Abstract
As a food consumed by more than half of the world’s population, rice (Oryza sativa) has always been a hot spot in plant science research. The three most important agronomic traits of rice, the yield, plant height, and flowering time, are controlled by many quantitative trait locus (QTLs). In this study, a newly identified QTL, qHD19, was found to be controlled by a single recessive gene. Using two populations with chromosome segment substitutions, qHD19 was narrowed to a 22.5-kb region containing three putative genes, one of which encoded a ZOS5-02-C2H2 zinc finger protein. This gene was regarded as the qHD19 candidate. Further analysis showed that the amino acid sequence encoded by the qHD19 gene included two amino acid mutations, (84): lysine (L) replaced by phenylalanine (F), and (169): alanine (A) replaced valine (V), and these mutations are expected to significantly alter the functions of the protein. Additionally, the CSSL87 and CSSL88 lines containing the qHD19 gene not only exhibited a shorter plant height but also exhibited a higher yield, which showed that the qHD19 gene presents good application prospects in rice breeding. Taken together, these data indicate that qHD19 probably plays an important role in the signaling network of photoperiodic flowering as well as the regulation of plant height and yield potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flowering time in plants is a critical determinant of the distribution and regional adaptability of plants (Andrés and Coupland 2012). As food for more than half of the world’s population, rice has always been a hot spot in plant science research. Rice yield is not only determined by spike number, grain weight, plant height, and the number of grains per panicle but is also affected by flowering time (Li et al. 2003; Wang et al. 2008). The adaptation of flowering plants is largely determined by their flowering time, which is mainly controlled by photoperiod and temperature (Izawa 2007).
Molecular genetic studies conducted in the past few decades have identified a number of flowering time locus. To date, 734 quantitative trait locus (QTLs) related to the heading stage have been identified, which are distributed among all of the chromosomes of rice (http://www.gramene.org/qtl/). A number of QTLs have been recently revealed in rice (Yano et al. 2000; Kojima et al. 2002; Xue et al. 2008; Wei et al. 2010; Gao et al. 2013; Wu et al. 2013; Gao et al. 2014; Liu et al. 2016; Kim et al. 2018; Zhang et al. 2019). Among these QTLs, dozens of early heading date genes have been identified. Ehd1, encoding a B-type response regulator, upregulates florigen gene expression under both short-day conditions and long-day conditions. Grain number, plant height, and heading date 7 (Ghd7), encoding a CO, CO-like, TOC1 (CCT) domain protein, and Days to heading 8 (DTH8), encoding a putative HAP3 subunit of a CCAAT box-binding transcription factor, act as LD-specific repressors of Ehd1 (Xue et al. 2008; Wei et al. 2010). On the other hand, several positive Ehd1 regulators have also been cloned. For example, it was shown that Early heading date 2 (Ehd2)/RID1/OsId1, encoding a Cs2/His2-type zinc finger protein; Early heading date 3 (Ehd3), encoding a putative plant homeo domain finger-containing protein; and Early heading date 4 (Ehd4), encoding a CCCH-type zinc finger protein, independently promote Ehd1 expression under both short-day conditions and long-day conditions (Matsubara et al. 2008; Matsubara et al. 2011; Gao et al. 2013).
The development of chromosome segment substitution lines (CSSLs), as suggested by Doi et al. (1997) and Kubo et al. (2002), allows the detection of QTLs for complex agronomical traits in plants and may well resolve issues related to the precise mapping of QTLs (Li et al. 2015). Specifically, CSSLs can be used for the detection and fine mapping QTLs as single Mendelian factors by blocking background genetic noise. Several CSSLs have been developed in rice, and many QTLs for traits of biological and economic interest have been detected (Kubo et al. 2002; Ebitani et al. 2005; Mei et al. 2006; Takai et al. 2007; Zhu et al. 2009; Chen et al. 2014; Subudhi et al. 2015; Qi et al. 2017; Liu et al. 2018; Balakrishnan et al. 2019; Sui et al. 2019). These achievements have undoubtedly enhanced the understanding of complex traits and promoted plant genomic studies.
