Abstract
Gibberella ear rot (GER), caused by the fungal pathogen Fusarium graminearum, is becoming one of the most prominent pathogens responsible for ear rot in maize. In this study three F2 populations, F2-C, F2-D, and F2-J, and their corresponding F2:3 families, were constructed by crossing three highly GER-resistant inbred lines—Cheng351, Dan598, and JiV203—with the susceptible line ZW18. We used this cross for genetic analysis and QTL mapping of resistance to GER. Analysis of variance of GER in the three F2 populations revealed the presence of significant differences among genotypes and between locations. The broad-sense heritability (H2) of GER resistance was estimated to be 0.68, 0.63, and 0.64 in the three F2 populations, indicating that genetic factors play a key role in the development of phenotypic variation. Seventeen QTLs conferring resistance to GER were detected in the three F2 populations, among which the QTL qRger7.1, originating from the resistant parent Cheng351, explained 20.16–41.84% of the phenotypic variation. The physical support interval of qRger7.1 exhibited approximately 2 Mb overlap with that of qRger7.2, which was derived from the resistant parent Dan598, supporting the identification of potential “hotspots” of the target QTLs. QTLs derived from the resistant parents Dan598 and JiV203 accounted for 59.67–61.28% and 65.82–66.90%, respectively, of the phenotypic variation. The GER-resistant QTLs identified in this study are useful candidates for improving the resistance to GER in maize using molecular marker-assisted selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gibberella ear rot (GER) is a fungal disease affecting the ears of maize. It is caused by Fusarium graminearum, which also causes rot diseases in the stalks and roots of maize, and affects many other cereal crop hosts, causing related diseases (Goswami and Kistler 2004). The symptoms of GER are rot and pink mold that occur in ears from the tip side (Mesterházy et al. 2012; Brauner et al. 2017). GER reduces the yield and quality of kernels and triggers the accumulation of two major mycotoxins, deoxysqualenol and zearalenone, which cause health problems in humans and animals (Wu 2007; Marin et al. 2013). Long-term high humidity and high temperatures of around 28 °C are conducive to the occurrence of GER (Reid et al. 1999; Goertz et al. 2010). As the use of fungicides and agronomic methods is neither effective nor environmentally friendly, the breeding of maize varieties resistant to GER will be the most effective measure for the prevention and control of the disease (Munkvold 2003).
Although GER has not received as much attention as Fusarium ear rot (FER)—another maize ear rot caused by Fusarium verticilloides—some research has been conducted regarding the inheritance of GER resistance. These results indicate that resistance to GER is inherited in a quantitative manner and is significantly affected by environmental conditions (Ali et al. 2005; Butrón et al. 2015). Additive and dominant effects were found to play an important role in the inheritance of GER resistance (Chungu et al. 1996; Martin et al. 2012). There have been few reports on mapping and exploring GER resistance loci. In 1996, 150 doubled haploid lines from a cross between the high GER-resistance inbred line UH006 and the moderate GER-resistant inbred line UH007 were used to conduct QTL mapping in four environments under artificial inoculation with Fusarium graminearum. Colocalization of QTLs was observed in bins 1.11 and 2.04 (Martin et al. 2011). A recombinant inbred population derived from the cross of a GER-resistant line, CO387, and a GER-susceptible line, CG62, was used for QTL mapping to GER resistance based on silk inoculation and kernel inoculation. Eleven QTLs were detected for silk inoculation, and eighteen for kernel inoculation. However, only two QTLs were detected in more than one test following silk inoculation, and only one was detected following kernel inoculation (Ali et al. 2005). On the basis of the QTLs for GER resistance reported in previous studies, six QTLs were investigated. However, the validation rates were low, indicating that GER resistance is controlled by many low-effect QTLs (Brauner et al. 2017). Previous research has supported this contention and found that the number of resistance QTLs stably inherited in different genetic backgrounds is limited (Ali et al. 2005; Brauner et al. 2017). Researchers have also tried to identify candidate resistance genes through transcriptome profiling of two inbred maize lines with distinct responses to GER. Several differentially expressed genes were detected; these were involved in processes such as membrane transport, hormone regulation, cell wall modification, cell detoxification, and biosynthesis of pathogenesis-related proteins and phytoalexins. These serve as candidates for genes contributing to resistance (Goswami and Kistler 2004).
