Abstract
Pueraria mirifica (PM) is a medicinal plant native to Thailand contained high amount of phytoestrogen and possesses anticancer activity. This study reports the effect of P. mirifica extract, phytoestrogen of diadzein and genistein for its benign prostate hyperplasia properties in testosterone-induced prostate hyperplasia in male Sprague Dawley rats. The P. mirifica extract was evaluated for its total phenols, flavonoid and antioxidant activity using DPPH, FRAP and metal chelating assay. The assessment of P. mirifica, diadzein and genistein against benign prostate hyperplasia was determined in testosterone-induced prostate hyperplasia in male Sprague Dawley rats. The total phenol was higher than flavonoid but showed low antioxidant activity of DPPH, FRAP and metal chelating. The aqueous PM extract at 1000 mg/kg significantly increased testosterone levels in testosterone-induced rats by 13% while diadzein and genistein increased it by 11% and 17% respectively. However, levels of FSH, LH, triglyceride and HDL are not affected by the oral administration of PM, diadzein and genistein to the rats. Similarly, total protein, albumin, globulin, total bilirubin, conjugated bilirubin, alkaline phosphatase, alanine aminotransferase, AST, and G-glutamyltransferase showed no significant difference as compared with negative control rats. The body weight of the rats, testis, kidney and liver showed no toxic effect. The zinc content increased significantly and the zinc transporter gen of ZnT4 and ZIP4 highly expressed suggesting that the PM, diadzein and genistein plays essential role in modulating prostate zinc homeostasis. Similarly, the expression of IL-6, AR and ER was significantly reduced indicating functioning in regulation of prostate growth and acts as anti-inflammatory role in preventing BPH. In conclusion, the results indicated that PM reduced BPH and contributed to the regulation in the zinc transport expression of the prostate cells in the benign prostate hyperplasia (BPH).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign prostatic hyperplasia (BPH) is an enlarged prostate that most commonly occur in elderly men [1]. It causes the lower urinary tract symptoms (LUTS) as a result of an increase in the total number of stromal and epithelial cells of the prostate gland. It has been reported that 50% men of more than 50 years of age show symptoms of BPH, and 45 million men are expected to be suffering from BPH by 2020 [2, 3]. Although the exact pathogenesis of BPH is still unclear, aging and androgens are known features of the growth and development of BPH in elderly men. A potent androgen DHT, which is produced from testosterone by the 5α-reductase [4] in the prostate promotes the growth of the prostate cells. Therefore, inhibition of 5α-reductase would lead to a reduction of DHT formation and prevent the development of BPH. Finasteride is a known synthetic 5α-reductase inhibitors are commonly used for BPH treatment [5]. However, it produced adverse effects such as gynecomastia due to its similarity in structure to steroid hormones [6]. At present, there are no natural plant compounds that are effective for curing BPH, which has been universally recognized. There are variety of dietary compounds has been shown associated with the decreasing of prostate cancer and BPH [7,8,9,10]. Therefore, new therapeutic methods are required, such as using medicinal plants for BPH treatment without adverse side effect from drug toxicity. There are several medicinal plants that possess anti-androgenic properties has been reported for the treatment of BPH [11,12,13,14] as well as able to inhibit growth of prostatic cancer cells sensitive to androgen and glucorticord hormones. Plants containing phytoestrogen compounds, especially genistein and diadzein, show the most potential for prostate hyperplasia treatment due to its ability to induce or mimic the action of oestrogen receptors [15, 16] as well as possessing anti-androgenic activities. Clemens et al. [17] reported on genistein being involved in the pathogenesis of BPH. Similarly, Denis et al. [18] and Griffiths et al. [19] has suggested that phytoestrogen is also involved in the development of BPH and prostate cancer. The genistein-inhibited BPH human cell culture [20], while genistein and diaizein induced apoptosis in prostate cancer [21]. There have been many human studies showing that that phytoestrogens inhibit BPH and the growth of prostate cancer in vitro and in vivo [22, 23]. Studies have also shown that phenols and flavonoids possessed an effects on BPH and prostate cancer. These compounds contributed its effects to BPH and prostate cancer via antioxidant properties in scavenging free radical produced by the cancer cells [24, 25].
The zinc is an important mineral required by the body metabolism process for growth and development [26]. The regulation of mechanism of zinc homeostasis involved the zinc transporter family of ZnT of solute link carrier 30(SLC30) and ZIP family of solute-linked carrier 39(SLC39) [27]. The SLC30 main role is to regulate the zinc ion to efflux from the cell cytoplasm into the intracellular lumen of organelles or outside of the cells. While for SLC39, it’s responsible to lets the zinc ions from extracellular space or organelle lumen influx into the cytoplasm [28, 29]. Hurley [30] reported that lack of zinc in the body may lead to stunted growth and serious metabolic disorder such as high rate of apoptosis in rapid cell turnover tissues. Several researches has reported that a low amount of zinc in cancer patient, indicating that there is correlation between amount of zinc and cancer development [31,32,33]. Kristal et al. [10] has suggested that the use of zinc supplement able to decrease the risk of prostate cancer. The prostate organ possess high amount of zinc as compared to other tissues organ in the body. In order to maintain a normal differential regulation of zinc intake in the cell, the ZnT and ZIP family of zinc transport expression must be activated to zinc cellular level. There was a down regulation of gene expression of ZIP and ZnT in prostate cancer tissue [34] and decreased in protein expression of ZnT4 [35] in prostate cancer tissue as compared to BPH tissue. This indicating that there is a decrease in expression of ZnT with the prostate cancer development. Therefore, it is essential to maintain high zinc content for prostate health and a low accumulation of zinc level may result in prostate cancer [34, 36].
Pueraria mirifica, from the family Leguminosae [37] has tuberous root containing many active phytoestrogens compounds such as diadzein, diadzin genestin, genistein, and puerarin [38,39,40]. In Thailand, it is traditionally consumed to treat menopausal symptoms. It possesses oestrogenic activity on rat bone [41], monkeys [42], and breast cancer in rats [43] by exerting its effect through estrogen receptor (ER) [44] and regulates the release of estrogen in BPH and prostate cancer [45, 46].
It has been reports that the phytoestrogen is effective in reducing prostate cancer and BPH [47]. The effect of phytoestrogen of diadzein and genistein in reducing BPH might involve in the gene expression of ZnT and ZIP family of zinc transporter to regulate the zinc homeostasis in the prostate organ.
In our previous work, we have reported the preventive effect of P. mirifica on testosterone induced prostatic hyperplasia in Sprague Dawley rats which showed that a dose-dependent reduction of prostate enlargement in testosterone-induced rats. The preventive effect is likely due to 5a-reductase inhibitory activity of the PM extract, daidzein and genistein [48]. In this study, the effect of the PM aqueous extract on testosterone-induced prostate hyperplasia in male Sprague–Dawley rats were evaluated.