In this study, using a bin map converted from an ultrahigh-quality physical map associated with the heading dates of 146 CSSLs, we identified a heading date gene, qHD19, by map-based cloning. The molecular cloning of qHD19 and phenotypic analysis of the qHD19 gene were also reported. Additionally, the CSSL87 and CSSL88 lines containing the qHD19 gene not only exhibited a shorter plant height but also exhibited a higher yield, which showed that the qHD19 gene presents good application prospects in rice breeding. Taken together, these data indicate that qHD19 probably plays an important role in the signaling network of photoperiodic flowering as well as the regulation of plant height and yield potential.
Materials and methods
Plant materials
A total of 146 chromosome segment substitution lines (CSSLs) derived from DongNanHui 810/ZhangPu wild rice with DongNanHui 810 as the recurrent parent were used to analyze QTLs controlling the heading date. Among the 146 CSSLs, only one CSSL carried two substituted segments, and the remaining 145 carried only one substituted segment. The physical map indicated that the average length of the substituted segments per chromosome was 95.47 Mb in the CSSLs. The total length of the substituted segments in the CSSLs was 1145.65 Mb, which was 3.04 times the total length of the rice genome, and all of the chromosomes exhibited 100% coverage in both cases (Yang et al. 2016).
Identification and substitution mapping of QTLs for heading date
DongNanHui 810, ZhangPu wild rice, and 146 CSSLs were grown in a paddy field under natural conditions at the experimental farm of the Fujian Academy of Agricultural Sciences (Fuzhou, China) at the end of 2017. The field experiment was designed in randomized plots with three plots per genotype. For the parents and each CSSL, 64 plants were planted in eight rows, and three plots were selected to investigate the characteristics of the heading date. The heading date was reported as the mean value from three plots, and QTLs were identified on the basis of significant differences between the parents and each CSSL, as determined by t tests. All plants were grown according to standard commercial practices, with spacing of 13.3 cm between plants within each row and 26.4 cm between rows. Field management essentially followed normal agricultural practices, and the amounts of N, P2O5, and K2O applied were 127.5 kg/hm2, 45.0 kg/hm2, and 30.0 kg/hm2, respectively.
Construction of the mapping population
The mapping population was constructed by crossing CSSL87 and CSSL88 with DongNanHui 810, and a total of 3769 recessive plants in the F2 population were selected for fine mapping.
PCR amplification and marker detection
Plant DNA was extracted from the frozen leaves of rice plants using the CTAB method (Murray and Thompson 1980) with minor modifications. The extracted DNA was dissolved in ddH2O. DNA amplification was performed by PCR with the following parameters: 5 min at 94 °C, 35 cycles of 1 min at 94 °C, 1 min at 60 °C (for Indels) or 55 °C (for SSRs), and 50 s at 72 °C, with a final extension of 10 min at 72 °C. For the PCR amplification of markers, each 20 μL reaction mixture contained 50 ng of DNA, 5 μmol of each primer, 10× PCR buffer [100 mM Tris (pH 8.3), 500 mM KCl, 15 mM MgCl2, 2 μg of gelatin], each dNTP at 250 μM and 0.5 U of Taq polymerase. The amplified PCR products were resolved by electrophoresis in 8% polyacrylamide denaturing gels with silver staining for SSR markers (Panaud et al. 1996).
Molecular mapping of the HD19 gene
A physical map of the target gene was constructed through bioinformatic analysis using (bacterial artificial chromosome) BAC and P1-derived artificial chromosome (PAC) clones of cvar Nipponbare released by the International Rice Genome Sequencing project (IRGSP, http://rgp.dna.affrc.go.jp/IRGSP/index.html). The clones were anchored with the target gene-linked markers, and the alignment of sequences was then carried out using the pair wise Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/blast/bl2seq/b12.html).
Bioinformatic analysis
Candidate genes were predicted according to the available sequence annotation databases (http://rice.plantbiology.msu.edu/;http://www.tigr.org/). DNA and amino acid sequences were employed for complete alignment using Clustal X version 1.81.