A small number of effective QTLs and candidate genes with minor effects have been identified, indicating that gene pyramiding of QTLs and genes from different sources could serve as an effective approach for improving resistance against GER. Thus, the identification of effective QTLs is needed, to provide resources for the molecular breeding of GER resistance. In this study, QTL mapping was performed using three F2 populations and their corresponding F2:3 families, from three GER-resistant inbred lines and a susceptible inbred line. The results from this study provide reference data for genetic improvement of resistance against GER in maize.
Materials and methods
Plant materials and field management
In 2016 we screened a range of inbred maize lines for GER resistance and identified three inbred lines that were resistant to GER. The three F2 populations were named F2-C, F2-D and F2-J, and their corresponding F2:3 families were developed for QTL mapping by crossing the three GER-resistant inbred lines Cheng351, Dan598, and JiV203 with the susceptible inbred line ZW18. Cheng351 is an inbred line from the Reid heterotic group in China. Dan598, derived from the inbred line Dan340, is a typical inbred line in the Lvdahonggu heterotic group. JiV203 is an inbred line from the Reid heterotic group, which was developed from a cross between Si278 and 98107. The inbred line ZW18 was derived from a Pioneer hybrid, 38P05, released in 2004 (registration No. Jishenyu2004025). The parental lines and F2:3 family lines were planted at two field trial locations in Gongzhuling (124.79°E, 43.52°N) and Songyuan (124.53°E, 44.79°N), in Jilin province, in May 2019. For each replication, each line was grown in single row five meters long, with 16 plants per line, spaced 0.60 m apart, producing a planting density of 67,000 plants/ha. Compound fertilizer (570 kg/ha) was used before sowing at both locations, and standard culture practices were followed throughout the growing season. All plants were open pollinated and artificially inoculated with a pathogen spore suspension.
Pathogen culture and artificial inoculation
A Fusarium graminearum strain kindly provided by prof. Xu (China Agricultural University, Beijing, China) was used for inoculation. The pathogen was first inoculated on Potato Dextrose Agar (PDA) medium plates and cultured in the dark at 25 °C until the hyphae filled the petri dish. The plates were then stored in a refrigerator at 4 °C. Each pathogen-medium plate was cut into pieces of about one square centimeter and suspended in 1 l of liquid medium containing 5% mung bean decoction, before being shaken at 150 r/min for 2 days at 25 °C. After filtering out the hyphae, the spores were collected and stored in a refrigerator at 4 °C. The day before the inoculation of the maize, a spore suspension with a concentration of 5 × 106 spores per milliliter was prepared using sterile distilled water. A surfactant, 2% Tween-80, was added to the spore suspension and mixed for final inoculation. Five days after the silking date, 2 ml of the prepared spore suspension was injected into the silk channel of each maize ear, using a syringe.
GER disease evaluations
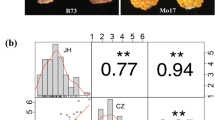
Parental inbred lines and F2:3 families were artificially inoculated with Fusarium graminearum and evaluated for their resistance to GER in two replicates at each of the two field trial locations. These four replicates were labeled G1 and G2 (Gongzhuling), and S1 and S2 (Songyuan). A scoring system with five ratings (1, 3, 5, 7, and 9) was used to evaluate GER disease severity, based on the percentages of the lesion areas. A rating of 1 indicated a lesion area of 0–1%, rating 3 had a lesion area of 2–10%, rating 5 had a lesion area of 11–25%, rating 7 had a lesion area of 26–50%, and rating 9 had a lesion area of 51–100%. Each individual was independently scored 30-day post inoculation (Fig. 1).
