between CAD stable patients and patients with fatal and non-fatal cardiovascular outcomes showed that there was not a particular variation between both groups except for urea (7.65 vs. 6.49; p < 0.01). Also inflammatory markers were significantly higher in both patients groups compared to controls.
Matrix marker levels were significantly higher in stable CAD patients, [ln MMP-8 (2.83 vs. 1.24; p < 0.01), ln MMP-9 (5.33 vs. 4.8; p < 0.01) and ln TIMP-1 (4.22 vs. 3.86; p < 0.01)] when compared to controls. Similarly, MMP-3, MMP-8 and MMP-9 were elevated in patients with fatal and non-fatal cardiovascular outcomes compared to controls. However, only ln MMP-3 level was found significantly higher in patients with fatal and non-fatal cardiovascular outcomes compared to stable CAD patients (2.59 vs. 2.28; p = 0.02) (Table 2).
Cox regression analysis for overall patients using clinical outcome and MMPs levels revealed only the MMP-3 as a cardiovascular predictor factor for the established models.
After adjustment for age and gender ((model 1) HR = 1.62 (95% CI: 0.93–2.83)) and then for major risk factor including hypertension, diabetes, dyslipidemia, smoking, CRP, urea and eGFR only the MMP-3 remained significant ((model 2) HR = 2.28 (95% CI: 2.23–4.23)).
After further adjustment for treatment (statins and β-blockers) (model 3), the MMP-3 showed up to be an independent predictor for cardiovascular outcomes ((model 3) HR = 3.01 (95% CI: 1.3–6.95)) (Table 3). Since MMP-3 persisted in the three models, cumulative effect of MMPs was evaluated, each time by adding a matrix marker to the model. The obtained result proved the persistence of the MMP-3 in each model (Table 4). The correlation analysis with biological parameters showed that MMP-3 correlated significantly to uric acid (p = 0.02; r = 0.17), urea (p = 0.02; r = 0.17), creatinine (p < 0.001; r = 0.28), neutrophils (p = 0.02; r = 0.17) and negatively to eGFR (p < 0.001; r = −0.33) (Fig. 2).
The MMP-3 analysis by ROC test was significant (p = 0.021) with an area under curve (AUC) equal to 0.613; the cut-off value of 9.3 ng/ml with a specificity equal to 0.47 and a sensitivity equal to 0.75 (Fig. 3). The found value was thereby used for Kaplan–Meier survival test according to MMP-3 basic level by the occurred events during the cohort period. The analysis showed that survival rate was elevated in patients having the MMP-3 level < 9.3 ng/ml. In contrast, the majority of occurring outcomes were present in patients having an MMP-3 level higher than 9.3 ng/ml. These results were illustrated in Fig. 4.
Discussion
The apparent power for the designed survey lies in the precision of the data. The recruited patients were followed after 5 years. Biological, anthropometric and matrix markers were measured when the patients were included. Also, cardiovascular outcomes were recorded and have been considered. Our major finding is the direct association of the MMP-3 to cardiovascular outcomes in CAD patients.
Matrix metalloproteinases and inhibitors variation
The comparison of MMPs and TIMPs level in baseline showed the existence of significant variations between controls, patients with and without cardiovascular outcomes. In relation with their endogenous tissue inhibitors, MMPs are involved in the activation, proliferation and migration of vascular cells. They are also implicated in the formation of new vessels and matrix remodeling including healing or ECM arteries destruction [25]. All of these functions explain the significant variation between controls and both groups of patients. It was shown that MMPs and TIMPs variation in plasma promotes the remodeling in myocardium and vessels indicating the existence of interaction between theses enzymes and the occurring outcomes.
In fact, cardiovascular occurring outcomes result from fibrous cap destruction surrounding the luminal lesion side of atherosclerosis plaque [26]. Henney et al. showed the accumulation of the MMP-3 in atherosclerosis lesions. Isolated plaque cells from individual with atherosclerosis plaques presented stromelysin mRNA transcripts in smooth muscle cells. This accumulation reflects vascular remodeling related to plaque growth which contributes to the pathological effect of matrix remodeling [27, 28].