Results
Substitution mapping of QTLs for heading date in the CSSLs
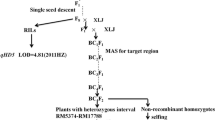
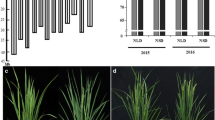
To evaluate the potential advantages of the CSSLs for QTL detection, phenotypic variations in the heading date were observed in the 146 CSSLs. There were two lines, CSSL87 and CSSL88, that showed earlier maturation (Fig. 1). Using three CSSLs, one QTL, qHD19, was identified and mapped within the marker interval between RM153 and RM17919, which spanned a genetic distance of 24.7 cM on rice chromosome 5 (Fig. 2).
Substitution mapping of the qHD19 gene. The substituted segments from ZhangPu wild rice are indicated by black bars. The substituted segments from ZhangPu wild rice are indicated by black bars with the assumption that a segment flanked by one marker of the donor type and one marker of the recipient type constitutes a 50% donor genotype. For the purposes of mapping, however, the full region between one marker of the donor type and one marker of the recipient type was used as the boundary on each end. The vertical bars through the CSSLs indicate the region to which the gene was mapped. Note: **Shows significance at the 0.01 level
Main agronomic characteristics of CSSL87 and CSSL88
To elucidate the genes that control the development of the rice heading date, we performed screening for the comparison of phenotypes between CSSL87, CSSL88, and DongNanHui 810. In addition to showing earlier maturation, CSSL87 and CSSL88 showed a number of special traits (Table 1). For example, CSSL87 and CSSL88 presented a shorter plant height and more effective panicles than DongNanHui 810, showing a difference at the 0.05 probability level or a significant difference at the 0.01 probability level. Additionally, CSSL87 and CSSL88 displayed shorter panicle lengths and fewer spikelets per panicle than DongNanHui 810, differing at the 0.05 probability level. However, we observed that the seed setting rate and the 1000-grain weight of CSSL87 and CSSL88 showed no difference compared with those of DongNanHui 810. It is worth mentioning that the yields of CSSL87 and CSSL88 were 44.06 g and 43.61 g, respectively, which were higher compared with that of DongNanHui 810 (Table 1).
Genetic analysis of the gene for the qHD19 trait
To determine whether qHD19 was controlled by a single gene or multiple genes, CSSL87 and CSSL88 were crossed with DongNanHui 810. All F1 hybrids showed normal phenotypes, and all F2 populations showed normal Mendelian segregation (Table 2). The segregation of the DongNanHui 810 and CSSL87 or CSSL88 plants fit a 3:1 segregation ratio in the two F2 populations (χ2 = 0.430~0.688, P > 0.05) (Table 2). Therefore, these results indicated that the early maturation phenotype was controlled by a single recessive gene.
Fine mapping of the qHD19 gene
To map the qHD19 gene to a smaller region, 3769 recessive individuals were identified from the two F2 populations (Table 2). Another map was constructed using published markers (http://archive.gramene.org/markers/) in the region between RM153 and RM17919, the qHD19 gene was mapped between molecular markers RM17777 and RM17790, and the physical distance between the two markers was 162 kb (Fig. 3b). To further map qHD19, two polymorphic markers were selected between molecular markers RM17777 and RM17790. The results showed that the qHD19 gene was mapped between molecular markers RM17777 and RM17783 on chromosome 5, and the physical distance between the two markers was 69 kb (Fig. 3c and Table 3).
Genetic and physical maps of the qHD19 gene. a Primary mapping of the qHD19 gene. The introgressed segment of qHD19 on the short arm of chromosome 5, and the gene was mapped to the region between markers RM153 and RM17919. b Further mapping of the qHD19 gene. The gene was mapped to the region between markers RM17777 and RM17790. c Fine mapping of the qHD19 gene. The gene was mapped to the region between markers RM17777 and RM17783. d High-resolution mapping of qHD19. The qHD19 gene was finally localized to a 22.5-kb region between markers IND-15 and IND-25, and the number of recombinants between the markers and the target genes was indicated under the linkage map
To fine map the qHD19 gene, seven polymorphic InDels were selected from 24 new InDels (Table 3). The InDel markers were designed from publicly available rice genome sequences, and the likelihood of detecting polymorphisms between ZhangPu wild rice and DongNanHui 810 was predicted by comparing sequences from Nipponbare (http://rgp.dna.affrc.go.jp/) and indica cultivar 93-11 (http://rice.genomics.org.cn/). First, the BAC clone sequences of japonica and indica were aligned; primers were then designed using Primer Premier 5.0 based on the polymorphic region between the two rice subspecies; and polymorphic markers were finally used for gene mapping. Recombinant screening with seven markers (IND-3, IND-5, IND-8, IND-12, IND-15, IND-18 and IND-25) located within the qHD19 locus detected seven, five, five, three, one, zero, and one recombinant, respectively. Thus, the qHD19 gene was precisely mapped within a 22.5-kb region between IND-15 and IND-25 (Fig. 3d).