Analysis of phenotypic data
A linear mixed model, Y = μ + αG + βL + (αβ)GL + εGLR, was used to calculate the best linear unbiased estimation (BLUE) values of disease ratings in the four replicates, where μ was the overall mean, αG represented the genotypic effect of the Gth line, βL was the effect of the Lth location, (αβ)GL was the effect of the line by location interaction, and εGLR was the residual effect. The overall mean and the genotypic effects were considered fixed, and all remaining terms were considered to be random effects (Sarinelli et al. 2019). BLUE values were calculated using the lmer function in the lme4 package of the R software (Bates et al. 2015).
Analysis of variance (ANOVA) was conducted using the R “anova” command (https://www.r-project.org/) to evaluate the significance of genotypes, location effects, and their interactions. Broad-sense heritability (H2) was estimated as H2 = σ2G/(σ2G + σ2GL/R + σ2e/LR) (Knapp et al. 1985), where σ2G is the genetic variance, σ2GL is the interaction variance of genotype by location, σ2e is the residual error, G is the number of genotypes, L is the number of locations, and R is the number of replications. Shapiro–Wilk tests and skewness tests were used to test whether the phenotype data conformed to a normal distribution, and whether there was skewness in the distribution of the residuals.
Genotyping, construction of linkage maps, and detection of QTLs for resistance to GER
The genomic DNA of each parental inbred line and plants from the F2 populations was genotyped using a GenoBaits Maize10K chip containing 10 K SNP markers, which was developed using a genotyping by target sequencing (GBTS) platform in maize (Guo et al. 2019). Three F2 populations were used to construct linkage maps using MAPMAKER3.0 with polymorphic SNP markers between two corresponding parents (Lincoln et al. 1992). There were 118 individuals in population F2-C, 200 individuals in population F2-D, and 175 individuals in population F2-J. Linkage groups were identified using the “Group” command with a logarithm of odds (LOD) score of 3.0. The recombination frequency was converted into cM using the mapping function of the software (Kosambi 1944).
The polymorphic markers between two parents were screened using genotype data obtained via sequencing with the GenoBaits Maize10K chip. Partial separations and duplicate markers were removed. A total of 466, 441, and 557 polymorphic markers were used to construct linkage maps using MAPMAKER 3.0 for populations F2-C, F2-D, and F2-J, respectively. Each linkage map included ten linkage groups, and the maps spanned a total of 2754.20 cM, 1862.00 cM, and 2086.10 cM, respectively. The average genetic distances between two adjacent markers were 5.91 cM for F2-C, 4.22 cM for F2-D, and 3.75 cM for F2-J. All markers were distributed evenly over each linkage group. Therefore, the constructed linkage maps were appropriate for use for QTL detection.
We constructed a consensus linkage map using JoinMap4.0 (Van Ooijen 2006), using three linkage maps constructed based on the F2-C, F2-D, and F2-J mapping populations, as the three populations have a common parental line, ZW18. After removing partial separation and duplicate markers, the remaining markers were placed into linkage groups using the “Grouping” command, with the Kosambi mapping function (Kosambi 1944). The common markers in the three populations were used to produce linkage groups with the “Combine Groups for Map Integration” command, and then a consensus linkage map was constructed using the “Calculate map” command. Unfortunately, there were not enough common markers and data for a consensus linkage map for a partial number of chromosomes. Then we chose to combine three individual maps, using the “combine maps” command, to generate a consensus linkage map for QTL mapping.
QTL detection was performed using the composite interval mapping method (CIM) (Zeng 1994) in QTL cartographer (Version 2.5), based on phenotypic data from four replications (G1, G2, S1, S2) and two locations (the average value of two replications from each location, designated G and S) as well as BLUE values calculated from the phenotypic data of four replicates (Basten et al. 1997). A significant threshold for confirming a putative QTL was obtained from 1000 permutations at P < 0.05 for each data set.
Results
Phenotypic analysis
The resistance of maize to GER was evaluated for in four parental lines and F2:3 families from three F2 populations, F2-C, F2-D, and F2-J, in Gongzhuling and Songyuan in 2019, with two replicates at each of the two locations. For each replication, each line of 16 plants was grown in single 5 m-long row. The extent of GER disease in each of the plants in each line was evaluated and the average was taken as the rating of that line. The resistant parent Cheng351 had ratings of 1.25, 1.33, 1.00, and 1.00 in G1, G2, S1, and S2, respectively (Fig. S1). The resistant parent Dan598 was consistently rated as 1.00 in four replicates (Fig. S2). JiV203 was rated as 1.15, 1.14, 1.00, and 1.00 in G1, G2, S1, and S2, respectively (S3). The susceptible parent ZW18 was rated as 8.00, 8.00, 9.00, and 7.92 in G1, G2, S1, and S2, respectively (Figs. S1, S2, S3).
