Abstract
There is a growing body of evidence about metformin being effective in cancer therapy. Despite controversies about the ways of its effectiveness, several ongoing clinical trials are evaluating the drug when used as an adjuvant or a neo-adjuvant agent. We aimed to investigate metformin’s effects on proliferation, metastasis, and hormone receptor expressions in breast cancer cell line MCF-7 incubated in two different glucose conditions. MCF-7 cells were incubated in high or low glucose media and treated with various doses of metformin. The cell viability was studied using MTT test. The Ki-67, estrogen and progesterone receptor expression were evaluated by ICC and galectin-3 expression was evaluated by ELISA or spectrophotometrically. The cell viability following consecutive metformin doses in either glucose condition for 24 and 48 h represented a significant decrease when compared to control. The proliferation detected in low glucose medium following metformin at doses < 20 mM was found significantly decreased when compared to high glucose medium at 48 h. In terms of galectin-3 levels, the increase in high glucose medium treated with metformin and the decrease in low glucose medium were found statistically significant when compared to control. Progesterone receptor staining demonstrated a significant increase in low glucose medium. Our findings represent better outcomes for cancer lines incubated in low glucose medium treated with metformin in terms of viability, receptor expression and metastatic activity, and highlight the potential benefit of metformin especially in restraining the cancer cell’s ability to cope energetic stress in low glucose conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperinsulinemia is shown to be an independent risk factor for cancer development in type II diabetes mellitus [1]. Hyperinsulinemia due to either insulin resistance or exogenous insulin injections is proven to induce the sensitivity of chemically induced carcinogenesis in animals and exogenous insulin and/or its secretagogues are shown to be related with increased cancer risk and cancer recurrence in human [2].
Hyperinsulinemia promotes carcinogenesis either directly via insulin receptors on relatively sensitive epithelial cells or indirectly via IGF, sex hormones, inflammatory processes and adipokines [2]. Apart from normal tissues, cancer cells cannot downregulate insulin receptors in case of hyperinsulinemia [2, 3]. Insulin–insulin receptor interaction on cancer cells activates proliferation and anti-apoptotic pathways via PI3K and MAPK pathways [2,3,4]. IGF-1 receptor (IGF1R) is overexpressed on cancer cells and bound either insulin or IGF-1 [2, 3, 5]. Hyperinsulinemia results elevated circulatory IGFs due to insulin–IGF1R binding and active IGFs result higher mitogenic activity via both IGF1R related pathways and PI3K and MAPK activation [2, 6]. Hyperinsulinemia or insulin resistance decreases the production of sex hormone binding globulins and increases circulatory sex hormones that ease sex-related cancer development like breast cancer. Moreover, insulin and inflammation are interacted in a reciprocal way. Hyperglycemia and insulin regulates inflammatory response while inflammation provokes insulin resistance. Adipokines like leptin, adiponectin are secreted from macrophages at the tissue level with cytokines and all result in an increased insulin resistance and exacerbated inflammatory response [2, 7].
Metformin is a biguanide antihyperglycemic agent used in the treatment of type II diabetes mellitus. It is known to inhibit the complex I of oxidative phosphorylation in mitochondria and thereby activate AMP-activated protein kinase (AMPK), the central energy sensor of the cell. Its upstream activator liver kinase B1 (LKB1) and AMPK, by increasing the cellular AMP/ATP ratio, inhibit hepatic gluconeogenesis and decrease glucose output [8, 9]. Moreover activation of AMPK, while causing no change at muscular basal glucose level, increases uptake of insulin activated glucose, thereby effecting insulin resistance in a positive manner. For the last decade, the retrospective studies evaluating coexistence of cancer and diabetes exposed a fact that metformin treatment for type II diabetes is related with a reduction of cancer incidence and cancer related mortality [10]. Despite the controversies of its main effect on cancer therapy, hundreds of ongoing clinical trials are evaluating anticancer effects of metformin when used as an adjuvant or a neo-adjuvant chemotherapeutic agent.