Candidate genes in the 22.5-kb region
There were three candidate genes (LOC_Os05g02370, LOC_Os05g02390, and LOC_Os05g02400) in the 22.5-kb region (Fig. 3d), according to the available sequence annotation databases (http://rice.plantbiology.msu.edu/;http://www.tigr.org/). LOC_Os05g02370 encodes an expressed protein, LOC_Os05g02390 encodes a ZOS5-02-C2H2 zinc finger protein, and LOC_Os05g02400 encodes an RNA recognition motif-containing protein.
Sequence analyses of the qHD19 gene
To investigate which gene was responsible for the observed phenotype, the sequencing of three genes in DongNanHui 810, ZhangPu wild rice, CSSL87, and CSSL88 revealed that two base substitutions (A to G and C to T) occurred in LOC_Os05g02390 (Fig. 4), whereas no differences in LOC_Os05g02370 and LOC_Os05g02400 were observed in DongNanHui 810, ZhangPu wild rice, CSSL87, and CSSL88. Thus, it was concluded that the LOC_Os05g02390 locus corresponded to qHD19.
The analysis of the open reading frame (ORF) region showed that the qHD19 gene (LOC_Os05g02390) had one exon and no introns. qHD19 was a two-point mutant, exhibiting the exchange of A for G (positions 269) and C for T (positions 506) in its cDNA (Fig. 5).
Further analysis showed that the amino acid sequence encoded by the qHD19 gene presented two amino acid mutations, (84): lysine (L) replaced by phenylalanine (F), and (169): alanine (A) replaced by valine (V) (Fig. 6). Lysine is a positively charged, basic amino acid, while phenylalanine is a nonpolar, hydrophobic amino acid. As such, these mutations would be expected to alter the function of the protein significantly.
Phylogenetic tree for the qHD19 gene
To gain insight into the function of qHD19, a phylogenetic tree was generated using the zinc finger protein sequences from rice and other plants (Fig. 7) according to the available sequence annotation databases (http://www.plant.osakafu-u.ac.jp/~kagiana/gcorn/p/). The phylogenetic tree analysis showed highly homologous genes of qHD19 in more than 20 different species (Fig. 7), and these genes all encoded zinc finger proteins (Table 4). These results indicated that the qHD19 gene presented high homology and conservation among different plants.
Discussion
qHD19 may regulate grain productivity, plant height, and heading date
In rice, the three most important agronomic traits are yield, plant height, and flowering time. The cloned QTLs/genes that control important agronomic traits can be classified according to their functions into the three following groups: genes (Gn1a and GIF1) that underlie grain productivity (Ashikari et al. 2005; Wang et al. 2008), genes (d2 and d11) that regulate plant height (Hong et al. 2003; Tanabe et al. 2005), and genes (Ehd1 and RID1) that regulate heading date (Doi et al. 2004; Wu et al. 2008). Although there are close correlations between yield, plant height, and flowering time, most of these genes have been reported to regulate only one of the three traits. Recently, several QTLs/genes, which contained ghd7, DTH8,DTH7, Ghd2, and OsNF-Y, have been proven to have large pleiotropic effects on an array of traits, including grain number, flowering time, and plant height (Xue et al. 2008; Wei et al. 2010; Gao et al. 2014; Liu et al. 2016; Yang et al. 2017). In this study, a rice early maturation gene, qHD19, was isolated by map-based cloning. Additionally, CSSL87 and CSSL88 containing qHD19 showed a shorter plant height and higher yields compared with DongNanHui 810 (Table 1). These results indicate that the qHD19 gene presents good application prospects for rice breeding in the future.
