Abstract
Rheumatoid arthritis (RA) is an inflammatory disorder of the joints that affects 0.5–1 % of adults. Excessive growth of the fibroblast-like synoviocytes (FLS) promotes hyperplasia of synovial tissues and causes its invasion into the bone and cartilage, which eventually causes deformity and dysfunction of affected joints. Interleukin 35 (IL-35) was shown to suppress the inflammatory responses to collagen-induced arthritis (CIA) via upregulation of T regulatory cells and suppression of T helper type 17 cells in a mouse model. To study the effects of IL-35 on the proliferation and apoptosis frequency of cultured FLS isolated from mice with CIA as well as to examine the effects of IL-35 on CIA in vivo. Thirty DBA/1 J mice, which are used as an animal model for RA, were divided randomly (ten mice per group) to a CIA group (collagen treatment), a CIA + IL-35 group (collagen and IL-35 treatments), and a control group (no treatment). Starting on the 24th day after collagen administration, IL-35 was injected intraperitoneally into mice of the CIA + IL-35 group once per day for 10 days. An arthritis index was calculated, and pathological analysis of synovial tissue was performed. FLS isolated from CIA mice were treated with various concentrations of IL-35 (12.5–100 ng/ml). The MTT assay was used to examine FLS proliferation, and apoptosis frequency of FLS was detected by flow cytometry. On day 24, the CIA mice began to exhibit arthritis symptoms, and the symptoms rapidly progressed with time. Treatment with IL-35 significantly alleviated arthritis symptoms and reduced the synovial tissue inflammation. In addition, IL-35 treatment inhibited proliferation and promoted apoptosis in cultured FLS from CIA mice in a dose-dependent manner. IL-35 could ameliorate the symptoms of arthritis in the CIA mouse model in vivo and inhibited FLS proliferation while promoting FLS apoptosis in vitro, thereby exhibited the potential in inhibiting the progression of RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an inflammatory disorder of the joint that affects up to 0.5–1 % of adults [1], chronic inflammation of the joints is the hallmark of RA and leads to symmetrical and erosive injury to the cartilage and bone. Although the pharmacological and non-pharmacological measures control joint inflammation and prevent joint deformity in certain patients, RA is still associated with considerably morbidity and mortality [2]. The precise pathogenesis of RA remains unclear, but multiple genetic and environmental factors are believed to contribute to the development of RA [1].
In RA, synovial tissue becomes hyperplastic and invasive in nature. Fibroblast-like synoviocytes (FLS) residing in the synovial intimal lining produce cytokines and inflammatory mediators, thereby contributing to the pathogenesis of RA [3]. In addition, FLS are the primary mediators of cartilage damage in RA due to their tendency to secrete multiple proteolytic enzymes such as matrix metalloproteinases (MMPs) [4]. Excessive growth of the FLS promotes hyperplasia of synovial tissues and induces its invasion into the bone and cartilage, eventually leading to joint deformity and dysfunction. Growth of FLS may result from an imbalance between cell proliferation and apoptosis. Several studies have demonstrated that FLS rarely undergo apoptosis and can survive even in highly genotoxic rheumatoid synovium that is rich in reactive oxygen species [5, 6]. Therefore, imbalance between synovial cell proliferation and apoptosis has been regarded as a contributing factor for the development of RA [7–9]. Promotion of FLS apoptosis might be a new strategy for treating RA.
Interleukin-35 (IL-35), a novel member of the IL-12 family, is specifically produced by regulatory T cells (Tregs) and considered as an anti-inflammatory cytokine, which is linked to many autoimmune diseases [10–12]. IL-35 has been shown to suppress inflammatory responses via Treg expansion and T helper type 17 (Th17) cell suppression in mice with collagen-induced arthritis (CIA) [13]. Moreover, patients with established RA have significantly higher serum IL-35 levels than those with controlled osteoarthritis [14]. Furthermore, IL-35 expression is greater in the synovial tissues of RA patients than in those of patients with osteoarthritis or psoriatic arthritis [15]. These clinical studies further suggest that IL-35 may be closely related to the pathogenesis of RA. However, the role of IL-35 in RA remains unknown, and the effects of IL-35 on FLS proliferation and apoptosis have yet to be elucidated.