The average scale of each F2:3 family was used to represent the disease scale of its corresponding F2 individual. No bimodal distribution in disease severity was observed in any of the three F2 populations (Figs. S1, S2, S3), indicating that GER resistance did not fit the genetic model of a single dominant gene. In contrast, the continuous distribution from highly resistant to susceptible implied that maize resistance to GER was quantitatively inherited. The Shapiro–Wilk tests showed that the resistance ratings of the three F2 populations in the four replicates did not conform to the normal distribution; however, there was no skewed distribution of the residuals in the three populations (Table 1; Fig. 2). Therefore, the GER disease ratings in the four replicates of the three F2 populations could be used for QTL mapping of GER resistance.
Genetic heritability of maize resistance to GER
The broad-sense heritability of GER resistance was estimated by H2 = σ2G/(σ2G + σ2GL/R + σ2e/LR) (Knapp et al. 1985), based on analysis of variance of GER resistance. The analysis was conducted using the GER disease rating of F2 individuals represented by the average GER disease rating of the corresponding F2:3 families in four replications. There were significant differences among genotypes and between locations, as well as among interactions between genotypes and locations, for the GER disease ratings of 118 F2-C individuals (Table 1). The GER disease rating of 200 F2-D individuals and 175 F2-J individuals in four replications also showed significant differences among genotypes and between both locations and repeats, as well as among interactions between genotypes and locations (Table 1). The broad-sense heritability for GER resistance in F2 populations was estimated to be 0.68 in F2-C, 0.63 in F2-D, and 0.64 in F2-J, indicating that phenotypic variance was predominantly controlled by genetic factors in the F2 populations.
QTL mapping for GER resistance
Three F2 populations, F2-C, F2-D, and F2-J, were used for mapping of QTLs conferring resistance to GER in maize. A total of 17 QTLs were detected based on the individual linkage maps of each population and the phenotypic data from the four replications from two locations, as well as BLUE values calculated from the phenotypic data of four replications. Two QTLs associated with resistance to GER were detected in population F2-C, which explained 23.76–52.02% of the phenotypic variation. The QTL qRger7.1, derived from the resistant parent Cheng351, which mapped to bin 7.02, accounted for 20.16–41.84% of the phenotypic variation (Fig. 3; Table 2). The resistance allele at qRger10.1 originating from the susceptible parent ZW18, localized in bins 10.01–10.03, accounting for 3.60–10.18% of the phenotypic variation (Fig. 3; Table 2). GER resistance conferred by qRger1 and qRger2 showed both additive and dominant effects (Table 2).
In population F2-D, we detected six GER-resistant QTLs: qRger2.1, qRger2.2, qRger4.1, qRger6.2, qRger7.2, and qRger9.1, which explained 68.04–71.75% of the phenotypic variation. The resistance alleles at qRger2.1, qRger2.2, qRger4.1, qRger7.2, and qRger9.1, derived from the resistant parent Dan598, mapped to bins 2.01–2.02, 2.02–2.03, 4.01–4.02, 7.01–7.02, and 9.01, respectively. These resistance alleles together explained 59.67–61.28% of the phenotypic variation (Fig. 4; Table 2). The resistance allele at qRger6.2, originating from the susceptible parent ZW18, was localized in bins 6.05–6.06 and accounted for 8.37–10.47% of the phenotypic variation (Fig. 4; Table 2). Resistance to GER conferred by all QTLs mapped in population F2-D showed both additive and dominant gene action (Table 2).