Levels of MMP-9, MMP-8, TIMP-1 and TIMP-2 did not witness any kind of variation between both patient groups. One of the reasons behind the reduction of the enzymes is taking treatments. Sixty-nine point nine percent of our patients were under statins, a lowering cholesterol drug that have been shown to decrease MMPs level and expression [29, 30]. Experimental evidence proved that statins dysregulate MMPs in different type of cells in vitro [31], since they have a pleotropic impact including an immunomodulatory, anti-inflammatory and matrix-modulating effects. However, recent meta-analyses showed that statins does not affect MMP-3 level [32,33,34]. This result is in accordance with ours since the only found difference was in MMP-3 and was proved in the further performed analysis in Table 3 in the 3rd model. Also, Beta- blockers reduce MMPs activities to prevent from arterial stiffing and thereby cardiac dysfunction [35].
Persistence of MMP-3 in CVD outcomes in CAD patients
The only persisting enzyme was the MMP-3 that remained as a significant predictor even after adjustment for probable risk factors. Known also as stromelysin, MMP-3 is widely expressed in human atherosclerotic lesions and has broad substrate specificity. Since it activates other MMPs, MMP-3 could be considered as a leader of MMPs, which explains its persistence in years [36, 37]. On animal models, Johnson et al. proved that MMP-3 knock-out mouse had a detectable reduced remodeling impact afterward ligation. They suggested that Pro MMP-9 was incapable of restoring vascular smooth muscle cells (VSMC) because of the lack of activation in MMP-3 absence [38].
In fact, as atherosclerosis persists, a reduction in elastin and an accumulation of collagen is witnessed inducing the decrease of elastin collagen ratio in the arterial matrix. This reduction leads to arterial compliance [39]. The existence of a common polymorphism 5A/6A in the MMP-3 promotor region could explain the association of the MMP-3 to the disease severity. On the one hand, the 6A allele is responsible of MMP-3 decrease and leads to the progression of atherosclerosis and to collagen accumulation in arterial matrix [40, 41]. On the other hand, the 5A allele is related to MMP-3 increase and therefore to plaque instability [2, 42]. The determined cut-off value used for Kaplan–Meier survival risk shows the MMP-3 can be used as a biomarker for CAD complications. A recent cohort study performed on male patients with and without acute coronary syndrome used the upper tertile of the MMP-3 value in order to predict MI [43].
According to Cavusoglu et al., patients having a higher MMP-3 level than 9.3 ng/mL had a lower survival rate, which means that MMP-3 can be used as predictor for cardiovascular complications [43]. The determined value of the MMP-3 level in our study (9.3 ng/mL) is almost nearly the half of that found in the study of Cavusoglu study (20.56 ng/mL).
It is true that the determined level (9.3 ng/mL) was not the same when we compared ours to Cavusoglu study (20.56 ng/mL) and this could be due to the difference of inclusion and exclusion criteria between the studied patients. The studied patients where in the chronic phase of the disease and they took in consideration only the MI as a complication [43]. While the recruited patients of the current study were with a history of CAD and the considered criteria included cardiovascular outcomes. Also the study used the upper tertile for the MMP-3 [43] whereas we used the ROC analysis to have a significant cut-off value.
MMP-3 association with biological markers
MMP-3 correlated positively to renal markers (creatinine, uric acid, and urea) and negatively to the eGFR. This association was not affected by other bias since patients with a renal dysfunction were excluded from the study. Shilpak et al. reported that atherosclerosis at non-renal sites is associated with renal function decline [44] explained by an overexpression of MMPs that have the ability to fragment ECM for elimination. Capable of cleaving also nonmatrix substrates, MMPs release growth factors engendering several complications [45]. MMP-3 activates other MMPs [46] and is expressed in podocytes and mesangial cells of glomerular basement membrane. Podocytes are linked to basement membrane by integrins. Integrin-linked kinase is induced by MMP-9 leading to podocyte release from glomerular basement membrane [47]. Also, the existent association between MMP-3 and neutrophils reveals its secretion as a consequence of an inflammatory response due to prior occlusion by atherosclerosis plaque. However, since it was not related to sensitive inflammatory markers like CRP, a low-grade inflammation phenomenon could be established [48, 49].
It was well established the MMP-3 has a predictive role in the disease complication. Inhibiting its effect by other endogenous inhibitors could be interesting despite the fact that the MMP-3 is implicated in other physiological roles.
To site, there are several limitations to the study, a group of controls followed for the same period of time could improve the founded cut-off value for MMP-3. Therefore, the strength of the actual study is the persistence of the MMP-3 as a predictor for cardiovascular complications. However, an intermediate time for another measure could give a better enzyme profile for the deceased patients. A larger cohort may ameliorate statistical analysis. Our major finding is the direct association of MMP-3 to CAD and to its cardiovascular complications and clinical outcomes. Effectively, MMP-3 revealed to be an important biomarker to CVD complications in patients with a history of CAD. MMP-3 could be serving as a predictive marker for a better treatment follow-up. An early performed analysis of MMP-3 level in patients provides a better diagnosis to avoid further complications.