To our knowledge metformin, as an anticancer agent, is capable of controlling the growth of various cancer cells both in vivo and in vitro in a time and dose dependent manner. This effect is mainly controlled by upstream activator LKB1 and AMPK [11, 12]. This activation inhibits downstream mammalian target of rapamycine (mTOR) and S6 kinase (S6K1) that regulate the protein translation of cellular growth regulators like cyclinD1, HIF1α and MYC [13]. mTOR inhibition via metformin also results a decrease in protein HER-2 expression, one of the major actors in cancer cell proliferation [14]. AMPK activation by metformin also activates tumor suppressor gene p53 and induces apoptosis [15]. Moreover, metformin decreases IGF1 levels thereby interrupting the cross talk between insulin, IGF1R and G-protein coupled receptor systems [2, 16,17,18]. Metformin, even though insufficient to treat cancer alone, is accepted to have cytostatic effects on tumor cells. However, metformin is also accepted to be cytotoxic in particular conditions like glucose deprivation, p53 mutation and synthetically lethal with glucose withdrawal [19, 20].
The aim of the study is to evaluate the effectiveness of metformin depending on low and high glucose conditions on cell viability, galectin 3 levels, estrogen and progesterone receptor expression and Ki-67 staining in MCF7 breast cancer line.
Materials and methods
Cell culture
MCF-7 cells were kindly provided from Prof. Isık Yulug (Bilkent University) and were grown in high (4.5 g/L) or low glucose (1 g/L) containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and a 1% penicillin/streptomycin mixture. Cells were incubated at 37 °C in a humidified 5.0% CO2 atmosphere.
Cell viability
Cells were seeded in 10% FBS containing DMEM at 2 × 104 cells/200 µL per well in 96 well plates for 24 h at 37 °C. After incubation for 24 h, MCF-7 cells were divided into two group as; high glucose and low glucose exposed and treated with a series of concentrations of metformin (2.5, 5, 10, 20, 40, 80, 160 mM) for 24 and 48 h. The metabolic activity of living cells, an indication of proliferation and viability, was determined by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After incubation time, 10 µL MTT solution (0.5 mg/mL) was added to each well. After 4 h of MTT incubation at 37 °C, 100 µL crystal dissolving buffer was added and the plates were gently shaken on an orbital shaker for 5 min. The absorbance at 570 nm was measured with a microplate reader. Each treatment was repeated at least four times. The mean absorbance of four wells was used as an indicator of relative cell growth.
Immunocytochemistry
Cells were seeded into 6 well plates at 2 × 105/3 mL per well for 24 h. After 24 h, they were incubated with 5 or 10 mM metformin in high or low glucose containing DMEM media. The cells were washed with ice cold PBS and removed with scrapers at 24 h metformin treatment. Scraped cell were fixed in formalin and embedded in paraffin for immunocytochemical staining protocol. For immunocytochemistry, a four micron thick section was cut from paraffin cell block and taken on poly-l-lysine coated glass slide. Sections were deparaffinized and rehydrated through graded alcohols and rinsed in phosphate buffer saline solution. All processes were performed with DAKO Autostainer (Agilent Technologies, USA). A brief description of immunohistochemistry process is as follows: A prediluted biotinylated antibody for Ki-67 (clone MIB-5), ER (clone EP1), PR(PgR 636) (obtained from Dako) was applied to sections and incubated for 30 min in humid chamber. Hydroperoxide (0.3%) then was applied to inhibit endogenous peroxidase activity. Sections then incubated with labelled streptavidin biotine peroxidase (LSAB, obtained from Dako). Reaction products were obtained with 1% solution of 3,3 diaminobenzidine (DAB, Obtained from Dako) in tris buffer. Slides then were counterstained with Mayer’s hematoxyline, dehydrated through graded alcohols, cleared in xylene and coverslipped. Positive reaction is identified as brown production on tumor cell nuclei. The magnification was ×40 of all ICC slides.