Therefore, in a CIA mouse model, we investigated how would IL-35 affect the arthritis symptoms in RA and evaluated its effects on the proliferation and apoptosis frequency of cultured FLS isolated from mice with CIA. The present study aimed at testing the hypothesis that IL-35 can inhibit FLS proliferation and promote FLS apoptosis.
Materials and methods
Animals
Institutional Animal Care and Use Committee (IACUC) of China Medical University approved the study. Thirty male DBA/1 J mice (Beijing HFK Bioscience Co. Ltd, China) aged between 8–10 weeks and weighing 22.6 ± 2.8 g were kept on a 12-h light/dark cycle and housed at room temperature with 60 % humidity. Mice were fed standard food and water ad libitum.
Reagents
Chicken type II collagen, Annexin V-FITC/PI (apoptosis detection kit), a cell cycle kit, and a 3-(4,5-dimethyl-thiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) kit were obtained from Sigma-Aldrich, USA. Trypsin, fetal bovine serum (FBS), complete Freund’s adjuvant (CFA), incomplete Freund’s adjuvant (IFA), and IL-35 (mouse): Fc (human IgG1) were purchased from R&D Systems, USA. High-glucose Dulbecco’s modified Eagle medium (H-DMEM) was obtained from Hyclone, USA. Goat anti-mouse monoclonal antibodies against CD68 and cylin D1 were purchased from Abcam, UK. Goat anti-mouse vimentin polyclonal antibody was purchased from Proteintech, USA.
Mouse model of CIA
Thirty mice were divided randomly into three groups with ten mice in each group, the CIA, CIA + IL-35 and negative control group. The CIA and CIA + IL-35 group was generated by treatment with collagen. After feeding for 3 days, intradermal immunization of mice with chicken type II collagen (0.2 ml of 2 mg/ml solution in acetic acid) was started, and a subcutaneous booster was given on day 21. Collagen was delivered as a 1:1 emulsification in CFA at a ratio of 1:1. On day 24, arthritis was successfully induced, according to an arthritis index (AI) >4, and one paw showing a >1.6-fold increase in the volume of footpad swelling.
Mice in the CIA + IL-35 group were treated intraperitoneally with IL-35 (2 μg) once per day for 10 days as previously described from day 24 [13]. The same volume of phosphate-buffered saline (PBS) was given to the animals in the control and CIA groups. For each mouse paw, the severity of arthritis was scored as 0 if normal, one if there was mild swelling with no joint deformities with or without erythema, two if there was severe swelling with no joint deformities, and three if there were joint deformities and ankylosis. The AI was the sum of the scores for all four paws, and the maximum AI was 12 points. The severity of swelling was assessed according to the measured footpad thickness.
Five mice in each group were sacrificed on day 48. The ankle joint of a hind limb was removed from each mouse, and hematoxylin and eosin (HE) staining was performed for pathological analysis of synovial tissues. Synovial hyperplasia, inflammation, cell infiltration, pannus, and bone erosion were scored as follows: (1) normal; (2) a small number of inflammatory cells with or without pannus, and without decalcification and bone erosion; (3) a moderate number of inflammatory cells with many pannus, and mild decalcification and bone erosion; (4) excessive inflammatory cells with a large amount of pannus, and moderate decalcification and bone erosion; and (5) excessive inflammatory cells with a large amount of pannus, and severe decalcification and bone erosion. For each joint, the minimum score was one and the maximum score was five.
