Summary
Pollen tube (PT) growth towards the micropyle is critical for successful double fertilization. However, the mechanism of micropyle-directed PT growth is still unclear in Brassica napus. In this study, two aspartate proteases, BnaAP36s and BnaAP39s, were identified in B. napus. BnaAP36s and BnaAP39s were localized to the plasma membrane. The homologues of BnaAP36 and BnaAP39 were highly expressed in flower organs, especially in the anther. Sextuple and double mutants of BnaAP36s and BnaAP39s were then generated using CRISPR/Cas9 technology. Compared to WT, the seed-set of cr-bnaap36 and cr-bnaap39 mutants was reduced by 50% and 60%, respectively. The reduction in seed-set was also found when cr-bnaap36 and cr-bnaap39 were used as the female parent in a reciprocal cross assay. Like WT, cr-bnaap36 and cr-bnaap39 pollen were able to germinate and the relative PTs were able to elongate in style. Approximately 36% and 33% of cr-bnaap36 and cr-bnaap39 PTs, respectively, failed to grow towards the micropyle, indicating that BnaAP36s and BnaAP39s are essential for micropyle-directed PT growth. Furthermore, Alexander's staining showed that 10% of cr-bnaap39 pollen grains were aborted, but not cr-bnaap36, suggesting that BnaAP39s may also affect microspore development. These results suggest that BnaAP36s and BnaAP39s play a critical role in the growth of micropyle-directed PTs in B. napus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In angiosperms, successful double fertilization plays a crucial role in plant reproduction. Mature pollen grains land on the stigma, and the compatible interaction between pollen and papilla cells triggers pollen hydration and germination (Palanivelu and Tsukamoto 2012). The germinated pollen tubes (PTs) grow towards the ovule, guided by internal signals, and then deliver two sperm cells into the embryo sac, which fuse with the egg and central cells to complete double fertilization (Swanson et al. 2004; Dresselhaus et al. 2016; Berger et al. 2008). Unlike animal sperm, plant sperm cannot move autonomously and rely on the transport of PTs to successfully reach the ovule. Therefore, the growth and guidance of PTs is key to complete double fertilization (Weterings and Russell 2004; Márton et al. 2005; Zhong and Qu 2019), which is controlled by a complex signaling cascade involving male and female gametes (Lausser and Dresselhaus 2010; Higashiyama and Takeuchi 2015).
Plant male and female gametes secrete a variety of signaling substances to regulate the growth and guidance of pollen grains. In Arabidopsis, the small peptide LURE1.2 secreted by the ovule is recognized by the pollen-specific receptor-like kinase 6 (PRK6), and directs the ovule to specifically attract its own pollen tubes, thereby promoting interspecies reproductive isolation (Zhong et al. 2019; Zhang et al. 2017b; Wang et al. 2016). The absence of receptor-like kinases in Arabidopsis, such as Feronia (FER), ANJEA (ANJ), and HERCULES RECEPTOR KINASE 1 (HERK1), results in the unrestricted elongation of PTs, even entering the micropylar and reaching the synergid cells (Escobar-Restrepo et al. 2007; Galindo-Trigo et al. 2020). Further studies indicate that the rapid alkalinization factor (RALF) peptides expressed by PTs are the ligands of FER, ANJ and HERK1 (Zhong et al. 2022). Mutation of these peptides results in the simultaneous emergence of multiple pollen tubes from the septum (Zhong et al. 2022). After fertilization, EGG CELL-SPECIFIC1 (ECS1 and ECS2) are secreted by the egg cells and degrade LURE1, preventing multiple sperm from entering the egg cell (Yu et al. 2021). In maize, down-regulation of the polypeptide ZmEA1 gene results in egg sterility, suggesting that ZmEA1 is involved in the signaling process of PTs attraction in maize (Márton et al. 2005). However, the mechanism of PTs growth and guidance is very complex and many regulatory pathways remain to be discovered.
Aspartate proteases (APs) are a class of important proteolytic enzymes that contain two Asp residues within the conserved Asp-Thr/Ser-Gly motifs at the active site (Simões and Faro 2004; Takahashi et al. 2008). The APs are activated under acidic pH condition, and the activates of APs are specifically inhibited by pepstatin A (Simões and Faro 2004; Takahashi et al. 2008). APs exist widely in many plants and play an indispensable role in plant development, stress responses, and gamete formation. For instance, tobacco CND41 is a DNA-binding aspartic protease localized in the chloroplast (Takahashi et al. 2008). Overexpression of CND41 in tobacco promotes leaf senescence, suggesting that CND41 plays an important role in leaf senescence (Takahashi et al. 2008; Kato et al. 2005). An atypical aspartic protease (ASPR1) modulates lateral root development in Arabidopsis (Soares et al. 2019). Overexpression of aspartate protease constitutive disease resistance (CDR1) in Arabidopsis and rice can enhance salicylic acid-mediated disease resistance (Xia et al. 2004; Prasad et al. 2009). The Arabidopsis AtASPG1 (ASPARTIC PROTEASE IN GUARD CELL 1) positively regulates ABA-mediated drought resistance (Yao et al. 2012). Subsequent studies suggest that AtASPG1 is also involved in the GA signaling-mediated storage protein degradation (Shen et al. 2018). Moreover, rice aspartate proteases, OsAP25, OsAP37 and OsAP65, are essential for tapetum degradation and pollen tube formation during pollen grain development (Huang et al. 2013; Niu et al. 2013). The Arabidopsis aspartic proteases encoding genes, AtA36 and AtA39, are highly expressed in pollen and pollen tubes (Gao et al. 2017b). Knocking-out of AtA36 and AtA39 leads to programmed apoptosis of microspores, degeneration of female gametophytes, and impaired pollen tube guidance (Gao et al. 2017b). These findings suggest functional specialization of plant APs and tight regulation of AP activity in different biological processes.
Brassica napus is an important oilseed crop derived from spontaneous hybridization between Brassica rapa and Brassica oleracea (Hu et al. 2022; Chen et al. 2023). We have previously identified 154 APs genes in B. napus (Huang et al. 2022). Here, two plasma membrane localized aspartate proteases, BnaAP36s and BnaAP39s, were identified in B. napus. The homologs of BnaAP36 and BnaAP39 were mainly expressed in flower organs, especially in the anther. The sextuple and double mutants of BnaAP36 and BnaAP39 were then generated by CRISPR/Cas9 genome editing technology. The sextuple and double mutants of BnaAP36 and BnaAP39 displayed lower seed-set, and PTs failed to grow towards the micropyle. Our results indicate that BnaAP36s and BnaAP39s are the first APs with an expected role in the regulation of PTs-guided growth in B. napus, which will elucidate the fundamental role of APs in pollen grain survival and egg-sperm communication in plants.