Protein isolation
Cells were seeded into 6 well plates at 2 × 105/3 mL per well for 24 h. After 24 h, they were incubated with 5 or 10 mM metformin in high or low glucose containing DMEM media. The cells were washed with ice cold PBS and removed from wells with scrapers at 24 h metformin treatment. For protein isolation, Cell Lysis Buffer (10×) (Cell Signaling #9803) was used and equivalent protein was measured with BCA Protein Assay (Thermo Fisher Scientific, USA).
Galectin-3 assay
Architect Galectin-3 was measured from cell protein lysates to measure quantitative galectin-3 by using Chemiluminescent Microparticle Immuno Assay (CMIA) which is the modified and advanced form of the Enzyme Linked Immuno Sorrbant Assay (ELISA) technique. Measurements were done in Abbott i1000 autoanalyzer.
Statistical analysis
Data was analyzed statistically by using SPSS 20.0 software (SPSS Inc, Chiago, IL, USA). One Way Anova, Mann–Whitney U and Fisher’s Exact Tests were used to analyze the qualitative data. A p value < 0.05 was considered as significant.
Results
Cell viability
The effects of varying metformin concentrations on viability of MCF7 breast cell lines in low and high glucose media for 24 and 48 h are presented as mean % ± SD in Fig. 1. Cell viability following increasing doses of metformin in either medium for 24 h represented a decrease when compared to control groups. The viability following consecutive metformin doses in low glucose (1 g/L) medium was even though lower when compared to high glucose (4.5 g/L) medium, the differences were not statistically significant (Fig. 1a). Likewise, the cell viability following increasing doses of metformin in either medium for 48 h represented a decrease when compared to control groups. The cell viability detected in low glucose medium following metformin at doses < 20 mM was found lower when compared to high glucose medium at 48 h (p < 0.05) (Fig. 1b).
a Cell viability of MCF7 cells treated with consecutive doses of metformin in high and low glucose conditions at 24 h (* and ƪ reflects p < 0.05 when compared to control group). b Cell viability of MCF7 cells treated with consecutive doses of metformin in high and low glucose conditions at 48 h (*p < 0.05 when compared to control group, #p < 0.05 when low glucose medium group compared to high glucose medium group)
Galectin-3 levels
Galectin-3 levels for 5 and 10 mM metformin treatment at 24 h in low and high glucose media are presented as % in Fig. 2. The increase in galectin-3 levels in high glucose medium with either metformin treatment was found statistically significant when compared to control group and the decrease in galectin-3 levels in low glucose medium was found statistically significant only with 10 mM of metformin. The differences between the cells in high and low glucose media with either metformin treatment were also found statistically significant (Fig. 2).
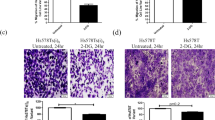
Estrogen receptor (ER), progesterone receptor (PR) and Ki-67 expression
Estrogen and progesterone receptor staining of 5 and 10 mM metformin treatment in low and high glucose media at 24 h are presented as % in Fig. 3. ER positive cells were significantly decreased with metformin treatment in low glucose media and they were significantly increased with only 10 mM metformin treatment in high glucose media. Also, ER positive cells treated with 10 mM metformin in high glucose media were significantly higher when compared to cells of same dose treated in low glucose media (Fig. 3a). The PR positive cells were significantly decreased with metformin treatment in high glucose media. Interestingly, PR positive cells were significantly higher in low glucose media in 5 mM metformin treated group (Fig. 3b). There was no statistically significant difference on Ki-67 staining between metformin treated groups and/or high and low glucose media (Fig. 3c).