References
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodeling. Nat Rev Mol Cell Biol 8:221–233. https://doi.org/10.1038/nrm2125
Galis ZS, Sukhova GK, Lark MW, Libby P (1994) Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 4(6):2493–2503. https://doi.org/10.1172/JCI117619
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516. https://doi.org/10.1146/annurev.cellbio.17.1.463
González-Pacheco H, Vargas-Barrón J, Vallejo M, Piña-Reyna Y, Altamirano-Castillo A, Sánchez-Tapia P et al (2014) Prevalence of conventional risk factors and lipid profiles in patients with acute coronary syndrome and significant coronary disease. Ther Clin Risk Manage 10:815
Heusch G, Libby P, Gersh B, Yellon D, Böhm M, Lopaschuk G et al (2014) Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383(9932):1933–1943. https://doi.org/10.1016/S0140-6736(14)60107-110
Brauer PR (2006) MMPs–role in cardiovascular development and disease. Front Biosci 11:447–478
Galis ZS, Sukhova GK, Lark MW, Libby P (1994) Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 94(6):2493–2503. https://doi.org/10.1172/JCI117619
Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T et al (2000) A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 102(16):1944–1949
Boluyt MO, O’Neill L, Meredith AL, Bing OH, Brooks WW, Conrad CH et al (1994) Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res 75(1):23–32
Rysä J, Leskinen H, Ilves M, Ruskoaho H (2005) Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension 45(5):927–933
Mittal B, Mishra A, Srivastava A, Kumar S, Garg N (2014) Matrix metalloproteinases in coronary artery disease. Adv Clin Chem 64:1–72
Jefferis BJ, Whincup P, Welsh P, Wannamethee G, Rumley A, Lennon L et al (2010) Prospective study of matrix metalloproteinase-9 and risk of myocardial infarction and stroke in older men and women. Atherosclerosis 208(2):557–563
Fertin M, Lemesle G, Turkieh A, Beseme O, Chwastyniak M, Amouyel P et al (2013) Serum MMP-8: a novel indicator of left ventricular remodeling and cardiac outcome in patients after acute myocardial infarction. PLoS ONE 8(8):e71280
Galis ZS, Sukhova GK, Kranzhofer R, Clark S, Libby P (1995) Macrophage foam cells from experimental atheroma constitutively produce matrix degrading proteinases. Proc Natl Acad Sci USA 92(2):402–406
Sundström J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB et al (2004) Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study. Eur Heart J 25(17):1509–1516
Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB et al (2001) Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation 104(16):1899–1904
Jefferis BJ, Whincup P, Welsh P, Wannamethee G, Rumley A, Lennon L, Thomson A et al (2010) Prospective study of matrix metalloproteinase-9 and risk of myocardial infarction and stroke in older men and women. Atherosclerosis 208(2):557–563
Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM et al (2006) Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 38(5):306–321. https://doi.org/10.1080/07853890600800103
Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH (2001) Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci 68(7):799–814
Li YY, Feldman AM, Sun Y, McTiernan CF (1998) Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation 98(17):1728–1734
DE Cutlip, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA et al (2007) Academic research consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115(17):2344–2351
K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease (2002) Evaluation, classification, and stratification Part 5 Evaluation of laboratory measurements for clinical assessment of kidney disease. Am J Kidney Dis 39:S76–S110
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease Study Group. Ann Intern Med 130(6):461–470
Levey AS, Greene T, Kusek J, Beck GJ, Group (2000) A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 11:A0828
Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT et al (2005) Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res 66(2):410–419. https://doi.org/10.1016/j.cardiores.2004.11.029
Libby P, Aikawa M, Jain MK (2006) Vascular endothelium and atherosclerosis. Handb Exp Pharmacol 176(2):285–306
Henney AM, Wakeley PR, Davies MJ, Foster K, Hembry R, Murphy G et al (1991) Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci USA 88(18):8154–8158
Libby P, Lee RT (2000) Matrix matters. Circulation 120:1874–1876
Luan Z, Chase AJ, Newby AC (2003) Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol 23(5):769–775. https://doi.org/10.1161/01.ATV.0000068646.76823.AE