Results
BnaAP36s and BnaAP39s are highly expressed in flower organs in B. napus
We previously identified 154 APs genes in B. napus (Huang et al. 2022). Among them, BnaAP36 and BnaAP39 homologs were significantly up-regulated after pollination (Fig. 1A and B). There were six and two homologs of BnaAP36 and BnaAP39 in B. napus, respectively (Table S1). Based on the chromosome localization, we named these BnaAP36 and BnaAP39 homologs as BnaA08.AP36, BnaC08.AP36, BnaA04.AP36, BnaC04.AP36, BnaA05.AP36, BnaC06.AP36, BnaA06.AP39 and BnaC03.AP39, respectively (Fig. S1, Table S1). The protein sequences of BnaAP36s and BnaAP39s shared high identity with Arabidopsis AP36 and AP39 homologs, ranging from 81.19% to 85.59%, and 83.01% to 83.05%, respectively (Fig. S2 and S3). Domain analysis revealed that both BnaAP36s and BnaAP39s contain signal peptide in N-terminal, and aspartic protease domain in C terminal (Fig. S2 and S3). Like other eukaryotic aspartic proteases, both BnaAP36s and BnaAP39s contain two catalytic Asp residues within two conserved active sites with the sequence Asp-Thr/Ser-Gly-Ser/Thr (Fig. S2 and S3), suggesting that these proteins could be functional as aspartate proteases.
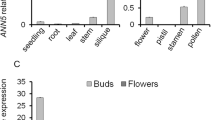
Expression patterns of BnaAP36s and BnaAP39s genes in B. napus. (A) and (B) Expression of homologues of BnaAP36s (A) and BnaAP39s (B) in stigmas before and after pollination. The data was obtained from our previous study (Zhang et al. 2017a) (C) and (D) The bar graphs showed the relative expression levels of BnaAP36s (C) and BnaAP39s (D) in vegetative tissues (root, stem, leaf), flower buds (2 mm 4 mm and 6 mm), seed, and silique. (E) and (F) The bar graphs showed the relative expression levels of BnaAP36s (E) and BnaAP39s (F) in sepal, petal, stigma, ovary, anther, and filament. (G) and (H) Images showing the GUS staining of BnaC08.AP36pro::GUS Arabidopsis transgenic lines (G) and BnaA06.AP39pro::GUS Arabidopsis transgenic lines (H). (I) and (J) The images showed the subcellular localization of BnaC08.AP36 (I) and BnaA06.AP39 (J) after transient expressed in N. benthamiana epidermal cells. The CBL-RFP protein was used as membrane marker. Scale bar=50 μm. In (C) to (F), values were the mean ± SE of three biological replicates. BnaActin7 acts an internal control. The expression level of each gene was calculated using the 2−ΔCT method. In (G) and (H), i: vegetative tissue; ii and iii: flower tissue; iv: pistil; v: stamen. i-iii: Scale bar = 1 mm, iv-v: Scale bar = 0.1 mm
Subsequently, the tissue expression data of BnaAP36s and BnaAP39s were obtained from BnTIR (http://yanglab.hzau.edu.cn/BnTIR) (Liu et al. 2021). Six BnaAP36 homologs were found to be highly expressed in 4 mm flower bud (Fig. S4 A-F). In particular, BnaA08.AP36, BnaC08.AP36, BnaA04.AP36 and BnaC04.AP36 were highly expressed in the anther (Fig. S4A-D). Compared to BnaAP36s, BnaA06.AP39 and BnaC03.AP39 were expressed in anther and 4 mm flower bud (Fig. S4G-H). To verify the RNA-seq results, the vegetative tissues (root, stem, leaf), flower buds (2 mm, 4 mm and 6 mm), open flower (sepal, petal, stigma, ovary, anther and filament), seed, and silique were collected, and the tissue expression pattern of BnaAP36 and BnaAP39 homologs was examined by RT-qPCR. In general, BnaAP36s and BnaAP39s were mainly expressed in flower buds, and less expressed in vegetative tissues (Fig. 1C and D). However, the expression of six BnaAP36 homologues was quite different. For example, BnaA08.AP36, BnaC08.AP36, BnaC04.AP36 and BnaC06.AP36 were highly expressed in 6 mm flower buds; BnaA04.AP36 was highly expressed in 4 mm and 6 mm flower buds; only BnaA05.AP36 was highly expressed in 2 mm flower buds (Fig. 1C). The expression of two BnaAP39s gradually increased with the anther development (Fig. 1D). In open flowers, all the homologs of BnaAP36 and BnaAP39 showed the highest expression in the anther (Fig. 1E and F). In addition, BnaC08.AP36 and BnaA06.AP39 were randomly selected to obtain the exact tissue expression pattern. Two GUS reporter constructs (pBnaC08.AP36::GUS and pBnaA06.AP39::GUS) were generated and individually transformed into Arabidopsis. The positive transgenic lines were obtained in T1 generation and GUS staining assay was performed. The results showed that no significant GUS signal was observed in vegetative tissues in pBnaC08.AP36::GUS and pBnaA06.AP39::GUS Arabidopsis transgenic lines (Fig. 1G (i) and H (i)). The strong GUS signal was found in floral organs, such as sepal (Fig. 1G (ii, iii) and H (ii, iii)), stigma (Fig. 1H (iv)), ovary (Fig. 1G (iv) and H (iv)), anther and filament (Fig. 1G (v) and H (v)) in Arabidopsis transgenic lines.
Sequence analysis revealed that BnaAP36s and BnaAP39s were GPI-anchored proteins (http://mendel.imp.ac.at/gpi/gpi_server.html) (Fig. S5). To determine the subcellular localization of BnaAP36s and BnaAP39s, we transiently expressed the BnaC08.AP36-GFP and BnaA06.AP39-GFP fusion genes in tobacco epidermal cells. The fusion proteins were predominantly localized to the plasma membrane (PM) (Fig. 1I and J), which was colocalized with the PM marker CBL-RFP (Yang et al. 2021). These results indicate that BnaAP36s and BnaAP39s belong to the membrane-anchored aspartic proteases.