Isolation and culture of FLS
After removal of the paw bones from mice in all groups, the joint cavity was cut open to reveal the synovial tissues, which were sliced into 1 mm3 pieces. These pieces (8–12 pieces) were washed with H-DMEM containing 15 % FBS and transferred to a culture bottle for culture in H-DMEM containing 15 % FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37 °C in 5 % CO2 for 24 h. Afterward, the tissue pieces were collected and cultured in culture media. Cell growth was observed under a microscope.
Cells subcultured to 70–80 % confluence were digested with 0.25 % of trypsin at 37 °C for 3–5 min. H-DMEM containing Ca2+ was added to inhibit trypsin activity. The cells were harvested and centrifuged at 1000 r/min. The supernatant was decanted and the pellet was re-suspended in H-DMEM. Cells were passaged at a ratio of 1:2, and passages 3–5 of the cells were used.
Cells seeded at 5 × 104 cells/ml within wells of 96-well plates were used in six different treatment groups: the negative control group, the CIA group, and four CIA + IL-35 groups that were treated with 12.5, 25, 50, or 100 ng/ml IL-35. After culture in H-DMEM supplemented with 15 % FBS, the cells were incubated at 37 °C in 5 % CO2 for 12 h. Next, IL-35 was added at the indicated concentrations for further culture for 24, 48, 72, 96, and 120 h.
Immunocytochemistry
FLS were plated at a density of 2 × 104 cells/ml, cultured for 48 h, and then fixed in 4 % paraformaldehyde. The fixed cells were washed in PBS three times (3 min each) and incubated in 3 % H2O2 for 15 min. To block nonspecific protein binding sites, the cells were incubated in 5 % BSA for 20 min at room temperature. Further, the cells were incubated with CD68 (goat anti-mouse CD68, dilution 1:100) and vimentin (goat anti-mouse vimentin, dilution 1:100) primary antibodies overnight at 4 °C, followed by incubation with rabbit anti-goat secondary antibodies with conjugated biotin (dilution 1:200) at 37 °C for 30 min. Cells were then incubated with streptavidin horseradish peroxidase for additional 20 min at 37 °C, followed by application of 3,3-diaminobenzidine (DAB) substrate. Cells were counterstained with hematoxylin. Samples incubated in PBS not containing primary antibodies served as negative controls. All cells were examined and photographed under a light microscope.
MTT assay
FLS proliferation was evaluated using the MTT assay. Briefly, 5 × 104 cells/ml were seeding into 96-well plates and treated with IL-35 at different concentrations (12.5, 25, 50, or 100 ng/ml) or vehicle for 0, 24, 48, 72, 96, and 120 h. The cell proliferation detection MTT dye was added to a final concentration of 5 mg/ml, and plates were incubated for 4 h. After that, 150 μl of dimethyl sulfoxide (DMSO) was added, and then the plate was incubated for 10 min in darkness at room temperature. The absorbance of the solution in each well was read at 490 nm in a microtiter plate reader, and the average values from triplicate readings were used to plot a FLS cell proliferation curve.
Flow cytometry
Flow cytometry was used to detect FLS apoptosis frequency and cell cycle. Briefly, 5 × 105 FLS were seeded into of 24-well plates. After incubation with IL-35 for 48 h, cells (1 × 105/ml) were collected by trypsin digestion, followed by centrifugation at 1000 rpm for 5 min. For cell apoptosis analysis, the cells were resuspended in 500 μl binding buffer and incubated with 5 μl Annexin-V-FITC. Then cells were incubated with 5 μl propidium iIodide solution for 15 min in darkness at room temperature. For cell cycle analysis, cells were incubated with 25 μl propidium iodide and 10 μl RNase A at 37 °C for 30 min. The cell apoptosis frequency and cell cycle was analyzed by flow cytometer (Becton Dickenson, USA).