Materials and methods
Plant materials
The dried tuberous root of P. mirifica was purchased from Anhui Bozhou Qiaocheng, Chinese Herbal Medicine Trading Center, Guangzhou, China. The distributor has confirmed it authentic and 100% purity. The root was ground into a fine powder and kept in airtight container prior to further study.
Preparation of PM extracts
The powdered PM was macerated with distilled water for 12 h at 40 °C. Aqueous extracts were filtered with Whatman filter paper (No.1) and the aqueous extract powder was obtained through vacuum-drying (yield—4.3% w/w).
High pressure liquid chromatography (HPLC)
The method used to separate diadzein and genistein was following as described by [43] with slight modification. The standards daidzein and genistein were purchased from Sigma (USA). The organic solvents for chromatography (HPLC grade) were purchased from Merck, Germany. Water with over 16 MΩ/cm for a component of the mobile phase of HPLC was prepared by ultrapure water system. HPLC system control and data processing were carried out using JASCO instrument. The reversed phase C18 column (250 nm × 4.6 nm) was used and 10 µL of PM extract was injected with the linear gradient system for 55 min from 100:0 to 55:45 with 0.1% acetic acid: acetonitrile at a flow rate of 1 mL/min and the compounds were detected at a wavelength of 254 nm.
Determination of total phenolic content
Total phenol content (TPC) was measured by the Folin–Ciocalteu method, as described by [49] with some modification. Briefly, 20 μL of sample extracts was mixed with 100 μL of Folin–Ciocalteu reagent (diluted 10-fold with distilled water) in a 96-well microplate and left incubated for 5 min at room temperature. Then, 75 μL of sodium carbonate solution (75 g/L) was added. After an incubation period of 2 h in darkness at room temperature, absorbance was measured at 740 nm with a microplate reader (Tecan Sunrise, Austria). Gallic acid monohydrate (1.0–20.0 mg/100 mL) was applied as a standard for calibration and construction of a linear regression line and water was used as blank. The total phenolic content was estimated as mg gallic acid equivalent (mg GAE)/g of dry extract.
Determination of total flavonoid content
Total flavonoid contents (TFC) were measured according to the method as described by [50]. Briefly, extracts (10 μL) were added with 60 μL of methanol and 10 μL of 10% aluminium chloride solution in a 96-well microplate. The solutions were well mixed and incubated for 6 min at room temperature. Then, 10 μL of potassium acetate (1 M) and 120 µL of methanol were added into the mixture. After 30 min of incubation, absorbance was measured at 415 nm with a microplate reader (Tecan Sunrise, Austria). Total flavonoid contents were estimated from quercetin (1.0–20.0 mg/100 mL) standard curve, and the results were expressed as mg quercetin equivalent (mg QE)/g of dry extract.
Antioxidant activity assays
DPPH radical scavenging activity
The free radical scavenging activity of the extracts was measured in terms of hydrogen donating ability, using DPPH radical as described by the [51]. with slight modification. Briefly, 40 μL of sample extracts of different concentrations (0.05–2 mg/mL) were mixed with 200 μL of 50μMDPPH solution in ethanol. The mixture was immediately shaken and incubated for 15 min in the dark at room temperature. The quenching of free radicals in absorbance was measured at 517 nm by a microplate reader (Tecan Sunrise, Austria). Ascorbic acid (1.56–200 µg/mL) was used as a standard and ethanol as the control. The percentage of scavenging activity of the extracts was calculated according to the following equation:
Percentage of scavenging activity for each concentration of the active extract was estimated from the graph plotted against the percentage inhibition and compared with the standard. The entire test was performed in triplicate.
Ferric reducing antioxidant power (FRAP)
The FRAP activities of the extracts were measured with a previously-described method by [52] with some modification. 20 mL of extracts in ethanol were mixed with 200 μL of daily prepared FRAP reagent, which contained 5 ml 10 mM TPTZ in 40 mM HCl, 5 mL of 20 mM FeCl3, and 50 mL of 0.3M acetate buffer (pH 3.6) in 96-well microplate. After 8 min of incubation time, the absorbance was measured at 595 nm with a microplate reader (Tecan Sunrise, Austria). Ethanol was used as blank. Ferrous sulfate (FeSO4) solution (0.2 mM–1 mM) was used for a standard calibration curve. The FRAP value was calculated according to the linear regression between standard solutions and the results were estimated as mmol Fe2+/g of dry extract from triplicated tests.
Metal chelating activity
The ferrous ion chelating activity of the extracts at different concentration was investigated according to the procedure as described by [51] by measuring the formation of the Fe2+-ferrozine complex. In this assay, extracts (100 μL) at different concentrations (20–100 μg/mL) were mixed with 120 µL distilled water and 10 μL FeCl2 (2 mM) in a 96-well microplate. The reaction was initiated by the addition of ferrozine (5 mM, 20 μL) to the mixture. The reaction mixture was incubated at room temperature for 20 min and absorbance at 562 nm was measured along with EDTA-Na2 (5–80 µg/mL) as a standard metal chelator. Ethanol (100 μL) was used as a control; blank was without ferrozine (20 μL of distilled water instead of ferrozine). The percentage inhibition of Fe2+–ferrozine complex formation was calculated according to the following formula:
The concentration of extracts required to chelate 50% of the Fe2+ ion (IC50) was calculated from the graph against the percentage of inhibition. All tests were performed in triplicate.
Experimental animals
Adult (12 weeks old) male Sprague Dawley rats, weighing 200–250 g, were purchased from Faculty of Veterinary, Universiti Putra Malaysia were used in this study. The rats were housed in polypropylene cages at room temperature (25 ± 2 °C) with light/dark cycles of 12 h in Animal House, University Malaya. The rats were fed with phytoestrogens deficient pellet diets (Altromin, Germany) and water ad libitum. After 7 days acclimatization, the rats were randomly distributed into experimental groups. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of National Institute of Health. The protocol was approved by the University of Malaya Animal Care and Use Committee (No: ISB/30/05/2012/SSM(R)). All efforts were made to minimize animal suffering.
Acute toxicity studies
Acute toxicity studies were carried out following OECD guidelines (OECD 423, Acute Toxic Class Method) [53]. In all cases, 2000 mg/kg oral dose of the P. mirifica extract, 200 mg/kg diadzein and genistein was found to be safe as no mortality was observed during the study. On the basis of this study, the doses of 10, 100, 1000 mg/kg were selected for P. mirifica. Whereas doses of 10, 100 mg/kg for diadzein and genistein respectively.