Generation the functionally deficient mutants of BnaAP36s and BnaAP39s in B. napus
To study the function of BnaAP36s and BnaAP39s in B. napus, we then generated mutant lines of BnaAP36s and BnaAP39s using the CRISPR/Cas9-mediated genome editing toolbox (Tang et al. 2018). Four single-guide RNAs (sgRNAs) were designed to target the homologous of BnaAP36 (Fig. 2A). Among them, sgRNA1 targeted the first exon of BnaA08.AP36, BnaC08.AP36, BnaA05.AP36 and BnaC06.AP36; sgRNA2 targeted the first exon of BnaA05.AP36 and BnaC06.AP36; sgRNA3 targeted the sixth exon of BnaA08.AP36, BnaC08.AP36, BnaA04.AP36, BnaC04.AP36, and BnaA05.AP36; and sgRNA4 targeted the seventh exon of all six BnaAP36s (Fig. 2A). Two sgRNAs, sgRNA5 and sgRNA6, were designed to target the first and second exons of the two BnaAP39s, respectively (Fig. 2C). These two constructs were then individually transformed into B. napus WT (Westar, self-compatible). Ten and nine mutants of BnaAP36s and BnaAP39s, respectively, were isolated in T0 generation (Tables 1 and 2). Short deletions or insertions at sgRNA target sites were validated by Hi-TOM sequencing (Fig. 2, Tables 1 and 2). The editing efficiency of the sgRNAs was quite variable. For example, no mutation was identified at the target sites of sgRNA1, sgRNA2 and sgRNA3, whereas sgRNA4 simultaneously generated mutations at six BnaAP36s (Fig. 2B, Table 1). In the T0 generation, cr-bnaap36-3 and cr-bnaap36-6 were homozygous sextuple mutants of BnaAP36s, while the other cr-bnaap36 lines and all cr-bnaap39 lines were heterozygous at different sgRNAs targeted sites (Tables 1 and 2). In the T1 generation, the progeny of cr-bnaap36 homozygous mutants (L3 and L6) were all homozygous at the sgRNA4 targeted site (Table S2), and the genotype of other sgRNA targeted sites was WT (sgRNA1, sgRNA2 and sgRNA3) (Table S2). The segregation ratio followed the Mendelian law (Table S2). At the sgRNA5 targeted site, the numbers of wild type : heterozygote : homozygote were 6: 14: 0 (cr-bnaap39, L4, BnaA06.AP39), 6: 14: 0 (cr-bnaap39, L4, BnaC03.AP39), 6: 2: 12 (cr-bnaap39, L9, BnaA06.AP39), and 6: 1:13 (cr-bnaap39, L9, BnaC03.AP39) (Table S3); while the numbers of wild type : heterozygote : homozygote were 11: 1: 8 (cr-bnaap39, L4, BnaA06.AP39), 9: 3: 8 (cr-bnaap39, L4, BnaC03.AP39), 20: 0: 0 (cr-bnaap39, L9, BnaA06.AP39), and 7: 0: 13 (cr-bnaap39, L4, BnaC03.AP39) (Table S3) at sgRNA6 targeted site. We speculate that the small number of populations were tested resulted in a segregation ratio of offspring that did not match the classical Mendelian segregation ratio of 1:2:1.
The genotypes of B. napus BnaA36s and BnaAP39s mutants. (A) The homologues of the BnaAP36 gene model. Grey squares: UTR regions; black squares: exon regions; solid lines: introns; and small red squares: sgRNA target sites. (B) Genotype of the cr-bnaap36 sextuple mutants. The protospacer adjacent motif (PAM) region was marked in green. The sgRNA was denoted in red. The mutation sites were indicated in blue. d#: # of bp deleted from target site; i#: # of bp inserted from target site; wt: wild type. (C) The homologues of the BnaAP39 gene model. Grey squares: UTR regions; black squares: exon regions; solid lines: introns; and small red squares: sgRNA targeted sites. (D) Genotype of the cr-bnaap39 double mutants. The protospacer adjacent motif (PAM) region was marked in green. The sgRNA was denoted in red. The mutation sites were indicated in blue. d#: # of bp deleted from target site; i#: # of bp inserted from target site; wt: wild type
Disruption of BnaAP36s or BnaAP39s genes result in lower seed setting rate
Subsequently, the homozygotes of cr-bnaap36 (sextuple mutant, L3 and L6) and cr-bnaap39 (double mutant, L4 and L9) were isolated by Hi-TOM sequencing in the T1 generation (Fig. 2B and D; Tables 1 and 2), and grown in the field for phenotype observation. Like WT (Westar) and transgenic negative plants (no editing), all mutants showed normal development during the vegetative stage, and the floral organs didn’t show any defect in these mutant lines (data not shown). Then, the silique lengths of cr-bnaap36 after self-crossing were approximately 3.8-4.9 cm, which was shorter than wild-type (WT) and transgenic negative plants (NC) after self-crossing (WT: 5.5 cm; NC: 5.6 cm) (Fig. 3A and B). Seed-set was also reduced in cr-bnaap36 after self-crossing, approximately 11-18 seeds per silique in cr-bnaap36, which was significantly less than WT and transgenic negative plants after self-crossing (WT: 28 seeds per silique; NC: 27 seeds per silique) (Fig. 3C). To further investigate the role of male or female gametophytes in responsible for the seed-set reduction, the silique length and seed set were calculated using cr-bnaap36 plants as the male or female parent in crosses with WT plants. When cr-bnaap36 was used as the male parent, there was no difference in the silique length and the seed-set between cr-bnaap36 × wild-type and wild-type × wild-type (Fig. 3A–C). When cr-bnaap36 was used as the female parent, the silique length and the seed-set were reduced (silique length: 3.9-5.2 cm; and seed set: 12-19 seeds/ silique) (Fig. 3A–C), similar to the self-crossing of cr-bnaap36. Like cr-bnaap36, the silique lengths and the seed-set were also reduced in cr-bnaap39 (Fig. 3D–F), and the seed-set reduction was observed after reciprocal crosses using cr-bnaap39 as the female parent (Fig. 3D–F).
Seed-set was reduced in cr-bnaap36 and cr-bnaap39 mutants. (A) The images showed the siliques of cr-bnaap36 sextuple mutants after self-cross and reciprocal crosses with WT. Scale bars: 1 cm. (B) and (C) The bar graph showed the silique length (B) and seed-set (C) in (A). (D) The images showed the siliques of cr-bnaap39 double mutants after self-cross and reciprocal crosses with WT. Scale bars: 1cm. (E) and (F) The bar graph showed the silique length (B) and seed-set (C) in (D). In (B), (C), (E), and (F), the experiment was repeated three times independently. In each replicate, five individual plants of each line were randomly selected. In each individual plant, at least ten stigmas were randomly pollinated. After pollination, 50 siliques from each line were measured. The seed-set was calculated as the number of seeds per each silique. In (B), (C), (E), and (F), all statistical analyses were calculated in SPSS version 19.0. Error bars were expressed as mean ± SE. P values were obtained by one-way ANOVA with multiple comparisons. P > 0.05 (ns), P < 0.05 (*), P < 0.01 (**). WT: Wild type; NC: transgenic negative plants; L3 and L6: two independent cr-bnaap36 lines; L4 and L9: two independent cr-bnaap39 lines
The viability of mature pollen grains in the BnaAP36 and BnaAP39 mutants was then examined by Alexander staining. No obvious differences were observed in the mature pollen grains of the cr-bnaap36 mutants compared to the WT (Fig. 4A and B). Approximately 9% of pollen grains of cr-bnaap39-4 (8.4%) or cr-bnaap39-9 (9.1%) mutants could not be stained by Alexander dye (Fig. 4A and B). These unstained pollen grains were smaller with abnormal cytoplasm (Fig. 4A). These results suggest that BnaAP39 is essential for pollen grain viability.