In population F2-J, nine GER-resistant QTLs were identified: qRger1.1, qRger2.3, qRger3.1, qRger4.2, qRger4.3, qRger5.1, qRger6.1, qRger7.3, and qRger9.2. The resistance alleles at qRger1.1, qRger2.3, qRger4.2, qRger4.3, qRger5.1, and qRger7.3 were derived from the resistant parent JiV203 and explained 65.82–66.90% of the phenotypic variation, while the resistance alleles at qRger3.1, qRger6.1, and qRger9.2, originating from the susceptible parent ZW18, together accounted for 21.54–29.99 of the phenotypic variation (Fig. 5; Table 2). Resistance to GER conferred by qRger1.1, qRger2.3, qRger3.1, qRger4.2, qRger4.3, qRger5.1, qRger7.3, and qRger9.2 showed both additive and dominant gene action, and resistance conferred by qRger6.1 was predominantly controlled by additive gene action (Table 2).
Almost no overlap of QTLs was found in the three different F2 populations, except for QTL-qRger7.1 and QTL-qRger7.2. The physical support interval of qRger7.1 had about 2 Mb overlap with that of qRger7.2 derived from resistant parents Dan598, supporting the validity of this potential “hotspot” of the target QTL.
We also conducted mapping of QTLs to GER resistance using a consensus linkage map based on the F2-C, F2-D, and F2-J mapping populations. However, this mapping resulted in the identification of obscure and controversial QTLs (Figs. S4, S5, S6). The consensus linkage map constructed using the current populations was therefore not suitable for use in mapping QTLs to GER resistance.
Discussion
The accurate evaluation of GER proved difficult in this study. Natural inoculation of GER is unreliable, so we performed artificial inoculation by injecting spores directly into the silk channel of the ear, to minimize variation in the developmental stages, flowering times, and seed maturation, which could confound the disease assays. Data on silking dates were recorded. Each individual plant was inoculated 5 days after silking, so all individuals were inoculated at almost the same stage of kernel development. We used an average disease rating scale for each F2:3 family to represent its parental F2 plant. This method proved to be valuable for obtaining accurate phenotypes for the parental lines, as the major resistance QTLs could be consistently detected in different replications. However, no QTLs were detected in all of the four replications and two locations tested, suggesting the existence of a complicated genetic mechanism of GER resistance involving sensitivity to the environment. There was very little difference between the two locations in the number of rainy days or the maximum temperature during the inoculation period and 30 days after inoculation. Obtaining a more accurate phenotype through multi-year and multi-point disease evaluation of GER may be important to obtain better mapping results.
Based on the GER disease ratings—8.0, 8.0, 9.0, and 7.9—in Gongzhuling and Songyuan, the susceptible parent ZW18 was heavily infected by Fusarium graminearum. The resistant parental lines Cheng351, Dan598 and JiV203, in comparison, were not infected, and the disease rating of all the three F2 populations was biased towards resistance to GER. According to the QTLs mapped in the three F2 populations, excluding four QTLs, qRger2.1, qRger9.1, qRger6.1, and qRger7.3, the dominant effects of the remaining fourteen QTLs were negative, indicating that individuals with heterozygous genotypes in the target QTL interval are likely to be biased towards resistance to GER, which explains to a certain extent the skewness of the distribution of phenotypes in the F2 populations. The broad-sense heritability for GER resistance was estimated to be 0.68, 0.63, and 0.64 in the three F2 populations, and it was shown that GER resistance was inherited in quantitative manner, with both additive and dominant effects, in accordance with previous reports (Ali et al. 2005; Butrón et al. 2015; Chungu et al. 1996; Martin et al. 2012). However, the value of the additive effects was higher than the dominant effect for all seventeen QTLs detected in the current study, indicating that GER resistance is predominantly governed by additive gene action. The additive effect of QTLs derived from the resistant parent Cheng351 was stronger than those from Dan598 and JiV203, as well as ZW18 (Fig. 6).