Utilization of qHD19 in rice breeding
Plant breeding aimed at improving the genetic basis of new varieties of crops with increased productivity and quality, combines art with science (Xi et al. 2006). In general, traditional breeding is predominantly based on phenotypic assays (Xu et al. 2010). However, this approach has targeted QTLs for very few traits for genetic improvement. On the other hand, CSSLs, which are selected at the level of the whole genome with multi-trait breeding objectives, have expanded the available targets and thus become important in improving the properties of plants (Xu et al. 2010). In this study, the qHD19 gene was associated with not only a shorter plant height but also a higher yield (Table 1). Therefore, the qHD19 gene presents good application prospects for rice breeding. First, breeders can develop excellent conventional rice varieties using qHD19. For example, CSSL87 and CSSL88 containing the qHD19 gene showed a shorter plant height and higher yield compared with DongNanHui 810. These two lines can be used as conventional rice lines that can be directly included in regional rice tests and may be certified as new rice varieties. Second, the qHD19 gene was found to be controlled by a single recessive gene (Table 2). Therefore, to breed a new hybrid rice variety, breeders can transfer this gene into both restorer and sterile lines with the assistance of molecular markers.
Why does qHD19 result in early maturation in DongNanHui 810?
Although the qHD19 gene came from the ZhangPu wild rice, ZhangPu wild rice did not show early maturation in the same culture environment. We transferred the qHD19 gene into DongNanHui 810 and obtained two stable lines (CSSL87 and CSSL88) via molecular marker-assisted selection. However, the two lines both showed early maturation (Fig. 2), giving rise to the question of why this occurs? Ehd1 is a pivotal convergence point that integrates multiple signaling pathways to regulate the flowering time of rice under diverse environmental conditions (Tsuji et al. 2011). Studies have shown that the Ehd4 gene encodes a new CCH zinc finger protein and that Ehd4 is upregulated by Ehd1, leading to the expression of the anthocyanin genes Hd3a and RFT1 to promote flowering, independent of other known Ehd1 regulators (Gao et al. 2013). We hypothesized that qHD19 may also promote flowering by upregulating the expression of genes related to anthocyanin through Ehd1. In cultivated rice, the qHD19 gene can normally regulate the expression of related genes and promote flowering, while in ZhangPu wild rice, due to the absence of related genes, flowering cannot be normally regulated, thus delaying heading.
Abbreviations
- CSSLs :
-
Chromosome segment substitution lines
- QTLs :
-
Quantitative trait locus
- SSR :
-
Simple sequence repeat
- INDEL :
-
Insertion/deletion
- ORF :
-
Open reading frame
- BAC :
-
Bacterial artificial chromosome
- PAC :
-
P1-derived artificial chromosome
References
Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005) Cytokinin oxidase regulates rice grain production. Science 309:741–745
Andrés F, Coupland G (2012) The genetic basis of flowering responses to seasonal cues. Nat Rev Genet 13(9):627–639
Balakrishnan D, Surapaneni M, Mesapogu S, Neelamraju S (2019) Development and use of chromosome segment substitution lines as a genetic resource for crop improvement. Theor Appl Genet 132(1):1–25
Chen JB, Li XY, Cheng C, Wang YH, Qin M, Zhu HT, Zeng RZ, Fu XL, Liu ZQ, Zhang GQ (2014) Characterization of epistatic interaction of QTLs LH8 and EH3 controlling heading date in rice. Sci Rep 4:4263
Doi K, Iwata N, Yoshimura A (1997) The construction of chromosome substitution lines of African rice (Oryza glaberrima Steud.) in the background of japonica rice (O. sativa L.). Rice Genet News 14:39–41
Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev 18:926–936
Ebitani T, Takeuchi Y, Nonoue Y, Yamamoto T, Takeuchi K, Yano M (2005) Construction and evaluation of chromosome segment substitution lines carrying overlapping chromosome segments of indica rice cultivar ‘Kasalath’ in a genetic background of japonica elite cultivar ‘Koshihikari’. Breed Sci 55:65–73