% ER positive (a), % PR positive (b), % Ki 67 positive (c) MCF7 cells treated with consecutive doses of metformin in high and low glucose conditions at 24 h (*p < 0.05 when metformin treated groups compared to control group in LG or HG media, #p < 0.05 when low glucose medium group compared to high glucose medium group at the same metformin dose). Axis data represented as log scale. ICC pictures represent the highest and lowest expression within metformin treated groups (×20)
Discussion
In this study, we aimed to evaluate the effectiveness of metformin on MCF7 cells incubated in high and low glucose media and found that metformin usage is related with better results in terms of cancer cell growth, proliferation and metastatic activities especially in low glucose medium.
p53 plays an important role as the central regulator of stress response at molecular and biochemical basis. p53 trigers oxidative phosphorylation through upregulation of cytochrome c oxidase and downregulates glycolytic pathway via inducing TIGAR [21]. In our experiments we used p53 wild type cancer cells to see the metformin’s effects that does not interfere with mutant p53’s effects on proliferation, receptor expression and metastatic activity in low and high glucose settlement.
Glucose is the main source for energy in proliferative cancer cells. The cancer cells possess elevated glucose uptake ability and they tend to make glycolysis instead of oxidative phosphorylation even in the presence of oxygen. This shift from respiration to fermentation in cancer cells results in lactate accumulation leading to acidosis and acidification of the microenvironment allows immune escape of the cancer cells and eases tumor invasion [22]. Otto Warburg posted this activity for the first time in 1920s and so-called Warburg effect is still a matter of debate in the oncology practice [22, 23]. Despite ongoing discussions, tumor cells seem to require ATP, NADH, NADPH in order to support their anabolic processes, cellular growth and proliferation and glucose is the main source for these activities [24]. Cancer cells incubated in only high glucose medium were shown to increase their viability and proliferation [20, 25,26,27]. In that manner, cutting off the glucose supply of cancer cells can lead inhibition of cellular growth and proliferation. Retrospective studies evaluating diabetic population associates the use of metformin with decreased cancer incidence and cancer related mortality [28]. With normalization of serum glucose and insulin levels, metformin may serve indirect anticancer effects, as normoglycemia blocks the free glucose use of tumor cells despite their high demand and normal insulin levels inhibits insulin/IGF signaling pathways. There is ample evidence that metformin has also direct anticancer effects partially through the activation of AMPK [2, 17, 18]. This activation changes the cells from energy consuming phenotype to energy conserving phenotype with the resultant cytostatic effects of the drug. Even though targeting ATP production at the mitochondrial level seems irrational treatment strategy for cancer cells relying glycolysis for ATP production, in case of limited glucose availability, the cancer cells became dependent on oxidative phosphorylation to produce ATP. In this scenario, inhibition of the mitochondrial metabolism with metformin results an energy crisis at the cellular level and the resultant cytotoxic effect of the drug [29]. However the in vitro studies evaluating the effects of metformin were designed with suprapharmacologic doses of the drug. The drug, used as an oral antidiabetic, is prescribed with the maximal approved daily dose of 2.5 g (35 mg/kg of body weight). After oral administration, metformin is transferred from enterocyte to hepatocytes via portal vein. Even the portal vein plasma concentration of the drug is 2–4 times greater than the systemic plasma concentration, the plasma peak concentration is only as high as 40 µM [30]. Suprapharmacologic doses used in in vitro studies are shown to inhibit respiratory chain complex 1 in mitochondria, increase AMP and suppress adenylyl cyclase activity with the resultant blockage of cAMP/PKA pathway [18].
In a study by Zhuang et al., they proposed the protective effect of high glucose against metformin induced cytotoxicity in cancer lines. They stated that high glucose supported the glycolytic activity and ATP production and also both AMPK activation and oxidative phosphorylation blockage due to metformin enhanced glycolytic activity leading to survival. They also stated that lowering glucose levels in the medium potentiated metformin’s cytotoxic effect by not only decreasing the ATP levels but also inhibiting the survival signaling pathways [31]. We studied the effects of metformin on MCF7 cell viability, proliferative activity and found that, even though either medium with metformin represented good results, better results are obtained from cells in low glucose medium. Cell viability significantly affected with lower doses of metformin in low glucose medium when compared to high glucose medium. Metformin and the low glucose level in the medium for cell culture may mimic a clinical scenario of regulated blood sugar levels in a diabetic patient using metformin. And this scenario is compatible with the finding of “less” cancer incidence and cancer related mortality in diabetic population using metformin.