The viability of mature pollen grains of cr-bnaap36 and cr-bnaap39 mutants. (A) The viability of mature pollen grains of different materials was observed by Alexander staining. (B) Quantification of pollen activities from different materials. I The experiment was repeated three times independently. In each replicate, five individual plants of each line were selected, and three flowers from each individual plant were randomly selected. More than 1000 pollen grains were counted for statistical analysis. In (B), the statistical analyses were calculated in SPSS version 19.0. Error bars were expressed as mean ± SE. P values were obtained by one-way ANOVA with multiple comparisons. P > 0.05 (ns), P < 0.05 (*), P < 0.01 (**). WT: Wild type; NC: transgenic negative plants; L3 and L6: two independent cr-bnaap36 lines; L4 and L9: two independent cr-bnaap39 lines
PTs in BnaAP36s and BnaAP39s mutants display abnormal micropylar guidance
The very low ratio of inactive pollen in the BnaAP36s and BnaAP39s mutants could not explain why the lower seed-set was significantly higher than that of WT plants (Fig. 3). We therefore monitored pollen tube growth after self- and cross-pollination using the aniline blue assay. The pollen grains of wild-type, cr-bnaap36 (L3 and L6), cr-bnaap39 (L4 and L9) germinated normally, and a large number of PTs could elongate and cross the stigma after 24 h (Fig. 5A and B), suggesting that the reduction in the seed-set isn’t due to the failure of pollen - stigma recognition and PTs elongation.
Abnormal pollen tube guidance in BnaAP36 and BnaAP39 mutants. (A) The images showed the pollen tube growth of cr-bnaap36 sextuple and cr-bnaap39 double mutants after self-crossing by aniline blue assay. Red arrows indicated the pollen tube bundles. Scale bars: 100 μm. (B) The images showed the pollen tube growth of cr-bnaap36 sextuple and cr-bnaap39 double mutants after reciprocal crosses with WT by aniline blue assay. Red arrows indicated the pollen tube bundles. Scale bars: 100 μm. (C) The images showed the defective navigation of cr-bnaap36 sextuple mutant pollen tubes. Pollen tubes were stained with aniline blue and visualized by fluorescence microscopy. i: WT self-pollination. WT pollen tubes enter the micropyle directly. ii and iii: cr-bnaap36-L3 self-pollination. The pollen tubes missed the micropyle (i) or twisted around the ovule surface (ii). Bar = 100 μm. Arrows indicate the pollen tubes. (D) The bar graph showed the statistical analysis of the percentage of pollen tubes targeting the ovule. The experiment was repeated three times independently. In each replicate, five individual plants from each line were selected, and ten stigmas of each plant were randomly pollinated. After pollination, 50 pistils from each line were stained and the number of ovule-directed pollen tubes was calculated for each pistil. In (E), the statistical analyses were calculated in SPSS version 19.0. Error bars were expressed as mean ± SE. P values were obtained by one-way ANOVA with multiple comparison. P > 0.05 (ns), P < 0.05 (*), P < 0.01 (**). WT: Wild type; NC: transgenic negative plants; L3 and L6: two independent cr-bnaap36 lines; L4 and L9: two independent cr-bnaap39 lines
We also followed the path of PTs growth towards the micropyles after self-pollination. Compared to the 88% that entered to the WT micropyles (Fig. 5C (i) and D), fewer PTs grew normally and directly entered the micropyles (cr-bnaap36: 64%-65%, cr-bnaap39: 66%-67%; Fig. 5D). Approximately 35%, and 33% of cr-bnaap36 and cr-bnaap39 pollen tubes showed abnormal guidance, and PTs grew on the ovule surface or twisting around the funicular surface, ultimately missing the micropyles (Fig. 5C (ii-iii) and D). The path of WT PTs was then followed after pollination of cr-bnaap36 and cr-bnaap39 stigma. After 24 h, approximately 34% and 36% of the WT PTs did not directly enter the micropyles (Fig. 5D). Therefore, micropylar guidance is impaired in the pollen tube of cr-bnaap36 and cr-bnaap39. We speculate that the reduction in seed-set in BnaAP36 and BnaAP39 knockout mutants might be due to the failure of recognition between PTs and ovules.
Discussion
The communication between female tissues and growing PTs is required for successful the double fertilization. Many components are involved in the regulation of micropyle-guided PTs growth (Müller et al. 2016). Here, we identified two aspartate proteases BnaAP36s and BnaAP39s in B. napus. Loss of function of BnaAP36s and BnaAP39s resulted in lower seed-set by mediating micropyle-guided PTs growth, indicating that the aspartate proteases play an important role in this process.
Aspartate proteases are hydrolytic enzymes that play an important role in plant reproduction (Ge et al. 2005). For example, rice aspartate proteases OsAP25, OsAP37 and OsAP65 are essential for tapetum degradation and pollen tube formation during pollen grain development (Huang et al. 2013). Further studies confirm that ETERNAL TAPETUM 1 (EAT1) promotes programmed cell death (PCD) of the tapetum by regulating the expression of OsAP25 and OsAP37 (Huang et al. 2013; Niu et al. 2013). We showed that anther-expressed BnaAP36s and BnaAP39s functioned as micropyle guides for PTs growth, while BnaAP39 was also essential for maintaining the survival of mature pollen grains (Fig. 4A and B). Similar to BnaAP36s and BnaAP39s, the Arabidopsis AP36 and AP39 (A36 and A39) are involved in PTs micropyle guidance (Gao et al. 2017b). These results indicate that the conserved function of AP36 and AP39 in Arabidopsis and B. napus. Egg cell-specific APs, ECS1 and ECS2, have been shown to participate in micropyle-directed PT growth by degrading LURE (Yu et al. 2021). The PTs could be germinated on the papilla cells of the BnaAP36 and BnaAP39 knockout lines and grown in style (Fig. 5A). Meanwhile, abnormal micropylar-guided PTs growth was observed in BnaAP36s and BnaAP39s mutants (Fig. 5D), implying that the reduction the seed-set in cr-bnaap36 and cr-bnaap39 could be due to the failure of recognition between pollen tubes and ovules. One possibility is that BnaAP36 and BnaAP39 may regulate the growth of micropylar-guided PTs through degradation of the proteins that block pollen guidance.