Administration and dosage
The powdered PM aqueous extract was suspended in distilled water for oral administration. Rats were given an oral dose of 10, 100 and (1000 mg/kg, p.o.) once daily for 30 days. Testosterone propionate was diluted in corn oil and injected subcutaneously at 3 mg/kg, daily for 30 days as described by [54]. Finasteride was suspended in Tween-20 (0.2% v/v) and administered per orally (1 mg/kg, p.o.). Daidzein and genestein were also included in the study in doses of 10 and (100 mg/kg, p.o.) and suspended in t
ween-20 (0.2% v/v) for p.o. administration.
Experimental design
The rats were divided in 10 groups consists of six rats in each group. Group 1: Sham (Control); Group 2: BPH (Testosterone induced BPH); Group 3: TFN (BPH + Fenisteride); Group 4: TLP (BPH + PM 10 mg/kg); Group 5: TMP (BPH + PM 100mg/kg); Group 6: THP (BPH + PM 1000mg/kg); Group 7: TLD (BPH + Diadzein 10 mg/kg); Group 8: THD (BPH + Diadzein 100 mg/kg); Group 9: TLG (BPH + Genistein 10 mg/kg); Group 10: THG (BPH + Genistein 100 mg/kg). The P. mirifica extract was suspended in distilled water for oral administration. Rats were given oral doses of 10, 100 and 1000 mg/kg once daily for 30 days. Testosterone propionate was diluted in corn oil and injected subcutaneously at 3 mg/kg, daily as described by [54]. Finasteride was suspended in Tween-20 (0.2% v/v) and administered orally (1 mg/kg, p.o). Daidzein and genistein were administered orally at doses of 10 and 100 mg/kg by suspending in Tween-20 (0.2% v/v). Prostate hyperplasia was induced by subcutaneous administration of testosterone (3 mg/kg) for 30 days in all groups except the control group. The rats were treated with vehicle Tween-20 (0.2% v/v, p.o.) or finasteride (1 mg/kg, p.o.), PM (10, 100 or 1000 mg/kg, p.o.), daidzein (10 or 100 mg/kg, p.o.) or genistein (10 or 100 mg/kg, p.o.) before administration of corn oil or testosterone (3 mg/kg), subcutaneously. Animals were weighed a day before the starting of the treatment and weekly thereafter. On day 30, animals were anesthetised ketamine and xylazine cocktail. The blood was collected by cardiac puncture and the organs were immediately dissected out and weighed.
Hormonal analysis
Blood was collected in the SST tube for hormonal analysis consists of testosterone, oestradiol, FSH and luteinizing hormone (LH). Serum was obtained by blood sample centrifugation at 1000 RCF for 10 min at 4 °C. The serums were taken to the Biohealth Science Laboratory, Institute of Biological Sciences, University Malaya for further analysis.
Biochemical tests
Blood was collected in the plain tubes. Serum was obtained through centrifugation at 1000 RCF for 10 min at 4 °C. Lipid profiles [total cholesterol, triglycerides, high-density lipoprotein (HDL) and low-density lipoprotein (LDL)] and liver function test (total protein, albumin, globulin, total bilirubin, conjugated bilirubin, alkaline phosphatase, alanine aminotransferase, AST and G-glutamyltransferase) were analyzed using the assay rat kit at the Biohealth Science Laboratory, Institute of Biological Sciences, University Malaya.
Prostate zinc analysis
Zinc concentration in the prostate tissue was determined by using an atomic absorption spectrophotometer (AAS), according to the method as described by [55]. Sample oxidation is accomplished by boiling 50 mg of formalinized prostate tissue in 25 mL water and 10 mL of a mixture of concentrated nitric acid (HNO3) and 60% perchloric acid (HClO4) in a ratio of 1:2. The samples were boiled until a clear solution appears. A final dilution of 50 mL with deionized water is performed and the sample is ready for AAS analysis.
Total RNA extraction, purification and quantifications
Purification of total RNA was carried out using formalin-fixed, paraffin embedded of a prostates tissue sample with RNeasy FFPE kit (Qiagen, Germany) according to the manufacturer’s protocol. The RNA was quantified by its absorption at 260 nm and its purity was determined by Nanodrop ND-100 spectrophotometer (Wilmington, USA). The purified RNA were aliquot before store at − 70 °C prior use.
Two step reverse transcriptase polymerase chain reaction (RTL PCR)
The expression of androgen receptor (AR), ER, interleukin 6, ZnT2 and Zip4 genes were evaluated by two steps RT-PCR (Qiagen, Germany). Expression of B-actin gene was used as internal control to ensure cDNA quality and loading accuracy. The specific primers (sense and antisense) used in the reaction were designed from NIH GenBank database. For each sample, 250 ng/µL of RNA solution were reverse transcripted into cDNA according to the manufacturer’s instructions (Invitrogen, USA). The reaction mixture was incubated at 42 °C for 30 min and then heated at 95 °C for 3 min. The resultant cDNA was used for PCR. The PCR reaction mix was prepared according to the manufacturer’s instructions with slight modification; 20 μL volume reactions were used instead of 50 μL volume reaction. Qualitative PCR amplification was performed with a 20 μL final volume consisting of 2 μL cDNA, 10 μL master mix (OnePCR, Taiwan), 1 μL of 10 pmol of each sense and antisense primer and 6 μL deionized water. The PCR was performed using BioRad iCycler (Bio-Rad) with reaction profile of; initial denaturation for 5 min at 940 °C and 30 cycles PCR amplification of denaturation at 940 °C for 40 s, annealing at 560 °C for 1 min and elongation at 720 °C for 2 min with a final elongation at 720 °C for 5 min. Subsequently, the PCR-amplified products were resolved by 2% agarose gel electrophoresis stained with ethidium bromide (Sigma, St Louis, USA) and visualized by UV transillumination (Vilber Lourmat, France). AR, ER, IL-6, ZnT2 and Zip4 expression in the test samples were normalized to the corresponding β-actin level and were reported as the relative band intensity to the β-actin gene expression.
Statistical analysis
Statistical analysis was performed with the use of Statistical Package for Social Science (SPSS) version 22.0. Significantly differences between means were analyzed by ANOVA test followed by Duncan’s and Dunnet’s post hoc test when appropriate. All results were expressed as the mean ± standard error (SEM). P < 0.05 was considered to be statistically significant.
Results
The results in Table 1 showed that the phenol contents of the PM aqueous extract (84.30 ± 0.12 mg GAE/g dry extract) are higher than flavonoid contents (0.62 ± 0.14 mg QE/g dry extract). The water extracts showed low antioxidant activity, with DPPH, FRAP and metal chelating at 24.91 ± 0.16%, 0.28 ± 0.11%, and 12.45 ± 0.12%, respectively, when compared with the antioxidant of standard gallic acid for DPPH, BHP for FRAP and EDTA for metal chelating with antioxidant activity at 82.09%, 1.389 and 99.89% respectively. The total phenols and total flavonoids do not correlate with the DPPH, FRAP and metal chelating activity. Cherdshewasart and Sutjitb [56] have reported that only isoflavonoid puerarin correlated with the antioxidant activity of DPPH in PM tubers and not with diadzein and genistein. The low antioxidant activity of the water extract could be due to the low content of the isoflavonoid puerarin in the PM [43, 56]. The low antioxidant activity of PM extract has been reported by [57].