Western blot
FLS were homogenized on ice in lysis buffer on ice after 48 h in culture. Proteins were first separated in SDS-PAGE gels by electrophoresis and then transferred to polyvinylidene fluoride (PVDF) membranes. The PVDF membranes were incubated with primary antibodies against cyclin D1 (goat anti-mouse monoclonal antibodies, diluted 1:100) for 2 h at room temperature. Then, the membranes were incubated with horseradish peroxidase-conjugated rabbit anti-goat secondary antibodies (dilution 1:5000) for 1 h at room temperature. β-actin was used as the internal control standard. Bands were detected using a chemiluminescence detection kit, and band intensities were analyzed with Quantity one software.
Statistical analysis
The All statistical analyses were completed using SPSS 17.0 (SPSS Inc., Chicago, IL). Data were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) or repetitive measure ANOVA was used to compare the significant differences among the groups, followed by least significant difference (LSD) tests. Probability values <0.05 were considered as statistically significant.
Results
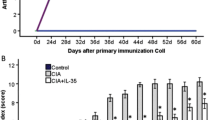
IL-35 ameliorated the arthritis severity in the mouse model of CIA
By day 24, The CIA collagen-treated mice of the CIA group exhibited arthritis symptoms rapidly progressed with time (Fig. 1), with the AI peaking on day 48. Treatment with IL-35 led to a significantly reduced AI score in CIA mice (P < 0.05), suggesting that IL-35 ameliorated the arthritis severity in mice with CIA.
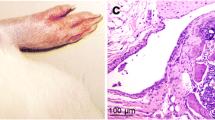
IL-35 prevents pathological damage of synovial tissue in CIA mice
HE staining showed no synovial hyperplasia, cell infiltration, dilatation, or congestion in blood vessels, pannus, or inflammation in synovial tissues of the control group. Synovial tissues of the CIA group exhibited obvious hyperplasia, inflammatory cell infiltration, dilatation and congestion in blood vessels, and pannus. Following treatment with IL-35, only a few infiltrating inflammatory cells and pannus were observed in synovial tissues of mice in the CIA group. The pathological score was significantly lower for the CIA + IL-35 group than for the CIA group (P < 0.0001; Fig. 2b), but significantly higher than in the control group (P < 0.0001, Fig. 2b).
Identification of cultured FLS
Under the microscope, FLS appeared spindle-like (Fig. 3a). According to the immunohistochemical studies, the cells isolated and cultured from CIA mice were vimentin-positive and CD68-negative, suggesting that they were indeed FLS cells (Fig. 3b).
IL-35 inhibits FLS proliferation in vitro
Figure 4 shows the time course of FLS proliferation upon treatment with different concentrations of IL-35. Cells proliferated slowly in the control group, whereas cells in the CIA group proliferated rapidly with time. IL-35 treatment significantly inhibited FLS proliferation at each time point (P < 0.05; Fig. 4), and this inhibition was dose-dependent.
IL-35 inhibits cell cycle progression in FLS in vitro
We examined the effect of IL-35 on FLS cell cycle progression in culture cells using flow cytometry. Compared with the CIA group, IL-35 (12.5–100 ng/ml) treatment for 48 h significantly increased the number of cells in G1 phase while significantly decreased the number of cells in S/G2 phases (Fig. 5, P < 0.05). These findings suggested that IL-35 slowed cell cycle progression and thereby inhibited cell proliferation.
IL-35 inhibited cell cycle progression in cultured FLS. a Representative flow cytometric results for cell cycle in FLS treated with different concentrations of IL-35. b–d The percentages of FLS in G1, S, and G2 phase after IL-35 treatment. Data are expressed as mean ± SD, n = 4. # P < 0.05 versus Control group, * P < 0.05 versus CIA group
IL-35 downregulates cyclin D1 expression in FLS in vitro
We next examined the expression of cyclin D1, which regulates cell cycle checkpoint in G1 phase, upon treatment with IL-35. Western blot results showed that cyclin D1 expression was significantly greater in the CIA group than in the normal group (P < 0.05; Fig. 6). Moreover, IL-35 treatment dose-dependently downregulated cyclin D1 expression.