There is complex signal regulation between pollen tube and ovule recognition. For instance, the Arabidopsis synergid cells secrete the pollen tube attraction signal LURE1, which is recognized by the pollen-specific receptor-like kinases, LIP1 and LIP2 (Lost in Pollen Tube Guidance 1 and 2), regulating pollen tube growth and guidance (Takeuchi and Higashiyama 2016; Liu et al. 2013). We identified BnaAP36s and BnaAP39s as GPI-modified aspartate proteases (Fig. 1 and Fig. 5). Approximately 35% the pollen tubes lose the ability to direct growth towards micropores in BnaAP36s and BnaAP39s mutants’ style (Fig. 5E). In loss-of-function mutants of other GPI-anchored proteins, A36, A39, and COBL10, PTs also fail to target the ovule (Gao et al. 2017a). GPI-anchored proteins are localized in lipid rafts, which serve as the platforms for protein trafficking and cell signaling transduction (Varma and Mayor 1998). We speculate that BnaAP36s and BnaAP39s might have some relationship with LIP1/ LIP2- LURE1 cassette, which needs to be investigated in the future.
Brassica napus (AACC) is an important allopolyploid crop derived from interspecific crosses between Brassica rapa and Brassica oleracea (Chalhoub et al. 2014; Ahmad et al. 2023). The Arabidopsis homologous genes in B. napus are multiple copies with high sequence similarity. Six and two homologs of BnaAP36 and BnaAP39, respectively, were identified in the B. napus genome with conserved protein domains (Fig. S2 and S3). Although the expression levels of BnaAP36 and BnaAP39 homologs were slightly different, these homologs showed the similar tissue expression patterns (Fig. 1C–D and S4), indicating BnaAP36s and BnaAP39s might function redundantly in B. napus. Indeed, the sextuple and double mutants of BnaAP36 and BnaAP39 showed reduced seed-set, and PTs failed to grow towards the micropyle (Fig. 5); whereas the single mutants of BnaAP36 and BnaAP39 didn’t show any defects in micropylar guidance, suggesting the functional redundancy among different homologs. Notably, the reduction in seed-set of BnaAP36 and BnaAP39 mutants was more severe than that of Arabidopsis A36 and A39 single gene mutants (Fig. 5) (Gao et al. 2017b), and the pollen abortion occurred in the BnaAP39 mutants, but not in the BnaAP36 mutants (Fig. 4), suggesting that the functional differentiation of BnaAP36s and BnaAP39s might be affected by polyploidization process.
Material and Methods
Plant materials and growth condition
All BnaAP36s and BnaAP39s mutants and wild-type (Westar, self-compatible) seeds were soaked in water for 7 days and then placed in soil and grown in the transgenic plant field at Huazhong Agricultural University. Arabidopsis thaliana transgenic lines, and tobacco (Nicotiana benthamiana) were grown in the greenhouse under a light intensity of 100 μmol m−2s−1 with a 16/8 h light/dark photoperiod at 22°C.
Plasmid construction
The genes were amplified from B. napus DNA library by PCR using gene-specific primers. For the GUS staining assay, the ~2kb promoters of BnaC08.AP36 and BnaA06.AP39 were amplified by PCR and cloned into the vector pCAMBIA2300::GUS, respectively. For the subcellular localization assay, the CDS of BnaC08.AP36 and BnaA06.AP39 were amplified by PCR and then cloned into pRI101-GFP vector. To generate BnaAP36s and BnaAP39s mutants, the GENE-sgRNA plant expression vectors were constructed as previously described with minor modifications (Xing et al. 2014). Briefly, four and two sgRNAs were designed to target the homologues of the BnaAP36 and BnaAP39 exons by CRISPR-P (http://cbi.hzau.edu.cn/cgi-bin/CRISPR), respectively (Lei et al. 2014). The sgRNAs cassettes were amplified by PCR using pCBC-DT1T2 as temple, and the PCR fragments were then inserted into pKSE401G by Golden Gate Assembly (Gao et al. 2013). All primers were listed in Table S4.
Plant transformation and genotyping
The procedure of Agrobaterium-mediated transformation of B. napus (Westar, self-compatible) was performed as previously described (Dai et al. 2020). To genotype the CRISPR/Cas9-mediated mutation, the CTAB method was used to extract the genomic DNA (Molecular Cloning 4th edition). The targeted mutations were determined in transgenic plants using the Hi-TOM platform (Liu et al. 2019). Target-specific and barcoding PCR, two rounds of PCR, were performed to amplify the genomic region containing the specific targets of independent samples, and the resulting PCR products were mixed in equal amounts and purified for next-generation sequencing (the Illumina HiSeq platform at Novogene Bioinformatics Institute, Beijing, China). The resulting sequencing data were then decoded using a dedicated online tool to track mutations at the target sites (http://www.hi-tom.net/hi-tom/). The target-specific primer sets are listed in Table S5.
RNA isolation and reverse-transcription quantitative PCR (RT-qPCR)
The B. napus root, stem, leaf, flower bud (2 mm 4 mm and 6 mm), seed, silique, sepal, petal, stigma, ovary, anther, and filament were collected and immediately frozen in liquid nitrogen. Total RNA was extracted using an RNAprep pure plant kit (TIANGEN Cat. #DP441) according to the manufacturer’s instructions. The cDNA was synthesized using a Transcript RT kit (TaKaRa, Japan, Cat. #RR047A). For RT-qPCR, a total volume of 10 μL of reaction mixtures was used, containing 5 μL of 2×SYBR Green Master Mix (BioRad, USA), 4.6 μL of 100× diluted cDNA, 0.2 μL of each primer. Amplification was performed using a CFX384 Real-Time System (TOYOBO, JAPAN). The amplification program was carried out as previously describe (Wu et al. 2020). BnaActin7 was used as an internal reference. The expression level of each gene was calculated using the 2−ΔCT method. All analyses were repeated at least three times. Primers used for RT-qPCR were listed in Supplemental Table S6.
Measurement of seeds per silique
The stigmas were emasculated one day before anthesis to avoid pollen contamination. The emasculated stigmas were then sufficiently pollinated with WT’s or mutant’s pollen. After pollination, the stigmas were covered with paper bags and kept for 10 days. The bags were then removed, and mature siliques were opened to calculate the number of seeds and silique length. The experiment was repeated three times independently. In each replicate, five individual plants of each line were randomly selected. In each individual plant, at least ten stigmas were randomly pollinated. After pollination, 50 siliques from each line were measured. The seed-set was calculated as the number of seeds per each silique.