The Sprague Dawley rats were induced to cause prostatic hyperplasia and treated with oral administration of PM extract, genistein and diazein. In, a testosterone-induced rats, the testosterone level increased significantly when compared with negative control and the testosterone-induced group treated with PM, diadzein and genestein, as illustrated in Table 2. The PM extracts at 1000 mg/kg increased significantly by 13%, whereas diadzein and genistein each at 100 mg/kg increased the testosterone level by 11% and 17% respectively. This showed that PM extracts at 1000 mg/kg have the capability to inhibit the high level of testosterone in the testosterone-induced rats. However, diadzein and genistein at 100 mg/kg demonstrated an ability to increase testosterone, which was slightly lower than PM extracts.
The total proteins were not significantly increased in the P. mirifica extract, diadzein and genistein as shown in Table 3. The total proteins were not significantly increased in the P. mirifica extract, diadzein and genistein as shown in Table 3. This shows that it had no effect on protein contents. Similarly, albumin and globulin do not show any significantly different after 30 days treatment with PM extract, diadzein and genistein. This gives indication that there are no damaged or infection of the liver of the rats. However, total bilirudin is slightly increased when treated with PM, diadzein and genistein. However, conjugated bilirudin remained constant and does not show significant difference level. PM, diadzein and genistein does not significantly alter the level of ALP, ALT and AST in the rats. Similarly, the GGT level remained the same throughout the treatment period. This indicates that the PM extract, genistein and diazein do not cause toxic effect of the organ of the rats.
The body weight of the rats in all groups was increased significantly within the period of 4 weeks (Table 4). The body weight of the rats in all groups was increased significantly within the period of 4 weeks. This showed that the PM extract promotes the growth and development of the rats. Similarly, the diadzein and genistein increased the body weight of the rats. However, the weight of the right and left testis reduced at high dose of PM as compared with the others rats group. The weight of the prostate organ, reduced significantly when treated with PM, diadzein and genistein, compared to testosterone-induced groups. This demonstrates that it gives effect to reduced benign prostatic hypertrophy (BPH) or prostate cancer in rats. The weight of kidney and liver did not show significant changes in weight compared with others in groups. This suggested that the intake of the PM extract, diadzein, and genistein does not cause damage to the organs of the rats.
The results in Fig. 1 shows that there was a significant decrease in prostatic zinc levels in the BPH group in comparison to sham and TFN group (P < 0.05). The TFN group have a significant similar level of prostatic zinc levels with the sham group. These indicated that the zinc concentrations in the prostate tissue were increased compared to the BPH group by 175%. Prostatic zinc levels in the testosterone-induced rat treated with P. mirifica extract were increased with increased dose compared to BPH group. TLP, TMP and THP group showed significant different when compared the prostatic zinc level with the BPH group at p < 0.05. Prostatic zinc levels in the TLP, TMP and THP were increased compared to BPH group at 113.46%, 213.46% and 155.77%, respectively. In comparison to the BPH group, the TLD, THD, TLG and THG groups showed a significant different at p < 0.05, where, the prostatic zinc levels were also increased at 119.23%, 71.15%, 148.07% and 94.23%, respectively. These results suggested that treatment with PM, phytoestrogen diadzein and genistein increase the zinc intake in the prostate cell.
Prostatic zinc content of rat groups. The histogram bars with different lower case letters (a, b, c) are significantly different at P < 0.05 (ANOVA, followed by Dunnett multiple comparison tests). a = P < 0.05 compared to sham control. b = P < 0.05 compared to BPH group. c = P < 0.05 compared to TFN group
Induction of BPH in rats significantly increased the number of mRNA tissue levels of IL-6 (Fig. 2), AR (Fig. 3) and ER (Fig. 4). The BPH group significantly showed a tremendous increase by 93.4% in IL-6 levels (Fig. 2) when compared to the sham group expression (P < 0.005). AR gene expression level also increased by 78.8% (Fig. 3) and oestrogen receptor (ER) gene level increase 78.2% (Fig. 4) in BPH group when compared to the sham group. The level of IL-6, AR and ERα genes in the TFN group significantly decreased when compared to BPH group (P < 0.005) by 90% (Fig. 2), 78% (Fig. 3) and 68% (Fig. 4), respectively.
Expression of IL-6 target genes in the ventral prostate of testosterone-induced rats after treatment. a The PCR product of IL-6 of all treated groups. (1) Sham group, (2) BPH, (3) TFN, (4) TLP, (5) TMP, (6) THP, (7) TLD, (8) THD, (9) TLG, and (10) THG. b The histogram bars with different lower case letters (a,b,c) are significantly different at P < 0.05 (ANOVA, followed by Dunnett’s multiple comparison tests). All values are expressed as mean ± SEM of relative band intensity (R.B.I) using β-actin as references. a = P < 0.05 compared to sham control. b = P < 0.05 compared to BPH group. c = P < 0.05 compared to TFN group
Expression of AR target genes in prostate rats after treatment. Expression of AR target genes in the ventral prostate of testosterone-induced rats after treatment. a The PCR product of IL-6 of all treated groups. (1) Sham group, (2) BPH, (3) TFN, (4) TLP, (5) TMP, (6) THP, (7) TLD, (8) THD, (9) TLG, and (10) THG. b The histogram bars with different lower case letters (a, b, c) are significantly different at P < 0.05 (ANOVA, followed by Dunnett’s multiple comparison tests). All values are expressed as mean + SEM of relative band intensity (R.B.I) using β-actin as references. a P < 0.05 compared to sham control. bP < 0.05 compared to BPH group. cP < 0.05 compared to TFN group
Expression of ER target genes in prostate rats after treatment. Expression of ER target genes in the ventral prostate of testosterone-induced rats after treatment. a The PCR product of IL-6 of all treated groups. (1) Sham group, (2) BPH, (3) TFN, (4) TLP, (5) TMP, (6) THP, (7) TLD, (8) THD, (9) TLG, and (10) THG. b The histogram bars with different lower case letters (a, b, c) are significantly different at P < 0.05 (ANOVA, followed by Dunnett’s multiple comparison tests). All values are expressed as mean + SEM of relative band intensity (R.B.I) using β-actin as references. aP < 0.05 compared to sham control. bP < 0.05 compared to BPH group. cP < 0.05 compared to TFN group
P. mirifica water extract treatment significantly decreased the IL-6 (Fig. 2), AR (Fig. 3) and ER (Fig. 4) with increased dose. The highest reduction between three doses of P. mirifica water extract can be seen in THP group (1000 mg/kg BW), where the reduction were 89% for IL-6, 64% for AR and 66% for ERα when compared to BPH group (P < 0.005). The medium dosage of P. mirifica water extract, the TMP group (100 mg/kg BW), showed an 88% reduction in IL-6, 55% reduction in AR and 39% reduction in ERα. While, the lowest dose of P. mirifica water extract, the TLP group (10 mg/kg BW) showed 63% reduction in IL-6, 45% reduction in AR and 8% reduction in ERα when compared to BPH group.