IL-35 downregulated cyclin D1 expression in FLS. a Representative Western blot showing cyclin D1 expression in FLS treated with different concentrations of IL-35. β-actin was used as a loading and normalization control. b Quantification of cyclin D1 expression normalized to β-actin expression. Data are expressed as mean ± SD, n = 4. # P < 0.05 versus Control mice, * P < 0.05 versus CIA mice. P < 0.05 for CIA + 12.5 ng/ml IL-35 versus CIA + 25 ng/ml IL-35 groups, P < 0.05 for CIA + 25 ng/ml IL-35 versus CIA + 50 ng/ml IL-35 groups, P < 0.05 for CIA + 50 ng/ml IL-35 versus CIA + 100 ng/ml IL-35 groups
IL-35 promotes FLS apoptosis in vitro
FLS apoptosis in each group was measured by flow cytometry. In the control group, the frequency of apoptotic FLS was 40.03 ± 1.49 %. In the CIA group, the frequency of apoptotic FLS was only 3.45 ± 1.00 %, which was significantly lower (all P < 0.05) than measurements in the CIA + 12.5 ng/ml IL-35 group (11.16 ± 1.32 %), CIA + 25 ng/ml IL-35 group (16.50 ± 1.11 %), CIA + 50 ng/ml IL-35 group (20.32 ± 1.00 %), and CIA + 100 ng/ml IL-35 group (25.81 ± 3.01 %) (Fig. 7), suggesting that IL-35 induced apoptosis in FLS in a dose-dependent manner. The late apoptotic rate, but not early apoptotic rate, significantly changed (Fig. 7a), suggesting that IL-35 promoted late apoptosis and necrosis in FLS. (In Fig. 7a, the right upper quadrant represents the late stage of FLS apoptosis, and the right lower quadrant represents the early stage of FLS apoptosis).
IL-35 increased FLS apoptosis. a Representative flow cytometric analysis of FLS apoptosis. b Percentages of apoptotic FLS after IL-35 treatment. Data are expressed as mean ± SD, n = 4. # P < 0.05 versus Control mice, * P < 0.05 versus CIA mice. P < 0.05 for CIA + 12.5 ng/ml IL-35 versus CIA + 25 ng/ml IL-35 groups, P < 0.05 for CIA + 25 ng/ml IL-35 versus CIA + 50 ng/ml IL-35 groups, P < 0.05 for CIA + 50 ng/ml IL-35 versus CIA + 100 ng/ml IL-35 groups
Discussion
Here, we studied the effect of IL-35 on CIA in DBA/1 J mice, a well-established mouse model of human RA [16]. Our experiments showed that IL-35 treatment attenuated the RA symptoms of CIA mice and, more specifically, protected synovial tissues against pathological damage in this mouse model. Furthermore, we demonstrated that IL-35 inhibited the proliferation of FLS from CIA mice while promoting apoptosis of these cells. Our results suggested that IL-35 might prevent the development of RA and thus may represent a potential strategy for treating this common and debilitating condition.
Proliferation and inflammation of the synovial intimal lining are the prominent pathological features of RA. FLS have been found to contribute to the pathogenesis of RA via the release of inflammatory cytokines and production of proteolytic enzymes such as MMPs [4]. Mutations in tumor protein p53 have been found in synovial tissues and FLS in RA patients, and mutant p53 differentially regulates the expression of human MMPs 13 (hMMP-13) in FLS [17], suggesting that dysregulation of hMMP-13 in FLS due to the inactivation of p53 may contribute to the pathogenesis of RA. In addition, FLS secrete various inflammatory cytokines and promote the development of inflammation and autoimmunity [18]. Therefore, effective inhibition of FLS proliferation and promotion of FLS apoptosis may be a therapeutic strategy against RA. In this study, IL-35 treatment significantly inhibited FLS proliferation and increased FLS apoptosis, suggesting that IL-35 may be a novel cytokine that can be used for the effective treatment of RA.