Pollen viability assay
Pollen grains from fully opened flowers were used pollen viability analysis. Pollen viability was determined as the percentage of stained pollen grains in Alexander. The experiment was repeated three times independently. In each replicate, five individual plants of each line were selected, and three flowers from each individual plant were randomly selected. More than 1000 pollen grains were counted for statistical analysis.
Aniline blue assay
The aniline blue staining assay was performed according to the established procedure, with minor modifications (Sankaranarayanan et al. 2015). The anther was removed the day before anthesis. The emasculated stigmas were pollinated with the corresponding pollen on the day of flowering. After 24 h, the pollinated pistils were placed in fixative buffer (3:1 ethanol, glacial acetic acid) for 2 h, and then the samples were incubated in 1 M NaOH at 65 °C water for 1 h. The pistils were then washed three times with distilled water and stained with the basic aniline blue stain (0.1% aniline blue staining in 0.1 M K3PO4) for 3 h. The stained pistils were placed on a glass slide in distilled water to detect the pollen germination and pollen tube growth using the blue channel (UV 340-380 nm) of a fluorescence microscope (SP8; Leica, Wetzlar, Germany). The experiment was repeated three times independently. In each replicate, five individual plants from each line were selected, and ten stigmas of each plant were randomly pollinated. After pollination, 50 pistils from each line were stained and the number of ovule-directed pollen tubes was calculated for each pistil.
Phytology tree and sequence alignment analysis
Amino acid sequences of BnaAP36 and BnaAP39 homologs were downloaded from BnIR (http://yanglab.hzau.edu.cn/BnIR) and Brassicaceae Database (http://brassicadb.cn). The MEGA software (version 7.0) was used for sequence alignment and clustering analysis by the nearest neighbor method with 1000 bootstrap replicates. The visualization of the sequence alignment results was performed using GeneDoc (version 2.7). The GPI-anchor sequence was predicted by big-PI Pre-dictor (http://mendel.imp.ac.at/gpi/gpi_server.html) (Eisenhaber et al. 1998).
Subcellular localization assay
The protein subcellular localization analysis in N. benthamiana was performed as previously described (Yang et al. 2021). Briefly, in N. benthamiana, Agrobacterium tumefaciens (strain GV3101) cells containing the desired constructs were co-infiltrated into 4- to 5-week-old N. benthamiana leaves with infiltration buffer (50 mM MES, pH=5.6, 2 mM Na3PO4 and 1 mM acetosyringone). The final bacteria concentrations were adjusted to an OD600 = 0.6 for each construct. Infected leaves were analyzed at 48 h after infiltration. GFP fluorescence (Excitation /Emission wavelength: 488 nm / 509 nm), and RFP fluorescence (Excitation /Emission wavelength: 559 nm / 583 nm) were observed under a fluorescence microscope (SPX8, Leica).
GUS staining assay
Two GUS reporter constructs (pBnaC08.AP36::GUS and pBnaA06.AP39::GUS) were generated and individually transformed into Arabidopsis by the floral dipping method (Clough and Bent 1998). Positive transgenic lines were obtained in the T1 generation and GUS staining assay was performed. For the GUS staining assay, the tissues were immersed in X-Gluc solution (Coolaber, SL7160), and incubated at 37 °C overnight. The stained tissues were then photographed under a microscope as previously described (Liu et al. 2020). The experiment was replicated three times independently. In each replicate, five individual plants from each line were selected.
Statistical analyses
Statistical analysis was conducted using SPSS version 19.0 (IBM; https://www.ibm.com). Error bars were expressed as mean ± SE. P values were obtained by one-way ANOVA with multiple comparisons. P > 0.05 (ns), P < 0.05 (*), P < 0.01 (**).
Data availability
Not applicable.
References
Ahmad A, Li W, Zhang H, Wang H, Wang P, Jiao Y, Zhao C, Yang G, Hong D (2023) Linkage and association mapping of ovule number per ovary (ON) in oilseed rape (Brassica napus L.). Mol Breed 43(2):1–16. https://doi.org/10.1007/s11032-023-01355-7
Berger F, Hamamura Y, Ingouff M, Higashiyama T (2008) Double fertilization - caught in the act. Trends Plant Sci 13(8):437–443. https://doi.org/10.1016/j.tplants.2008.05.011
Chalhoub B, Denoeud F, Liu S, Parkin IA, Tang H, Wang X, Chiquet J, Belcram H, Tong C, Samans B, Corréa M, Da Silva C, Just J, Falentin C, Koh CS, Le Clainche I, Bernard M, Bento P, Noel B et al (2014) Plant genetics. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345(6199):950–953. https://doi.org/10.1126/science.1253435
Chen D, Jin Q, Pan J, Liu Y, Tang Y, E Y, Xu L, Yang T, Qiu J, Chen X, Wang J, Gong D, Ge X, Li Z, Cui C (2023) Fine mapping of genes controlling pigment accumulation in oilseed rape (Brassica napus L.). Mol Breed 43 (3):19. https://doi.org/10.1007/s11032-023-01365-5
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x
Dai C, Li Y, Li L, Du Z, Lin S, Tian X, Li S, Yang B, Yao W, Wang J, Guo L, Lu S (2020) An efficient Agrobacterium-mediated transformation method using hypocotyl as explants for Brassica napus. Mol Breed 40(10). https://doi.org/10.1007/s11032-020-01174-0
Dresselhaus T, Sprunck S, Wessel GM (2016) Fertilization Mechanisms in Flowering Plants. Curr Biol 26(3):R125–R139. https://doi.org/10.1016/j.cub.2015.12.032
Eisenhaber B, Bork P, Eisenhaber F (1998) Sequence properties of GPI-anchored proteins near the omega-site: constraints for the polypeptide binding site of the putative transamidase. Protein Eng 11(12):1155–1161. https://doi.org/10.1093/protein/11.12.1155
Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U (2007) The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317(5838):656–660. https://doi.org/10.1126/science.1143562
Galindo-Trigo S, Blanco-Touriñán N, DeFalco TA, Wells ES, Gray JE, Zipfel C, Smith LM (2020) CrRLK1L receptor-like kinases HERK1 and ANJEA are female determinants of pollen tube reception. EMBO Rep 21(2):e48466. https://doi.org/10.15252/embr.201948466
Gao H, Li R, Guo Y (2017a) Arabidopsis aspartic proteases A36 and A39 play roles in plant reproduction. Plant Signal Behav 12(4):e1304343. https://doi.org/10.1080/15592324.2017.1304343
Gao H, Zhang Y, Wang W, Zhao K, Liu C, Bai L, Li R, Guo Y (2017b) Two Membrane-Anchored Aspartic Proteases Contribute to Pollen and Ovule Development. Plant Physiol 173(1):219–239. https://doi.org/10.1104/pp.16.01719
Gao X, Yan P, Shen W, Li X, Zhou P, Li Y (2013) Modular construction of plasmids by parallel assembly of linear vector components. Anal Biochem 437(2):172–177. https://doi.org/10.1016/j.ab.2013.02.028
Ge X, Dietrich C, Matsuno M, Li G, Berg H, Xia Y (2005) An Arabidopsis aspartic protease functions as an anti-cell-death component in reproduction and embryogenesis. EMBO Rep 6(3):282–288. https://doi.org/10.1038/sj.embor.7400357
Higashiyama T, Takeuchi H (2015) The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol 66:393–413. https://doi.org/10.1146/annurev-arplant-043014-115635
Hu J, Chen B, Zhao J, Zhang F, Xie T, Xu K, Gao G, Yan G, Li H, Li L, Ji G, An H, Li H, Huang Q, Zhang M, Wu J, Song W, Zhang X, Luo Y et al (2022) Genomic selection and genetic architecture of agronomic traits during modern rapeseed breeding. Nat Genet 54(5):694–704. https://doi.org/10.1038/s41588-022-01055-6
Huang C, Liang X, Dai C, Wen J, Yi B, Tu J, Shen J, Fu T, Ma C (2022) Genome wide analysis of BnAPs gene family in Brassica napus. Acta Agronomica Sinica (China) 48(3):597–607. https://doi.org/10.3724/SP.J.1006.2022.14023
Huang J, Zhao X, Cheng K, Jiang Y, Ouyang Y, Xu C, Li X, Xiao J, Zhang Q (2013) OsAP65, a rice aspartic protease, is essential for male fertility and plays a role in pollen germination and pollen tube growth. J Exp Bot 64(11):3351–3360. https://doi.org/10.1093/jxb/ert173
Kato Y, Yamamoto Y, Murakami S, Sato F (2005) Post-translational regulation of CND41 protease activity in senescent tobacco leaves. Planta 222(4):643–651. https://doi.org/10.1007/s00425-005-0011-4
Lausser A, Dresselhaus T (2010) Sporophytic control of pollen tube growth and guidance in grasses. Biochem Soc Trans 38(2):631–634. https://doi.org/10.1042/bst0380631
Lei Y, Lu L, Liu HY, Li S, Xing F, Chen LL (2014) CRISPR-P: a web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol Plant 7(9):1494–1496. https://doi.org/10.1093/mp/ssu044
Liu D, Yu L, Wei L, Yu P, Wang J, Zhao H, Zhang Y, Zhang S, Yang Z, Chen G, Yao X, Yang Y, Zhou Y, Wang X, Lu S, Dai C, Yang QY, Guo L (2021) BnTIR: an online transcriptome platform for exploring RNA-seq libraries for oil crop Brassica napus. Plant Biotechnol J 19(10):1895–1897. https://doi.org/10.1111/pbi.13665
Liu J, Zhong S, Guo X, Hao L, Wei X, Huang Q, Hou Y, Shi J, Wang C, Gu H, Qu LJ (2013) Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol 23(11):993–998. https://doi.org/10.1016/j.cub.2013.04.043
Liu Q, Wang C, Jiao X, Zhang H, Song L, Li Y, Gao C, Wang K (2019) Hi-TOM: a platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Sci China Life Sci 62(1):1–7. https://doi.org/10.1007/s11427-018-9402-9
Liu Y, Ye S, Yuan G, Ma X, Heng S, Yi B, Ma C, Shen J, Tu J, Fu T, Wen J (2020) Gene silencing of BnaA09.ZEP and BnaC09.ZEP confers orange color in Brassica napus flowers. Plant J 104 (4):932-949. https://doi.org/10.1111/tpj.14970
Márton ML, Cordts S, Broadhvest J, Dresselhaus T (2005) Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 307(5709):573–576. https://doi.org/10.1126/science.1104954
Müller LM, Lindner H, Pires ND, Gagliardini V, Grossniklaus U (2016) A subunit of the oligosaccharyltransferase complex is required for interspecific gametophyte recognition in Arabidopsis. Nat Commun 7:10826. https://doi.org/10.1038/ncomms10826
Niu N, Liang W, Yang X, Jin W, Wilson ZA, Hu J, Zhang D (2013) EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nat Commun 4:1445. https://doi.org/10.1038/ncomms2396
Palanivelu R, Tsukamoto T (2012) Pathfinding in angiosperm reproduction: pollen tube guidance by pistils ensures successful double fertilization. Wiley Interdiscip Rev Dev Biol 1(1):96–113. https://doi.org/10.1002/wdev.6
Prasad BD, Creissen G, Lamb C, Chattoo BB (2009) Overexpression of rice (Oryza sativa L.) OsCDR1 leads to constitutive activation of defense responses in rice and Arabidopsis. Mol Plant-Microbe Interact 22(12):1635–1644. https://doi.org/10.1094/mpmi-22-12-1635
Sankaranarayanan S, Jamshed M, Samuel MA (2015) Degradation of glyoxalase I in Brassica napus stigma leads to self-incompatibility response. Nat Plants 1:15185. https://doi.org/10.1038/nplants.2015.185
Shen W, Yao X, Ye T, Ma S, Liu X, Yin X, Wu Y (2018) Arabidopsis Aspartic Protease ASPG1 Affects Seed Dormancy, Seed Longevity and Seed Germination. Plant Cell Physiol 59(7):1415–1431. https://doi.org/10.1093/pcp/pcy070
Simões I, Faro C (2004) Structure and function of plant aspartic proteinases. Eur J Biochem 271(11):2067–2075. https://doi.org/10.1111/j.1432-1033.2004.04136.x
Soares A, Niedermaier S, Faro R, Loos A, Manadas B, Faro C, Huesgen PF, Cheung AY, Simões I (2019) An atypical aspartic protease modulates lateral root development in Arabidopsis thaliana. J Exp Bot 70(7):2157–2171. https://doi.org/10.1093/jxb/erz059
Swanson R, Edlund AF, Preuss D (2004) Species specificity in pollen-pistil interactions. Annu Rev Genet 38:793–818. https://doi.org/10.1146/annurev.genet.38.072902.092356