Gao H, Zheng XM, Yuan DY, Wang MQ, Zhang Z, Sheng P, Ma WW, Jiang L, Wang HY, Yuan LP (2014) Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc Natl Acad Sci U S A 111(51):16337–16342
Gao H, Zheng XM, Fei GL, Chen J, Jin MN, Ren YL, Wu WX, Zhou KN, Sheng PK, Zhou F, Jinag L, Wang J, Zhang X, Guo XP, Wang LJ, Cheng ZJ, Wu CY, Wang HY, Wan JM (2013) Ehd4 encodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice. PLoS Genet 9:e1003281
Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M (2003) A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell 15:2900–2910
Izawa T (2007) Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J Exp Bot 58(12):3091–3097
Kim SR, Torollo G, Yoon MR, Kwak J, Lee CK, Prahalada GD, Choi IR, Yeo US, Jeong OY, Jena KK, Lee JS (2018) Loss-of-function alleles of heading date 1 (Hd1) are associated with adaptation of temperate Japonica rice plants to the tropical region. Front Plant Sci 9:1827
Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of hd1 under short-day conditions. Plant Cell Physiol 43(10):1096–1105
Kubo T, Aida Y, Nakamura K, Tsunematsu H, Doi K, Yoshimura A (2002) Reciprocal chromosome segment substitution series derived from japonica and indica cross of rice (Oryza sativa L.). Breed Sci 52:319–325
Liu D, Yan Y, Fujita Y, Xu D (2018) Identification and validation of QTLs for 100-seed weight using chromosome segment substitution lines in soybean. Breed Sci 68(4):442–448
Li XY, Qian Q, Fu ZM, Wang YH, Xiong GS, Zeng DL, Wang XQ, Liu XF, Teng S, Hiroshi F, Yuan M, Luo D, Han B, Li JY (2003) Control of tillering in rice. Nature 422:618–621
Li XN, Wang WK, Wang Z, Li KN, Lim YP, Piao ZY (2015) Construction of chromosome segment substitution lines enables QTL mapping for flowering and morphological traits in Brassica rapa. Front Plant Sci 6:432. https://doi.org/10.3389/fpls.2015.00432
Liu JH, Shen JQ, Xu Y, Li XH, Xiao JH, Xiong LZ (2016) Ghd2, a CONSTANS-like gene, confers drought sensitivity through regulation of senescence in rice. J Exp Bot 67(19):5785–5798
Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, Yano M (2008) Ehd2, a rice ortholog of the maize indeterminate1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol 148:1425–1435
Matsubara K, Yamanouchi U, Nonoue Y, Sugimoito K, Wang XZ, Minobe Y, Yano M (2011) Ehd3, encoding a phd finger-containing protein, is a critical promoter of rice flowering. Plant J 66(4):603–612
Mei HW, Xu JL, Li ZK, Yu XQ, Guo LB, Wang YP, Ying CS, Luo LJ (2006) QTLs influencing panicle size detected in two reciprocal introgressive line (IL) populations in rice (Oryza sativa L.). Theor Appl Genet 112(4):648–656
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Panaud O, Chen X, Mccouch SR (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607
Qi L, Sun Y, Li J, Su L, Zheng XM, Wang XN, Li KM, Yang QW, Qiao WH (2017) Identify QTLs for grain size and weight in common wild rice using chromosome segment substitution lines across six environments. Breed Sci 67(5):472–482
Sui F, Zhao D, Zhu H, Gong Y, Tang Z, Huang XY, Zhang G, Zhao FJ (2019) Map-based cloning of a new total loss-of-function allele of OsHMA3 causing high cadmium accumulation in rice grain. J Exp Bot 70(10):2857–2871. https://doi.org/10.1093/jxb/erz093
Subudhi PK, Leon TD, Singh PK, Parco A, Cohn MA, Sasaki T (2015) A chromosome segment substitution library of weedy rice for genetic dissection of complex agronomic and domestication traits. PLoS One 10(6):e0130650
Takai T, Nonoue Y, Yamamoto S, Yamanouchi U, Matsubara K, Liang ZW, Lin HX, Ono N, Uga Y, Yano M (2007) Development of chromosome segment substitution lines derived from backcross between indica donor cultivar ‘Nona bokra’ and japonica recipient cultivar ‘Koshihikari’. Breed Sci 57:257–261
Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, Kato H, Iwasaki Y (2005) A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17:776–790