As an anti-inflammatory cytokine with a protective role in RA pathogenesis, IL-35 also has anti-inflammatory properties in animal models [13]. However, by increasing the severity of inflammation, IL-35 enhanced Lyme arthritis in Borrelia-vaccinated and infected mice [19]. IL-35 produces anti-inflammatory effects by promoting proliferation and differentiation of Tregs, increasing production of IL-10 (anti-inflammatory cytokine), and inhibiting growth and differentiation of effector T cells [20–24]. Collison et al. reported that IL-35 is specifically secreted by CD4 + CD25 + Tregs and is necessary for the maximal inhibitory effects of these cells [11]. Niedbala et al. found that IL-35 reduced the incidence of CIA and inhibited arthritis symptoms [13]. Consistent with these previous findings, we observed that arthritis symptoms were significantly reduced after treatment of CIA mice with IL-35. Furthermore, IL-35 inhibited proliferation and cell cycle progression in FLS harvested from CIA mice in a dose-dependent manner, accompanied by downregulation of cyclin D1.
The cell cycle is a fundamental processes of cell life cycle; the start of G1 being a key regulatory step. Cell cycle proteins (cyclins), cyclin-dependent kinases, and CDK inhibitors are the major factors involved in cell cycle regulation. Cyclin D1 is a key protein that regulates cell cycle at G1 and is essential for cell cycle progression through G1/S. Overexpression of cyclin D1 is the leading cause of detection of point defects in G1/S phase. Our results demonstrate that IL-35 can inhibit the expression of cyclin D1, thereby suppressing the progression of cell cycle, inhibiting the proliferation of synovial cells. Our data indicate that IL-35 may protect against arthritis by inhibiting FLS proliferation via downregulation of cyclin D1 and slowing cell cycle progression. In addition, we found that IL-35 treatment significantly promoted FLS apoptosis, which may be another mechanism by which IL-35 prevents arthritis.
It has been reported that IL-35 affects cell apoptosis, but the corresponding role differs among different cells. Several papers show both pro- and anti-apoptotic roles for IL-35 in different diseases. IL-35 suppresses cancer activity and cell growth, and increases the sensitivity of human cancer cells to apoptosis by regulating genes associated with the cell cycle and apoptosis [25]. Other studies show that IL-35 directly reduces apoptosis of acute myeloid leukemia blasts and promotes their proliferation [26]. IL-35 inhibits apoptosis and induces proliferation in association upregulation of cyclin D and downregulation of p27 expression in pancreas cancer [27]. IL-35 slightly enhanced ICAM-1 surface expression and increased IL-32 mRNA expression in Ao-SMCs, but IL-35 did not affect Ao-SMC apoptosis, necrosis, or viability [28]. The mechanisms of FLS apoptosis are complex, involving multiple pro-apoptotic factors such as Bcl and anti-apoptotic factors such as Bax. However, in the present study, we did not investigate the mechanisms through which IL-35 promoted FLS apoptosis. Further studies are required to identify the signaling pathway that mediates IL-35-induced apoptosis in FLS.
Conclusions
IL-35 ameliorated arthritis index by blocking synovial tissue hyperplasia and inflammation in mice with CIA. Furthermore, IL-35 inhibited FLS proliferation in vitro, possibly by downregulating cyclin D1 and slowing cell cycle progression. We also found that IL-35 promoted FLS apoptosis. Our results indicated the potential of IL-35 in RA therapy by inhibiting FLS proliferation and promoting FLS apoptosis.