Daidzein treatment of testosterone-induced BPH rats showed a reduction in IL-6 mRNA tissue levels of 60%, 43% reduction in AR and 58% reduction in ERα for low dosage (TLD). While, 54% reduction in IL-6, 17% reduction in AR and 56% reduction in ERα for high dosage (THD). Whereas, for genistein treatment of testosterone-induced BPH rat groups showed that a reduction in IL-6 mRNA tissue levels of 47%, 23% reduction in AR and 58% reduction in ERα for low dosage (TLG). While, for high dosage (THG), 54% reduction in IL-6 level expression, 14% reduction in AR gene level expression and 50% reduction in ERα when compared to the BPH group. All observation was significantly differed when compared to the BPH group at p < 0.05. This results clearly suggested that the treatment of the testosterone-induced rats with PM, diadzein and genistein reduced the gene expression of IL-6, AR and ER in reducing BPH development.
The induction of BPH was significantly changed the amount of mRNA level of ZIP4 (Fig. 5) and ZnT2 (Fig. 6) between the group. Based on the Fig. 4, the Zip4 gene level of all treatment of testosterone-induced BPH rats group showing an increased level of Zip4 except in BPH group. The BPH group showed a significant decrease of Zip4 gene level compared to the sham group (P < 0.05). While the TFN group, showed an increased level of Zip4 when compared to BPH group with 96% increment. The P. mirifica water extract treatment (TLP, TMP and THP) showed similar pattern to TFN group, where zip4 level were increased compared to BPH group with 56%, 82% and 58% increment, respectively. The outcome in the daidzein and genistein-treated groups also showed an increased level of Zip4 when compared to BPH group (P < 0.05).
Expression of Zip4 target genes in prostate rats after treatment. Expression of Zip4 target genes in the ventral prostate of testosterone-induced rats after treatment. a The PCR product of IL-6 of all treated groups. (1) Sham group, (2) BPH, (3) TFN, (4) TLP, (5) TMP, (6) THP, (7) TLD, (8) THD, (9) TLG, and (10) THG. b The histogram bars with different lower case letters (a, b, c) are significantly different at P < 0.05 (ANOVA, followed by Dunnett’s multiple comparison tests). All values are expressed as mean + SEM of relative band intensity (R.B.I) using β-actin as references. aP < 0.05 compared to sham control. bP < 0.05 compared to BPH group. cP < 0.05 compared to TFN group
Expression of ZnT2 target genes in prostate rats after treatment. Expression of ZnT2 target genes in the ventral prostate of testosterone-induced rats after treatment. a The PCR product of IL-6 of all treated groups. (1) Sham group, (2) BPH, (3) TFN, (4) TLP, (5) TMP, (6) THP, (7) TLD, (8) THD, (9) TLG, and (10) THG. b The histogram bars with different lower case letters (a, b, c) are significantly different at P < 0.05 (ANOVA, followed by Dunnett’s multiple comparison tests). All values are expressed as mean ± SEM of relative band intensity (R.B.I) using β-actin as references. aP < 0.05 compared to sham control. bP < 0.05 compared to BPH group. cP < 0.05 compared to TFN group
Based on the Fig. 6, the ZnT2 gene level of all treatment of testosterone-induced BPH rats group showed a significant increased level of ZnT2 when compared to the sham group (P < 0.05). The BPH group showed an increase of ZnT2 level compared to sham group. While the TFN, P. mirifica water extract treated group (TLP, TMP and THP), daidzein and genistein-treated groups showed insignificant differences in level of ZnT2 mRNA when compared to BPH group (P < 0.05).
Discussion
The uses of medicinal plants for the treatment of prostate hyperplasia have become one of the important treatments. There are many medicinal plants which have been shown to be effective for the treatment of prostate hyperplasia, such as Saw palmetto and Ganoderma lucidum. In most cases, these are taken in the form of aqueous extracts, either as decoction or as infusions. Since this commonly practice method due to its easy, cheap and simple way of intake in traditional medicinal plants for prostatic hyperplasia treatment, in this study, the aqueous extract of PM tuberous root was fed into testosterone-induced prostate hyperplasia in male Sprague–Dawley rats to evaluate its effectiveness for prostatic hyperplasia treatment. At present, there is no report or research has been carried out of PM tuberous root on neither prostate hyperplasia nor prostate cancer. The increasing of the testosterone level could be due to the inhibition of 5α-reductase that will prevent DHT being synthesized from testosterone by the PM extract. This agreed with reports [54] which also showed that administration of Ganoderm lucidum in rats increased the testosterone level. Similarly, Agraval et al. [58] reported that Echinops echinatus increased serum testosterone concentration in adult male Sprague–Dawley rats. The PM, diadzein and genistein may also show antiandrogenic activity in the testosterone-induced rats by inhibiting the 5α-reductase enzyme that reduced the formation of DHT from testosterone. The PM extracts, diadzein and genistein, significantly increased the oestradiol level when compared to the testosterone-induced rat group. This may be due to the activation of aromatease CYP19A1 to convert the testosterone into estradiol. This indicates that the oestradiol is indirectly involved in the regulation of prostate hyperplasia through the repression of the hypotalamine–pituitary–gonad axis and directly affect the testis [59] in reducing the development of prostate cancer. The previous researcher reported that estrogen may help keep the prostate gland from proliferating abnormally. They also showed that PM, diadzein, and genistein have an effect on the alpha estrogen receptor (ERα) in releasing oestradiol hormone. The PM extract activates the ERα more than diadzein and genistein in testosterone-induced rats. The genistein has been shown to inhibit the growth of human and rat prostate cell lines, preventing the development of prostate cancer [60].
The PM extracts, diadzein and genistein does not show any significant effect on the FSH and LH hormones. This gives an indication that it maintained FSH and LH level in preventing the formation of prostate hyperplasia. However, genistein of soy phytoestrogen decreased serum levels of LH in polycystic ovary syndrome (PCOS) women [61]. However, observations by Amani et al. [62] have shown that FSH, HDL and triglyceride level did not change significantly in hypercholesterolemic men treated with purified alcohol-extract soy protein isoflavones (SFI). The result in Table 2 showed the amount of triglycerides is increased slightly with P. mirifica at high doses, but not with diadzein and genistein. Total cholesterol showed no effect with Pueria mirifica, diadzein and genistein. Similarly, Pueria mirifica, diadzein and genistein did not make any significant difference in HDL level of testosterone-induced rats. This demonstrates that during the development of prostate hyperplasia, the treatment with PM, diadzein and genistein does not affect triglycerides, total cholesterol, and HDL level, but keep maintain during the treatment period. This would help to suppress the development of the prostate hyperplasia. In this way it could help prevent the triglycerides and cholesterol from being used or converted to testosterone during the cholesterol metabolism that eventually lead to an increase in the circulating testosterone level which may further convert into dihydrotestosterone (DHT). Kalo et al. [63] reported that cholesterol biosynthesis simvastatin reduces the testosterone level in normal rats. The genistein and diadzein inhibited hepatocyte apoβ in cultures, human hepatoma (HepG2) cell in cholesterol synthesis [64].