Takahashi K, Niwa H, Yokota N, Kubota K, Inoue H (2008) Widespread tissue expression of nepenthesin-like aspartic protease genes in Arabidopsis thaliana. Plant Physiol Biochem 46(7):724–729. https://doi.org/10.1016/j.plaphy.2008.04.007
Takeuchi H, Higashiyama T (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531(7593):245–248. https://doi.org/10.1038/nature17413
Tang T, Yu X, Yang H, Gao Q, Ji H, Wang Y, Yan G, Peng Y, Luo H, Liu K, Li X, Ma C, Kang C, Dai C (2018) Development and Validation of an Effective CRISPR/Cas9 Vector for Efficiently Isolating Positive Transformants and Transgene-Free Mutants in a Wide Range of Plant Species. Front Plant Sci 9:1533. https://doi.org/10.3389/fpls.2018.01533
Varma R, Mayor S (1998) GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394(6695):798–801. https://doi.org/10.1038/29563
Wang T, Liang L, Xue Y, Jia PF, Chen W, Zhang MX, Wang YC, Li HJ, Yang WC (2016) A receptor heteromer mediates the male perception of female attractants in plants. Nature 531(7593):241–244. https://doi.org/10.1038/nature16975
Weterings K, Russell SD (2004) Experimental analysis of the fertilization process. Plant Cell 16:S107–S118. https://doi.org/10.1105/tpc.016873
Wu J, Yan G, Duan Z, Wang Z, Kang C, Guo L, Liu K, Tu J, Shen J, Yi B, Fu T, Li X, Ma C, Dai C (2020) Roles of the Brassica napus DELLA Protein BnaA6.RGA, in Modulating Drought Tolerance by Interacting With the ABA Signaling Component BnaA10.ABF2. Front. Plant Sci 11:577. https://doi.org/10.3389/fpls.2020.00577
Xia Y, Suzuki H, Borevitz J, Blount J, Guo Z, Patel K, Dixon RA, Lamb C (2004) An extracellular aspartic protease functions in Arabidopsis disease resistance signaling. EMBO J 23(4):980–988. https://doi.org/10.1038/sj.emboj.7600086
Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ (2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14:327. https://doi.org/10.1186/s12870-014-0327-y
Yang B, Zhang K, Jin X, Yan J, Lu S, Shen Q, Guo L, Hong Y, Wang X, Guo L (2021) Acylation of non-specific phospholipase C4 determines its function in plant response to phosphate deficiency. Plant J 106(6):1647–1659. https://doi.org/10.1111/tpj.15260
Yao X, Xiong W, Ye T, Wu Y (2012) Overexpression of the aspartic protease ASPG1 gene confers drought avoidance in Arabidopsis. J Exp Bot 63(7):2579–2593. https://doi.org/10.1093/jxb/err433
Yu X, Zhang X, Zhao P, Peng X, Chen H, Bleckmann A, Bazhenova A, Shi C, Dresselhaus T, Sun MX (2021) Fertilized egg cells secrete endopeptidases to avoid polytubey. Nature 592(7854):433–437. https://doi.org/10.1038/s41586-021-03387-5
Zhang T, Gao C, Yue Y, Liu Z, Ma C, Zhou G, Yang Y, Duan Z, Li B, Wen J, Yi B, Shen J, Tu J, Fu T (2017a) Time-Course Transcriptome Analysis of Compatible and Incompatible Pollen-Stigma Interactions in Brassica napus L. Front Plant Sci 8:682. https://doi.org/10.3389/fpls.2017.00682
Zhang X, Liu W, Nagae TT, Takeuchi H, Zhang H, Han Z, Higashiyama T, Chai J (2017b) Structural basis for receptor recognition of pollen tube attraction peptides. Nat Commun 8(1):1331. https://doi.org/10.1038/s41467-017-01323-8
Zhong S, Li L, Wang Z, Ge Z, Li Q, Bleckmann A, Wang J, Song Z, Shi Y, Liu T, Li L, Zhou H, Wang Y, Zhang L, Wu HM, Lai L, Gu H, Dong J, Cheung AY et al (2022) RALF peptide signaling controls the polytubey block in Arabidopsis. Science 375(6578):290–296. https://doi.org/10.1126/science.abl4683
Zhong S, Qu LJ (2019) Peptide/receptor-like kinase-mediated signaling involved in male-female interactions. Curr Opin Plant Biol 51:7–14. https://doi.org/10.1016/j.pbi.2019.03.004
Zhong S, Liu M, Wang Z, Huang Q, Hou S, Xu YC, Ge Z, Song Z, Huang J, Qiu X, Shi Y, Xiao J, Liu P, Guo YL, Dong J, Dresselhaus T, Gu H, Qu LJ (2019) Cysteine-rich peptides promote interspecific genetic isolation in Arabidopsis. Science 364(6443). https://doi.org/10.1126/science.aau9564
Acknowledgements
The authors extend their appreciation to the support from National Key Laboratory of Crop Genetic Improvement and Hubei Hongshan Laboratory, Huazhong Agricultural University.
Funding Support
This study was supported by the NSFC (No. 32172070) to Chaozhi Ma, and NSFC (No. 32072105) to Cheng Dai.
Author information
Authors and Affiliations
Contributions
C.D., and L.W. designed the research. L.W., and X.L. performed the experiments. B.Y., T.F., and C.M. provided lab support. S.D., and L.W. analyzed the data. C.D., and L.W. wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors approved the submission.
Consent to participate
N/A
Consent for publication
Yes.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1:
Supplemental Figures and Tables. Supplemental Figure S1. Phylogenetic analysis of amino acid sequences of BnaAP36 and BnaAP39 in different plant species. Supplemental Figure S2. Multiple alignments of AP36 from A. thaliana, and B. napus. Supplemental Figure S3. Multiple alignments of AP39 from A. thaliana, and B. napus. Supplemental Figure S4. The tissue expression pattern of BnaAP36 and BnaAP39 homologs. Supplemental Figure S5. Prediction of potential C-terminal GPI-modification sites. Supplemental Table S1. Gene ID of BnaAP36 and BnaAP39 homologs. Supplemental Table S2. Segregation of self-progeny BnaAP36 mutants. Supplemental Table S3. Segregation of self-progeny BnaAP39 mutants. Supplemental Table S4. The list of primers used for making constructs. Supplemental Table S5. The list of primers used for Hi-TOM sequencing. Supplemental Table S6. The list of primers used for RT-qPCR. (DOCX 3575 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Liang, X., Dou, S. et al. Two aspartic proteases, BnaAP36s and BnaAP39s, regulate pollen tube guidance in Brassica napus. Mol Breeding 43, 27 (2023). https://doi.org/10.1007/s11032-023-01377-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-023-01377-1