Abbreviations
- RA:
-
Rheumatoid arthritis
- FLS:
-
Fibroblast-like synoviocytes
- IL-35:
-
Interleukin-35
- Th17:
-
T helper type 17
- CIA:
-
Collagen-induced arthritis
- MTT:
-
3-(4,5-Dimethyl-thiazoyl-2-yl)-2,5-diphenyltetrazolium bromide
- MMPs:
-
Matrix metalloproteinases
- IACUC:
-
Institutional Animal Care and Use Committee
- CFA:
-
Complete Freund’s adjuvant
- IFA:
-
Incomplete Freund’s adjuvant
- FBS:
-
Fetal bovine serum
- HE:
-
Hematoxylin and eosin
- H-DMEM:
-
High glucose dulbecco’s modified eagle’s medium
- H2O2 :
-
Hydrogen peroxide
- DAB:
-
3,3-Diaminobenzidine
- SDS-PAGE:
-
Sodium dodecylsulphate-polyarcylamide gel electrophoresis
- ANOVA:
-
One-way analysis of variance
- SD:
-
Standard deviation
- LSD:
-
Least significant difference
- Ao-SMCs:
-
Human aortic smooth muscle cells
References
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108. doi:10.1016/S0140-6736(10)60826-4
Jutley G, Raza K, Buckley CD (2015) New pathogenic insights into rheumatoid arthritis. Curr Opin Rheumatol 27(3):249–255
Bartok B, Firestein GS (2010) Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev 233(1):233–255. doi:10.1111/j.0105-2896.2009.00859.x
Tolboom TC, Pieterman E, van der Laan WH, Toes RE, Huidekoper AL, Nelissen RG, Breedveld FC, Huizinga TW (2002) Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis 61(11):975–980
Firestein GS, Yeo M, Zvaifler NJ (1995) Apoptosis in rheumatoid arthritis synovium. J Clin Invest 96(3):1631–1638. doi:10.1172/JCI118202
Nakajima T, Aono H, Hasunuma T, Yamamoto K, Shirai T, Hirohata K, Nishioka K (1995) Apoptosis and functional fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum 38(4):485–491
Li H, Wan A (2013) Apoptosis of rheumatoid arthritis fibroblast-like synoviocytes: possible roles of nitric oxide and the thioredoxin 1. Mediators Inflamm 2013:953462
Yoon CH, Chung SJ, Lee SW, Park YB, Lee SK, Park MC (2013) Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine 80(3):274–279. doi:10.1016/j.jbspin.2012.08.010
Yi JK, Kim HJ, Yu DH, Park SJ, Shin MJ, Yuh HSB, Ji YR, Kim R, Park J, Kim Y, Lee HS (2013) Regulation of inflammatory responses and fibroblast-like synoviocyte apoptosis by calcineurin-binding protein 1 in mice. Arthritis Rheum 64(7):207
Castellani M, Anogeianaki A, Felaco P, Toniato E, De Lutiis M, Shaik B, Fulcheri M, Vecchiet J, Tete S, Salini V (2009) IL-35, an anti-inflammatory cytokine which expands CD4 + CD25 + Treg cells. J Biol Regul Homeost Agents 24(2):131–135
Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA (2007) The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450(7169):566–569. doi:10.1038/nature06306
Neurath MF (2008) IL-12 family members in experimental colitis. Mucosal Immunol 1(Suppl 1):S28–S30. doi:10.1038/mi.2008.45
Niedbala W, Xq Wei, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY (2007) IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 37(11):3021–3029
Senolt L, Sumova B, Jandova R, Hulejova H, Mann H, Pavelka K, Vencovsky J, Filkova M (2015) Interleukin 35 synovial fluid levels are associated with disease activity of rheumatoid arthritis. PLoS One 10(7):e0132674. doi:10.1371/journal.pone.0132674
Filkova M, Vernerova Z, Hulejova H, Prajzlerova K, Veigl D, Pavelka K, Vencovsky J, Senolt L (2015) Pro-inflammatory effects of interleukin-35 in rheumatoid arthritis. Cytokine 73(1):36–43. doi:10.1016/j.cyto.2015.01.019