It is known that 5α-reductase catalyses the testosterone conversion into DHT [65,66,67] and then DHT binds to AR within the prostate to regulate the prostate growth. Therefore, when the expression of AR becomes excessive, it may associate with the pathogenesis of BPH in aging men [68, 69]. The oestrogen receptor increases the expression with increasing age. In the normal prostate, ERα is not expressed in the prostate epithelial cells. However, the expression of ERα is elevated in BPH [70]. In current study, RT-PCR results revealed that P. mirifica water extracts administered groups showed a significant reduction in AR and ER expression in the prostate tissue of the testosterone-induced BPH rats when compared to the BPH model group. The daidzein groups and the genistein groups, as well as finasteride group also showed a significant reduction in the AR and ER expression. This suggested that P. mirifica water extract able to down-regulate the gene expression of AR and ER in BPH. Inflammation plays a crucial role in BPH. Differential expressions of cytokine have been highlighted in BPH tissue suggesting a role for inflammation in the BPH propagation [71, 72]. However, in the current study revealed an increase of IL-6 expression levels in the prostate samples harvested from the BPH model rat compared to the sham animals. In contrary, the administration of P. mirifica water extracts, daidzein and genistein, as well as finasteride significantly reduced IL-6 levels. This concluding that P. mirifica has an anti-inflammatory role in preventing BPH.
Zinc is one the essential markers for normal function of prostate. Mamalian prostate gland contains extremely high levels of zinc compared to the other organs [73]. There is a strong association between zinc and prostate cancer development been reported, where low zinc status been observed in cancer patients [74]. Low zinc levels in the prostate also associated with aging and decreased fertility [75]. The Zip4 is one of the important zinc transporters. The expression of Zip4 gene have been expressed in BPH and prostate cancer samples in human [76]. The zinc concentration in the prostate as shown in Fig. 7 revealed that BPH model rats significantly reduced when compared to the sham group. Whereas the P. mirifica water extracts, daidzein and genistein, as well as finasteride groups showed significantly increased zinc level in the prostate compared to the BPH rats’ model. This showed that the treatment improved the prostate zinc concentration level and it suggests maintaining the prostate health well-being. Our study consistent with the previous study reported that tissue zinc is substantially lowered in the BPH compared to the normal prostate [77, 78]. Michelle et al. [79] have also reported that zinc contributed in the prevention of prostate cancer and benign prostate hyperplasia. In our study, the Zip4 level was significantly decreased in BPH model rats when compared to sham rats. The administration of P. mirifica water extracts, daidzein and genistein, as well as finasteride significantly increased the Zip4 level when compared to the BPH model rats. While for ZnT2 gene expression result showed BPH model rats as well as the rats that receive P. mirifica water extracts, daidzein and genistein, and finasteride significantly increased the ZnT2 level compared to the sham group. The HPLC analysis of the PM water extract showed the presence of daidzein and genistein (Fig. 6) which clearly suggesting that the zinc transporter expression is due the contribution of diadzein and genestein. The reverse transcription polymerase chain reaction (RT-PCR) analysis was carried out to study the effect of the treatment on IL-6, AR, ERα, Zip4 and ZnT2 expression. IL-6 is one of the major physiologic mediators of acute phase reaction that influence immune response and inflammatory reactions. AR plays a key role in the androgen signal transduction system, mediating the androgen biological activity and plays a role in BPH development and occurrence. Oestrogen level are elevated with increase of age may result in increase of ER-α expression. In normal prostate, ERα is not expressed but the expression increase with the incidence of BPH.
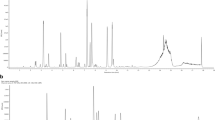
HPLC profile of aP. mirifica aqueous extract and b standard (1) daidzein (2) genistein. This figure Shows the HPLC profile of the PM aqueous extract and it contained diadzein at 5.1 mg/100 g dry weight and genistein at 2.3 mg/100 g dry weight. These were used as bookmaker to characterize and calibrated the concentration of the doses of the PM aqueous extracts that was administered to the rats at 10 mg/kg, 100 mg/kg and 1000 mg/kg body weight
In summary, the present findings showed that the testosterone-induced rats in hyperplasia prostate when treated with P. mirifica, diadzein and genistein showed increased in zinc concentration in the prostate cells. The increased of zinc was supported by the increased of ZnT and ZIP expression that activated the zinc transporter to influx zinc into the prostate cell. Similarly, the expression of IL-6, AR and ER was decreased indicating that there is reducing of inflammation in the BPH development.
Conclusion
The P. mirifica extract showed that it possessed the ability to reduce the testosterone that indirectly inhibited the growth of prostate hyperplasia. It increased the eostradiol level that leads to the decrease the formation of prostate cancer and does not affect the FSH, LH, triglycerides, total cholesterol, HDL and liver function enzymes indicating that there was no damage to the liver. The weight of the rats’ body, testis, prostate, kidney and liver were not affected by intake of P. mirifica extract. Thus, the results of the study showed that the P. mirifica extract able to reduce benign prostate hyperplasia (BPH) and prostate cancer.