In this study, we evaluated Ki-67 expression, a proliferation marker, and found it to be decreased in either medium with metformin, but the decrease was not statistically significant. Ki-67 is a prognostic factor in breast cancer and its high levels are related with higher relapse risk and worse survival. Hadad et al. demonstrated in vitro and in vivo evidences for AMPK dysfunction for cancer cells and reactivation of AMPK with metformin could have therapeutic potential especially in breast cancer. They attributed the effects of metformin as cytostatic rather than cytotoxic and they demonstrated significant decrease in Ki-67 and cleaved caspase 3 activities [32,33,34].
Estrogen and progesterone receptor expression of the cancer cells were also evaluated in this study. Estrogen receptors were found decreased after drug incubation in low glucose medium whereas progesterone receptors represented a prominent increase in the same medium. Berstein et al. demonstrated interesting clinical data about hormone receptor expression and metformin usage in diabetic women with breast cancer. They showed similar estrogen receptors but more progesterone receptors expression in breast tumors of diabetic women treated with metformin when compared to other antihyperglycemic agents. They attributed no change in estrogen receptor and elevation in progesterone receptor to different modes of antidiabetic therapy as the study groups were similar in terms of HER-2/neu expression. They stated that such increase in receptor expression due to metformin can be related to the amelioration of estrogenic signal transduction, tumor sensitivity to hormones and better outcomes [35].
Galectin-3, a member of beta-galactoside-binding protein family, is known to promote neoplastic transformation, ease tumor adhesion to extracellular matrix and enhance metastatic spreading of the tumor [36]. As its overexpression is related with metastasis, we also aimed to evaluate the effectiveness of metformin on galectin-3 levels of MCF7 cells in high and low glucose conditions. We showed galectin-3 levels were significantly decreased with metformin in low glucose medium when compared to control and high glucose medium groups. Interestingly the cells in the high glucose medium represented significantly elevated galectin-3 activity after drug administration.
The overall findings of this study represent better outcomes for MCF7 incubated in low glucose media treated with metformin in terms of viability, receptor expression and metastatic activity. Even metformin blocks oxidative phosphorylation, high glucose inhibits metformin cytotoxicity by providing a power supply for glycolysis in cancer cells. Thus, it will be better to affiliate energetic and microenvironmental changes and incorporate them to the comments of metformin studies in terms of analyzing its antiproliferative and antimetastatic effects. Future research with different cells and animal models will help to better define metformin’s role as a potential cancer therapy.