Anthony DD, Haqqi TM (1999) Collagen-induced arthritis in mice: an animal model to study the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 17(2):240–244
Sun Y, Cheung JM, Martel-Pelletier J, Pelletier JP, Wenger L, Altman RD, Howell DS, Cheung HS (2000) Wild type and mutant p53 differentially regulate the gene expression of human collagenase-3 (hMMP-13). J Biol Chem 275(15):11327–11332
Perlman H, Bradley K, Liu H, Cole S, Shamiyeh E, Smith RC, Walsh K, Fiore S, Koch AE, Firestein GS, Haines GK 3rd, Pope RM (2003) IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J Immunol 170(2):838–845
Kuo J, Nardelli DT, Warner TF, Callister SM, Schell RF (2011) Interleukin-35 enhances lyme arthritis in Borrelia-vaccinated and -infected mice. Clin Vaccine Immunol 18(7):1125–1132. doi:10.1128/CVI.00052-11
Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF (2012) IL-35 is a novel responsive anti-inflammatory cytokine—a new system of categorizing anti-inflammatory cytokines. PLoS One 7(3):e33628. doi:10.1371/journal.pone.0033628
Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA (2010) IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 11(12):1093–1101. doi:10.1038/ni.1952
Collison LW, Pillai MR, Chaturvedi V, Vignali DA (2009) Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35-and IL-10-dependent manner. J Immunol 182(10):6121–6128
Whitehead GS, Wilson RH, Nakano K, Burch LH, Nakano H, Cook DN (2012) IL-35 production by inducible costimulator (ICOS)-positive regulatory T cells reverses established IL-17-dependent allergic airways disease. J Allergy Clin Immunol 129(1):207–215. doi:10.1016/j.jaci.2011.08.009 e201-205
Wirtz S, Billmeier U, Mchedlidze T, Blumberg RS, Neurath MF (2011) Interleukin-35 mediates mucosal immune responses that protect against t-cell–dependent colitis. Gastroenterology 141(5):1875–1886
Long J, Zhang X, Wen M, Kong Q, Lv Z, An Y, Wei XQ (2013) IL-35 over-expression increases apoptosis sensitivity and suppresses cell growth in human cancer cells. Biochem Biophys Res Commun 430(1):364–369. doi:10.1016/j.bbrc.2012.11.004
Tao Q, Pan Y, Wang Y, Wang H, Xiong S, Li Q, Wang J, Tao L, Wang Z, Wu F, Zhang R, Zhai Z (2015) Regulatory T cells-derived IL-35 promotes the growth of adult acute myeloid leukemia blasts. Int J Cancer 137(10):2384–2393. doi:10.1002/ijc.29563
Nicholl MB, Ledgewood CL, Chen X, Bai Q, Qin C, Cook KM, Herrick EJ, Diaz-Arias A, Moore BJ, Fang Y (2014) IL-35 promotes pancreas cancer growth through enhancement of proliferation and inhibition of apoptosis: evidence for a role as an autocrine growth factor. Cytokine 70(2):126–133. doi:10.1016/j.cyto.2014.06.020
Skowron W, Zemanek K, Wojdan K, Gorzelak P, Borowiec M, Broncel M, Chalubinski M (2015) The effect of interleukin-35 on the integrity, ICAM-1 expression and apoptosis of human aortic smooth muscle cells. Pharmacol Rep 67(2):376–381. doi:10.1016/j.pharep.2014.10.015
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (Nos. 81172867, 81471542).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Rights and permissions
About this article
Cite this article
Li, Y., Wu, S., Li, Y. et al. Interleukin-35 (IL-35) inhibits proliferation and promotes apoptosis of fibroblast-like synoviocytes isolated from mice with collagen-induced arthritis. Mol Biol Rep 43, 947–956 (2016). https://doi.org/10.1007/s11033-016-4034-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-016-4034-7