Abbreviations
- PM:
-
Pueraria mirifica
- BPH:
-
Benign prostatic hyperplasia
- PW:
-
Prostatic weight
- PI:
-
prostatic index
- mRNA:
-
Messenger ribonucleic acid
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- FRAP:
-
Ferric ion reducing antioxidant power
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- HDL:
-
High density lipid
- AST:
-
Aspartate aminotransferase
- ZnT4:
-
Zinc transporter ZnT4
- ZIP4:
-
Zrt-Irt-like protein
- IL-6:
-
Interleukin 6
- AR:
-
Androgen receptor
- ER:
-
Estrogen receptor
References
Beduschi MC, Beduschi R, Oesterling JE (1999) Alpha-blockade therapy for benign prostatic hyperplasia: from a nonselective to a more selective alpha1A- adrenergic antagonist. Urology 51:861–872
Napalkov P, Maisonneuve P, Boyle P (1995) Worldwide patterns of prevalence and mortality from benign prostatic hyperplasia. Urology 46(3A):41–46
Flannery MT, Ramsdell J, Ranhosky A, Davidai G, Ruoff G (2006) Efficacy and safety of tamsulosin for benign prostatic hyperplasia: clinical experience in the primary care setting. Curr Med Res Opin 22:721–730
Russell DW, Wilson JD (1994) Steroid 5-reductase: two genes/two enzymes. Ann Rev Clin Biochem 63:25–26
Vaughan D, Imperato-McGinley J, McConnell J, Matsumoto AM, Bracken B (2002) Long-term (7–8-year) experience with finasteride in men with benign prostatic hyperplasia. Urology 60:1040–1044
Uygur MC, Gur E, Arik AI, Altug U, Erol D (1998) Erectile dysfunction following treatments of benign prostatic hyperplasia: a prospective study. Andrologia 30:5–10
Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional prevention of cancer study group. JAMA 276:1957–1963
Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC (2002) A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst 94:391–398
Rohrmann S, Giovannucci E, Willett WC, Platz EA (2007) Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr 85:523–529
Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE (1999) Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol Biomark Preview 8:887–892
Takeuchi T, Nishii O, Okamura T, Yaginuma T (1991) Effect of paeoniflorin, glycyrrhizin and glycyrrhetic acid on ovarian androgen production. Am J Chin Med 19(1):73–78
Grant P, Dworakowska D (2012) Tea and Diabetes: the laboratory and the real world. In: Preedy V (ed) Tea in health & disease prevention. Elsevier Academic Press, New York
Boyle P, Robertson C, Lowe F, Roehrborn C (2004) Updated meta-analysis of clinical trials of Serenoa repens extract in the treatment of symptomatic benign prostatic hyperplasia. BJU Int 93(6):751–756
Wilt T, Ishani A, Mac Donald R (2002) Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Revis 3:CD001423
Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggbald J (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870
Setchell KDR, Cassidy A (1999) Dietary isoflavones—biological effects and relevance to human health. J Nutr 129:758S–767S
Clemens B, Karin AP, Arin P, Klaus P, Marco A (2004) Phytoestrogen tissue levels in benign prostatic hyperplasia and prostate cancer and their association with prostatic diseases. Urology 64(4):707–711
Denis L, Morton MS, Griffiths K (1999) Diet and its preventive role in prostatic diseases. Eur Urol 35:377–387
Griffiths K, Denis L, Turkes A (2002) Oestrogens, phytoestrogens and the pathogenesis of prostatic diseases. Martin Dunitz, London
Geller J, Sionit L, Partido C, Li L, Tan X (1998) Genistein inhibits the growth of human-patient BPH and prostate cancer in histoculture. Prostate 34:75–79
Hsu A, Bray TM, Helferich WG, Doerge DR, Ho E (2010) Differential effects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp Biol Med 235:90–97
Stephens FO (1999) The rising incidence of breast cancer in women and prostate cancer in men. Dietary influences: a possible preventative role for nature’s sex hormone modifiers: the phytoestrogens. Oncol Rep 6:865–870
Choi YH, Lee WH, Park KY, Zhang I (2000) p53-Independent induction of p21 (WAF1/CIPI), reduction of cyclin B1 and G2/M arrest by the isoflavone genistein in human prostate carcinoma cells. Jpn J Cancer Res 91:164–173
Vance TM, Su J, Fontham ET, Koo SI, Chun OK (2013) Dietary antioxidants and prostate cancer. Nutr Cancer 65(6):793–801
Lall RK, Syed DN, Khan MI, Adhami VM, Gong Y, Lucey JA, Mukhtar H (2015) Dietary polyphenols in prevention and treatment of prostate cancer. Int J Mol Sci 16:3350–3376
Taylor KM, Morgan HE, Smart K, Zahar NM, Pumford S, Ellis IO, Nicholson RI (2007) The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med 13(7–8):396–406
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281:24085–24089
Eide DJ (2006) Zinc transporters and the cellular trafficking of zinc. Biochim Et Biophys Acta 1763(7):711–722
Liuzzi JP, Cousins RJ (2004) Mammalian zinc transporters. Annu Rev Nutr 24:151–172
Hurley LS (1981) Teratogenic aspects of manganese, zinc, and copper nutrition. Physiol Rev 61(2):249–295
Michael F, Leitzmann MJ, Stampfer K, Wu (2003) Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst 95(13):1004–1007
Christudoss P, Selvakumar R, Fleming JJ, Gopala Krishnan G (2011) Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J Urol 27(1):14–18
Rahman MT, Mowladad C (2016) Zinc and benign prostatic hyperplasia (BPH) & prostate cancer (PCa) association. Med Res Arch 4:7
Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC (2005) hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer 4:32
Henshall SM, Afar DE, Rasiah KK, Horvath LG, Gish K, Caras I (2003) Expression of the zinc transporter ZnT4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene 22:6005–6012
Huang L, Kirschke CP, Zhang Y (2006) Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int 6:10
Kashemsanta MCL, Suvatabandhu K, Airy SHK (1952) A new species of Pueraria (Leguminosae) from Thailand, yielding an oestrogenic principle. Kew Bull 7:263–266
Chansakaow S, Ishikawa T, Sekine K, Okada M, Higuchi Y (2000) Isoflavonoids from Pueraria mirifica and their estrogenic activity. Plant Med 66:572–575
Chansakaow S, Ishikawa T, Seki H, Sekine K, Okada M (2000) Identification of deoxymiroestrol as the actual rejuvenating principle of “Kwao Keur”, Pueraria mirifica. The known miroestrol may be an artefact. J Nat Prod 63:173–175
Cherdshewasart W, Panriansaen R, Picha P (2007) Pretreatment with phytoestrogen-rich plant decreases breast tumor incidence and exhibits lower profile of mammary ERalpha and ERbeta. Maturitas 58(2):174–181
Urasopon N, Hamada Y, Asaoka K, Cherdshewasart W, Malaivijitnond M (2007) Pueraria mirifica, a phytoestrogen-rich herb, prevents bone loss in orchidectomized rats. Maturitas 56:322–331
Malaivijitnond SH, Cherdshewasart W, Watanabe G, Taya K (2007) The influence of Pueraria mirifica herb containing phytoestrogens on the urinarygonadotropin and estradiol levels in aged menopausal monkeys. Anim Sci J 78(4):378–386