We also attempted to conduct QTL mapping to GER resistance using a consensus linkage map based on the F2-C, F2-D, and F2-J mapping populations, but unfortunately, this proved to be ineffective. In this study, QTL mapping based on the consensus linkage map tended to produce very high LOD thresholds and fewer QTLs could be detected (Figs. S4, S5, S6). QTLs qRger10.1, qRger2.1, qRger4.1, qRger6.2, qRger7.2, qRger1.1, and qRger4.2 could be detected in QTL mapping based on the consensus linkage map at consistent physical intervals. The QTL qRger7.1, which explained 20.16–41.84% of the phenotypic variation, and could be detected in different replications, was not identified using the mapping based on the consensus linkage map. However, there was an obvious peak LOD value in the target support interval, which implied the possibility that QTL-qRger7.1 was authentic. In the process of constructing the consensus linkage map, there were not enough common markers and data among three populations to combine groups for map integration of partial numbers of chromosomes. This problem may be due to the relatively large genetic differences among the three resistant parents. We therefore chose to combine the three individual maps to produce a consensus linkage map for QTL mapping. In any of the three populations there may be some cases in which genotypes at some markers were absent, so the threshold of the LOD value was high, and the effectiveness of mapping was reduced.
One of the most valuable results of this study was the identification of the QTL qRger7.1, which explained 20.16–41.84% of the phenotypic variation. The QTL qRger7.1, derived from the resistant parent Cheng351, mapped to bin 7.02, and had about 2 Mb overlap with qRger7.2, derived from the resistant parent Dan598. The QTL qRger7.2 was located in bins 7.01–7.02, and explained 14.09–15.70% of the phenotypic variation. The QTL qRger2.3, derived from the resistant parent JiV203, was identified in bins 2.04–2.07, and accounted for 9.97–10.39% of the phenotypic variation, and QTLs colocalization in bin 2.04 has previously been reported (Martin et al. 2011). The overlaps of the QTLs mentioned above provide some support for their authenticity.
In the past, Fusarium verticilloides has been the major pathogen that causes Fusarium ear rot. Breeders have paid the most attention to breeding for resistance to FER. For Fusarium graminearum, germplasm resources in maize with GER resistance are lacking due to limited research, a situation that has led to the widespread occurrence of GER in recent years. In order to identify more GER resistance QTLs and resistance genes, alternative approaches such as genome wide association studies, transcriptome sequencing, proteome sequencing, and metabolome sequencing could be adopted. The results of this study provide a basis for future research on the genetic improvement of resistance to GER in maize.
References
Ali ML, Taylor JH, Jie L, Sun G, William M, Kasha KJ, Reid LM, Pauls KP (2005) Molecular mapping of QTLs for resistance to Gibberella ear rot, in corn, caused by Fusarium graminearum. Genome 48:521–533. https://doi.org/10.1139/g05-014
Basten CJ, Weir B, Zeng Z (1997) QTL cartographer: a reference manual and tutorial for QTL mapping. Department of Statistics, North Carolina State University, Raleigh
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:133–199. https://doi.org/10.18637/jss.v067.i01
Brauner PC, Melchinger AE, Schrag TA, Utz HF, Schipprack W, Kessel B, Ouzunova M, Miedaner T (2017) Low validation rate of quantitative trait loci for Gibberella ear rot resistance in European maize. Theor Appl Genet 130:175–186. https://doi.org/10.1007/s00122-016-2802-3
Butrón A, Reid LM, Santiago R, Cao A, Malvar RA (2015) Inheritance of maize resistance to gibberella and fusarium ear rots and kernel contamination with deoxynivalenol and fumonisins. Plant Pathol 64:1053–1060. https://doi.org/10.1111/ppa.12351
Chungu C, Mather DE, Reid LM, Hamilton RI (1996) Inheritance of kernel resistance to Fusarium graminearum in maize. J Hered 87:382–385. https://doi.org/10.1093/oxfordjournals.jhered.a023019
Goertz A, Zuehlke S, Spiteller M, Steiner U, Dehne HW, Waalwijk C, de Vries I, Oerke EC (2010) Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur J Plant Pathol 128:101–111. https://doi.org/10.1007/s10658-010-9634-9