Massaro M, Zampolli A, Scoditti E, Carluccio MA, Storelli C, Distante A et al (2010) Statins inhibit cyclooxygenase-2 and matrix metalloproteinase-9 in human endothelial cells: anti-angiogenic actions possibly contributing to plaque stability. Cardiovasc Res 86(2):311–320. https://doi.org/10.1093/cvr/cvp375
Izidoro-Toledo TC, Guimaraes DA, Belo VA, Gerlach RF, Tanus-Santos JE (2011) Effects of statins on matrix metalloproteinases and their endogenous inhibitors in human endothelial cells. Naunyn Schmiedebergs Arch Pharmacol 383(6):547–554
Davignon J (2004) Beneficial cardiovascular pleiotropic effects of statins. Circulation 109(23):39–43
Sahebkar A, Serban C, Ursoniu S, Mikhailidis DP, Undas A, Lip GY et al (2016) The impact of statin therapy on plasma levels of von Willebrand factor antigen. Systematic review and meta-analysis of randomised placebocontrolled trials. Thromb Haemost 115(3):520–532
Sahebkar A, Simental-Mendia LE, Pedone C, Ferretti G, Nachtigal N, Simona Bo S et al (2016) Statin therapy and plasma free fatty acids: a systematic review and meta-analysis of controlled clinical trials. Br J Clin Pharmacol 81(5):807–818
Senzaki H, Paolocci N, Gluzband YA, Lindsey ML, Janicki JS, Crow MT et al (2000) Beta-blockade prevents sustained metalloproteinase activation and diastolic stiffening induced by angiotensin II combined with evolving cardiac dysfunction. Circ Res 86(7):807–815. https://doi.org/10.1161/01.RES.86.7.807
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92(8):827–839. https://doi.org/10.1161/01.RES.0000070112.80711.3D
Ugwu F, Van Hoef B, Bini A, Collen D, Lijnen HR (1998) Proteolytic cleavage of urokinase-type plasminogen activator by stromelysin-1 (MMP-3). Biochemistry 37(20):7231–7236
Johnson JL, Dwivedi A, Somerville M, George SJ, Newby AC (2011) Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler Thromb Vasc Biol 31(9):35–44. https://doi.org/10.1161/ATVBAHA.111.225623
Roman MJ, Saba PS, Pini R, Spitzer M, Pickering TG, Rosen S et al (1992) Parallel cardiac and vascular adaptation in hypertension. Circulation 86(6):1909–1918
Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM et al (1996) Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J Biol Chem 271(22):13055–13060
Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ (2003) Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ Res 92(11):1254–1261. https://doi.org/10.1161/01.RES.0000076891.24317.CA
Beyzade S, Zhang S, Wong YK, Day IN, Eriksson P, Ye S (2003) Influences of matrix metalloproteinase-3 gene variation on extent of coronary atherosclerosis and risk of myocardial infarction. J Am Coll Cardiol 41(12):2130–2137
Cavusoglu E, Marmur JD, Kassotis JT, Yanamadala S, Chopra V, Eng C (2016) Usefulness of plasma matrix metalloproteinase-3 levels to predict myocardial infarction in men with and without acute coronary syndrome. Am J Cardiol 117(6):881–886. https://doi.org/10.1016/j.amjcard.2015.12.022
Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, Sarnak MJ (2009) Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis 204(1):298–303. https://doi.org/10.1016/j.atherosclerosis.2008.08.016
Zeisberg M, Neilson EG (2010) Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21(11):1819–1834. https://doi.org/10.1681/ASN.2010080793
Ogata Y, Enghild JJ, Nagase H (1992) Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem 267(6):3581–3584
Kang YS, Li Y, Dai C, Kiss LP, Wu C, Liu Y (2010) Inhibition of integrin-linked kinase blocks podocyte epithelial–mesenchymal transition and ameliorates proteinuria. Kidney Int 78(4):363–373. https://doi.org/10.1038/ki.2010.137
Barbaresko J, Koch M, Schulze MB, Nöthlings U (2013) Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev 71(8):511–527. https://doi.org/10.1111/nure.12035
Chase AJ, Newby AC (2003) Regulation of matrix metalloproteinase (matrixin) genes in blood vessels: a multi-step recruitment model for pathological remodelling. J Vasc Res 40:329–343. https://doi.org/10.1159/000072697
Acknowledgements
The study was supported by a grant from the “Ministry of High Education, Scientific Research and Technologies of Tunisia”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guizani, I., Zidi, W., Zayani, Y. et al. Matrix metalloproteinase-3 predicts clinical cardiovascular outcomes in patients with coronary artery disease: a 5 years cohort study. Mol Biol Rep 46, 4699–4707 (2019). https://doi.org/10.1007/s11033-019-04914-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04914-4