Tsuji H, Taoka KI, Shimamoto K (2011) Regulation of flowering in rice: two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol 14:45–52
Wang E, Wang JJ, Zhu XD, Hao W, Wang LY, Li Q, Zhang LX, He W, Lu BR, Lin HX, Ma H, Zhang GQ, He ZH (2008) Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat Genet 40(11):1370–1374
Wei XJ, Xu JF, Guo HN, Jiang L, Chen SH, Yu CY, Zhou ZL, Hu PS, Zhai HQ, Wan JM (2010) DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol 153:1747–1758
Wu CY, You CJ, Li CS, Long T, Chen GX, Mary EB, Zhang QF (2008) RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc Natl Acad Sci U S A 105:12915–12920
Wu WX, Zheng XM, Lu GW, Zhong ZZ, Gao H, Chen LP, Wu CY, Wang HJ, Wang Q, Zhou KN, Wang JL, Wu FQ, Zhang X, Guo XP, Cheng ZJ, Lei CL, Lin QB, Liang L, Wang HY, Ge S, Wan JM (2013) Association of functional nucleotide polymorphisms at DTH2 with the northward expansion of rice cultivation in Asia. Proc Natl Acad Sci U S A 110(8):2775–2780
Xue WY, Xing YZ, Weng XY, Zhao Y, Tang WJ, Wang L, Zhou HJ, Yu SB, Xu CG, Li XH, Zhang QF (2008) Natural variation in ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40(6):761–767
Xi ZY, He FH, Zeng RZ, Zhang ZM, Ding XH, Li WT, Zhang GQ (2006) Development of a wide population of chromosome single-segment substitution lines in the genetic background of an elite cultivar of rice (Oryza sativa L.). Genome 49:476–484
Xu JJ, Zhao Q, Du P, Xu CW, Wang BH, Feng Q, Liu QQ, Tang SZ, Gum H, Han B, Liang GH (2010) Developing high throughput genotyped chromosome segment substitution lines based on population whole-genome re-sequencing in rice (Oryza sativa L.). BMC Genomics 24:656
Yang DW, Ye XF, Zheng XH, Cheng CP, Ye N, Huang FH (2016) Development and evaluation of chromosome segment substitution lines carrying overlapping chromosome segments of the whole wild rice genome. Front Plant Sci 7:01737
Yang WJ, Lu ZH, Xiong YF, Yao JL (2017) Genome-wide identification and co-expression network analysis of the OsNF-Y gene family in rice. Crop J 5(1):21–31
Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12(12):2473–2483
Zhang ZH, Zhu YJ, Wang LS, Fan YY, Zhuang JY (2019) Importance of the interaction between heading date genes Hd1 and Ghd7 for controlling yield traits in rice. Int J Mol Sci 20:516
Zhu WY, Lin J, Yang DW, Zhao L, Zhang YD, Zhu Z, Chen T, Wang CL (2009) Development of chromosome segment substitution lines derived from backcross between two sequenced rice cultivars, indica recipient 93-11 and japonica donor Nipponbare. Plant Mol Biol Report 27(2):126–131
Author’s contribution statement
DY drafted the manuscript. DY, XY, XZ, CC, and NY contributed to the data analysis. DY participated in the design of the study and the interpretation of the results and wrote and edited the manuscript.
Funding
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest of Fujian Province (No. 2017R1021-5, 2017R1021-2, 2016R1020-13, 2016R1020-7), the Youth Technology Innovation Team of the Fujian Academy of Agricultural Sciences (No. STIT2017-3-3), the Fujian Provincial Natural Science Foundation of China (No. 2019J01102), the General Project of the Fujian Academy of Agricultural Sciences (No. A2017-13), the Science and Technology Innovation Project of the Fujian Academy of Agricultural Sciences (No. PC2018-2), and the Free Exploration Project of the Fujian Academy of Agricultural Sciences (No. AA2018-21).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors declare that the experiments comply with the current laws of Italy and the P. R. of China.
Competing interests
The authors declare that they have no competing interests.
Additional information
Open access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, D., Cheng, C., Zheng, X. et al. Identification and fine mapping of a major QTL, qHD19, that plays pleiotropic roles in regulating the heading date in rice. Mol Breeding 40, 30 (2020). https://doi.org/10.1007/s11032-020-1109-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-020-1109-x