Goswami RS, Kistler HC (2004) Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 5:515–525. https://doi.org/10.1111/j.1364-3703.2004.00252.x
Guo Z, Wang H, Tao J, Ren Y, Xu C, Wu K, Zou C, Zhang J, Xu Y (2019) Development of multiple SNP marker panels affordable to breeders through genotyping by target sequencing (GBTS) in maize. Mol Breed 39(3):37. https://doi.org/10.1007/s11032-019-0940-4
Knapp SJ, Stroup WW, Ross WM (1985) Exact confidence intervals for heritability on a progeny mean basis. Crop Sci 25:192–194. https://doi.org/10.2135/cropsci1985.0011183x002500010046x
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugenics 12:172–175. https://doi.org/10.1111/j.1469-1809.1943.tb02321.x
Lincoln S, Daly M, Lander E (1992) Mapping genetic mapping with MAPMAKER/EXP3.0. Cambridge, Whitehead Institute Technical Report
Marin S, Ramos AJ, Cano-Sancho G, Sanchis V (2013) Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol 60:218–237. https://doi.org/10.1016/j.fct.2013.07.047
Martin M, Miedaner T, Dhillon BS, Ufermann U, Kessel B, Ouzunova M, Schipprack W, Melchinger AE (2011) Colocalization of QTL for Gibberella ear rot resistance and low mycotoxin contamination in early european maize. Crop Sci 51:1935–1945. https://doi.org/10.2135/cropsci2010.11.0664
Martin M, Dhillon BS, Miedaner T, Melchinger AE (2012) Inheritance of resistance to Gibberella ear rot and deoxynivalenol contamination in five flint maize crosses. Plant Breed 131:28–32. https://doi.org/10.1111/j.1439-0523.2011.01908.x
Mesterházy Á, Lemmens M, Reid LM (2012) Breeding for resistance to ear rots caused by Fusarium spp. in maize-a review. Plant Breed 131:1–19. https://doi.org/10.1111/j.1439-0523.2011.01936.x
Munkvold GP (2003) Cultural and genetic approaches to managing mycotoxins in maize. Annu Rev Phytopathol 41:99–116
Reid LM, Nicol RW, Ouellet T, Savard M, Miller JD, Young JC, Stewart DW, Schaafsma AW (1999) Interaction of Fusarium graminearum and F. moniliforme in maize ears: disease progress, fungal biomass, and mycotoxin accumulation. Phytopathology 89:1028–1037. https://doi.org/10.1094/phyto.1999.89.11.1028
Sarinelli JM, Murphy JP, Tyagi P, Holland JB, Johnson JW, Mergoum M, Mason RE, Babar A, Harrison S, Sutton R, Griffey CA, Brown-Guedira G (2019) Training population selection and use of fixed effects to optimize genomic predictions in a historical USA winter wheat panel. Theor Appl Genet 132:1247–1261. https://doi.org/10.1007/s00122-019-03276-6
Van Ooijen JW (2006) Joinmap 4.0, software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen
Wu F (2007) Measuring the economic impacts of Fusarium toxins in animal feeds. Anim Feed Sci 137:363–374. https://doi.org/10.1016/j.anifeedsci.2007.06.010
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Acknowledgments
We are grateful to Prof. Mingling Xu and his lab from China Agricultural University (Beijing, China) for kindly providing the Fusarium graminearum strain for use in this research.
Funding
This study was financially supported by the National Natural Science Foundation of China (NSFC, Grant No. 31701504).
Author information
Authors and Affiliations
Contributions
Yan Zhang and Dongyun Hao conceived and designed the project. Yan Zhang and Jing Wen wrote the paper with input from all authors. Yanqi Shen performed data analysis and disease evaluation. Yuexian Xing and Ziyu Wang prepared the F1, F2 generation and F2:3 families and conducted artificial inoculation. Siping Han, Shijie Li, and Chunming Yang performed other work, including planting in the field, pollination, data processing, and preparation of spore suspension.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable. This manuscript has not been published and is not under consideration for publication elsewhere. The manuscript has been seen and approved by all listed authors.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1794 kb)
Rights and permissions
About this article
Cite this article
Wen, J., Shen, Y., Xing, Y. et al. QTL mapping of resistance to Gibberella ear rot in maize. Mol Breeding 40, 94 (2020). https://doi.org/10.1007/s11032-020-01173-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-020-01173-1