References
Kasznicki J, Sliwinska A, Drzewoski J (2014) Metformin in cancer prevention and therapy. Ann Transl Med 2(6):57
Jalving M, Gietema JA, Lefrandt JD, de Jong S et al (2010) Metformin: taking away the candy for cancer? Eur J Cancer 46(13):2369–2380
Poloz Y, Stambolic V (2015) Obesity and cancer, a case for insulin signaling. Cell Death Dis 6:e2037
Denduluri SK, Idowu O, Wang Z, Liao Z et al (2015) Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis 2(1):13–25
Li R, Pourpak A, Morris SW (2009) Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J Med Chem 52(16):4981–5004
Xue M, Cao X, Zhong Y, Kuang D et al (2012) Insulin-like growth factor-1 receptor (IGF-1R) kinase inhibitors in cancer therapy: advances and perspectives. Curr Pharm Des 18(20):2901–2913
Sen S, He Y, Koya D, Kanasaki K (2014) Cancer biology in diabetes. J Diab Investig 5(3):251–264
Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S et al (2014) Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife 3:e02242
Hawley SA, Ross FA, Chevtzoff C, Green KA et al (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11(6):554–565
Jara JA, Lopez-Munoz R (2015) Metformin and cancer: between the bioenergetic disturbances and the antifolate activity. Pharmacol Res 101:102–108
Memmott RM, Mercado JR, Maier CR, Kawabata S et al (2010) Metformin prevents tobacco carcinogen–induced lung tumorigenesis. Cancer Prev Res 3(9):1066–1076
Zhou G, Myers R, Li Y, Chen Y et al (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108(8):1167–1174
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12(1):9–22
Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA (2009) The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle 8(1):88–96
Ben Sahra I, Laurent K, Giuliano S, Larbret F et al (2010) Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res 70(6):2465–2475
Rozengurt E, Sinnett-Smith J, Kisfalvi K (2010) Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res 16(9):2505–2511
Salani B, Del Rio A, Marini C, Sambuceti G et al (2014) Metformin, cancer and glucose metabolism. Endocr Relat Cancer 21(6):R461–R471
Pryor R, Cabreiro F (2015) Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem J 471(3):307–322
Daugan M, Dufay Wojcicki A, d’Hayer B, Boudy V (2016) Metformin: an anti-diabetic drug to fight cancer. Pharmacol Res 113(Pt A):675–685
Menendez JA, Oliveras-Ferraros C, Cufi S, Corominas-Faja B et al (2012) Metformin is synthetically lethal with glucose withdrawal in cancer cells. Cell Cycle 11(15):2782–2792
Madan E, Gogna R, Bhatt M, Pati U et al (2011) Regulation of glucose metabolism by p53: emerging new roles for the tumor suppressor. Oncotarget 2(12):948–957
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41(3):211–218
Xu XD, Shao SX, Jiang HP, Cao YW et al (2015) Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat 38(3):117–122
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033
Han L, Ma Q, Li J, Liu H et al (2011) High glucose promotes pancreatic cancer cell proliferation via the induction of EGF expression and transactivation of EGFR. PLoS ONE 6(11):e27074
Sinnett-Smith J, Kisfalvi K, Kui R, Rozengurt E (2013) Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK. Biochem Biophys Res Commun 430(1):352–357
Choi YW, Lim IK (2014) Sensitization of metformin-cytotoxicity by dichloroacetate via reprogramming glucose metabolism in cancer cells. Cancer Lett 346(2):300–308
Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK et al (2014) Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res 7(9):867–885
Weinberg SE, Chandel NS (2015) Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol 11(1):9–15
He L, Wondisford FE (2015) Metformin action: concentrations matter. Cell Metab 21(2):159–162
Zhuang Y, Chan DK, Haugrud AB, Miskimins WK (2014) Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PLoS ONE 9(9):e108444
Hadad SM, Jordan LB, Roy PG, Purdie CA et al (2016) A prospective comparison of ER, PR, Ki67 and gene expression in paired sequential core biopsies of primary, untreated breast cancer. BMC Cancer 16(1):745
Hadad SM, Coates P, Jordan LB, Dowling RJ et al (2015) Evidence for biological effects of metformin in operable breast cancer: biomarker analysis in a pre-operative window of opportunity randomized trial. Breast Cancer Res Treat 150(1):149–155
Hadad SM, Baker L, Quinlan PR, Robertson KE et al (2009) Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer 9:307
Berstein LM, Boyarkina MP, Tsyrlina EV, Turkevich EA et al (2011) More favorable progesterone receptor phenotype of breast cancer in diabetics treated with metformin. Med Oncol 28(4):1260–1263
Reticker-Flynn NE, Malta DF, Winslow MM, Lamar JM et al (2012) A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nat Commun 3:1122
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dogan Turacli, I., Umudum, H., Pampal, A. et al. Do MCF7 cells cope with metformin treatment under energetic stress in low glucose conditions?. Mol Biol Rep 45, 195–201 (2018). https://doi.org/10.1007/s11033-018-4152-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4152-5