Cherdshewasart W, Subtang S, Dahlan W (2007) Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J Pharmacol Biomed Anal 43:428–434
Turner JV, Snezana AK, Beverley DG (2007) Molecular aspects of phytoestrogen selective binding of estrogen receptors. J Pharm Sci 96(8):1879–1885
Härkönen PL, Mäkelä SI (2004) Role of estrogens in development of prostate cancer. J Steroid Biochem Mol Biol 92(4):297–305
Soronen P, Laiti M, Törn S, Härkönen P, Patrikainen L (2004) Sex steroid hormone metabolism and prostate cancer. J Steroid Biochem Mol Biol 92(4):281–286
Feng Y, Xia XY, Huang YF (2007) Effects of phytoestrogens on prostate cancer and benign prostatic hyperplasia. Zhonghua Nan Ke Xue 13(5):57–61
Masrudin SS, Mohamad J (2015) Preventive effect of Pueraria mirifica on testosteroneinduced prostatic hyperplasia in sprague dawley rats. Andrologia 47:1153–1159
Singleton VL, Rossi JAJ (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. J Enol Vitic 16:144–158
Chang ST, Wu JH, Wang SY, Kang PL, Yang NS (2001) Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem 49:3420–3424
Ablat A, Mohamad J, Awang K, Jamil AS, Aditya A (2014) Evaluation of antidiabetic and antioxidant properties of Brucea javanica seed. Sci World J. https://doi.org/10.1155/2014/786130
Müller L, Susanne G, Anne MP, Volker B (2010) Antioxidant capacity and related parameters of different fruit formulations. Food Sci Technol 43:992–999
OECD 423 (2001) OECD guidelines for testing of chemical acute oral toxicity-acute toxic class method adopted: 17th December 2001
Nahata A, Dixit VK (2012) Ganoderma lucidum is an inhibitor of testosteronw-induced prostatic hyperplasia in rats. Andrologia 44:160–174
O’Halloran J, Walsh AR, Fitzpatrick PJ, Christiansen N, Mathrani IM, Ahring BK (1998) The Determination of trace elements in biological and environmental samples using atomic absorption spectroscopy. In: Sheehan D, Totowa NJ (eds) Methods in biotechnology, Vol. 2. Humana Press, New York, pp 201–211
Cherdshewasart W, Sutjitb W (2008) Correlation of antioxidant activity and major isoflavonoid contents of the phytoestrogen-rich Pueraria mirifica and Pueraria lobata tubers. Phytomedicine 15:38–43
Buran P, Supan P (2007) Antioxidant capacities of Pueraria mirifica, Stevia rebaudiana Bertoni, Curcuma longa Linn., Andrographis paniculata (Burm.f.) Nees. and Cassia alata Linn. for the development of dietary supplement. Kasetsart J 41:548–554
Agraval M, Nahata A, Dixit VK (2012) Protective effects of Echinatus on testosterone-induced prostatic hyperplasia in rats. Eur J Integr Med 4:e177–e185
Huggins C, Hodges CV (1941) Studies on prostate cancer: effect of castration, of estrogen, and of androgen injection on serum phosphatase in metastatic carcinoma of the prostate. Cancer Res 1:293–297
Wang J, Isam-Eldin E, Lamartiniere CA (2002) Dietary genistein suppresses chemically induced prostate cancer in Lobund-Wistar rats. Cancer Lett 186:11–18
Khani B, Mehrabian F, Khalesi E, Eshraghi A (2011) Effect of soy phytoestrogen on metabolic and hormonal disturbance of women with polycystic ovary syndrome. J Res Med Sci 16(3):297–302
Amani R, Zand-Moghaddam A, Jalali MT, Hatamizadeh MA (2002) Effects of soy protein isoflavones on lipid profile and serum hormones in hypercholesterolemic men. Biochem J 366(2):531–539
Kalo MS (2009) Effect of cholesterol biosynthesis inhibitor on some biochemical parameters in normal male rats. Iraqi J Vet Sci 23(1):5–12
Nica MB, Linda ED, Lisa JW, Jane YE, Murray WH (2002) Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem J 366:531–539
Andriolep GL, Humphrey P, Ray P, Gleave ME, Trachtenberg J, Thomas LN, Lazier CB, Rittmaster RS (2004) Effect of the dual 5α-reductase inhibitor dutasteride on markers of tumor regression in prostate cancer. J Urol 172(3):915–919
Bartsch G, Rittmaster RS, Klocker H (2002) Dihydrotestosterone and the concept of 5α-reductase inhibition in human benigh prostatic hyperplasia. World J Urol 19:413–425
Steers WD (2001) 5a-reductase activity in the prostate. Urology 58:17–24
Izumi AM, Wen-JyeLin, Kuo-PaoLai, Chawnshang C (2013) Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol 182(6):1942–1949
Suzuki S, Platz EA, Kawachi I (1992) Benign prostatic hyperplasia (BPH) is a common problem among older men. J Clin Endocrinol Metab 75:1022
Royuela M, de Miguel MP, Bethencourt FR, Sánchez-Chapado M, Fraile B, Arenas MI, Paniagua R (2001) Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol 168(3):447–454
Minutoli L, Altavilla D, Marini H, Rinaldi M, Irrer N, Pizzino G, Morgia G (2014) Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: effects of serenoa repens, selenium and lycopene. J Biomed Sci 21:19
Rick FG, Schally AV, Block NL, Halmos G, Perez R, Fernandez JB, Vidaurre I, Szalontay L (2011) LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate 15(7):736–747
Costello LC, Franklin RB, Feng P, Tan M, Omar Bagasra (2005) Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States). Cancer Causes Control 16(8):901–915
Federico A, Lodice P, Federico P, Del Rio A, Mellone MC, Catalano G, Federico P (2001) Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. Eur J Clin Nutr 55(4):293–297
Elzanaty S (2007) Association between age and epididymal and accessory sex gland function and their relation to sperm motility. Arch Androl 53:149–156
Chen QG, Zhang Z, Yang Q, Shan GY, Yu XY, Kong CZ (2012) The role of zinc transporter ZIP4 in prostate carcinoma. Urol Oncol 30(6):906–911
Christudoss P, Selvakumar R, Joseph FJ, Ganesh Gopalakrishnan (2011) Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J Urol 27(1):14–18
Malm J, Hellman J, Hogg P, Lilia H (2000) Enzymatic action of prostate-specific antigen (PSA or hK3): substrate specificity and regulation by Zn (2+), a tight-binding inhibitor. Prostate 45(2):132–139
Michelle Y, Karin H, Emily H (2010) Differential response to zinc-induced apoptosis in benign prostate hyperplasia and prostate cancer cells. J Nutr Biochem 21:687–694
Acknowledgements
We are grateful to the Institute of Biological Sciences for providing laboratory facilities to conduct the research works.
Funding
This research was supported by the University of Malaya, Grant No. PV010-2012A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Ethical approval
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of National Institute of Health. The protocol was approved by the University of Malaya Animal Care and Use Committee (No: ISB/30/05/2012/SSM(R)).
Informed consent
Informed consent was obtained from all researches participates in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamad, J., Masrudin, S.S., Alias, Z. et al. The effects of Pueraria mirifica extract, diadzein and genistein in testosterone-induced prostate hyperplasia in male Sprague Dawley rats. Mol Biol Rep 46, 1855–1871 (2019). https://doi.org/10.1007/s11033-019-04638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04638-5