Abstract
Thousand grain weight (TGW) is one of the three crucial determinants of wheat (Triticum aestivum) grain yield. The identification of functional genes and development of closely linked markers are urgently needed for wheat yield improvements. In this study, quantitative trait locus (QTL) mapping for TGW was carried out using one RIL population in four environments. A total of 8 QTL controlling TGW were detected across four chromosomes. Of them, QGW4B.4-17 was confirmed as a major and stable QTL for TGW increases of 2.19–3.06 g and high PVE of 22.5–36.3% in three environments. It was located in an interval (Rht1-TaGW-4B) of 2 Mb in physical distance that contains the Rht1 locus. Three high-confidence (HC) genes were predicted as potential candidates according to their annotated functions and expression profiles. Furthermore, a cleaved amplified polymorphic sequence (CAPS) marker, TaGW-4B, was developed and verified in a panel of 205 wheat cultivars and showed a highly significant correlation with TGW, demonstrating high value for efficient marker-assisted selection. The study provided a basis for cloning the functional genes underlying the QTL and a practical and accurate marker for molecular breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat is one of the three major food crop species worldwide, in addition to rice and maize, and provides approximately one-fifth of the calories and one-quarter of the protein consumed by humans (FAOSTAT 2015). Breeding high-yield wheat varieties has become increasingly important in modern times to meet the ever-growing demand for high volumes of wheat grains for human population growth and arable land reduction.
Thousand grain weight (TGW) is one of the three major components of wheat yield and serves as an important means for increasing wheat grain yield in the breeding process (Acreche and Slafer 2006). Grain size, including kernel length (KL), kernel width (KW), and kernel thickness (KT), usually contributes indirectly to yield by affecting the TGW (Chastain et al. 1995). Furthermore, grain size is also considered a predictor of wheat quality since it affects kernel hardness as well as milling and processing quality (Osborne and Anderssen 2003).
Most yield and quality traits of wheat are controlled by multiple genes. It is essential to identify major TGW quantitative trait locus (QTL) in modern wheat breeding programmes. In recent years, with the rapid development of single-nucleotide polymorphism (SNP) detection and analysis technology, high-density SNP have been widely used in wheat QTL mapping and haplotype analysis, helping clarify the complex relationships between phenotype and genotype (Xu et al. 2019; Liu et al. 2018).
Moreover, comparative genomics has developed rapidly with the complete genome sequencing of rice, maize, brachypodium, and wheat. Chromosomal collinear maps (Salse et al. 2008) and linear comparison evolution maps (Bolot et al. 2009) have been constructed based on DNA sequences and bioinformatic data. Additionally, a good linear relationship between them was reported by Sorrells et al. (2003). Furthermore, Girin et al. (2014) considered the close genetic relationships of both wheat and barley to brachypodium, and the expressed sequence tag (EST) sequence information of wheat was combined with the genome sequences of barley and brachypodium. This combination is highly important for the study of wheat genomics and provides an effective way to finely map functional genes and facilitate their development. Recently, the fully annotated reference genome has shifted the limits of wheat research and breeding (IWGSC 2018), serving both as a resource that can drive disruptive innovation in wheat improvement and as a foundation for accelerating wheat research and genomics-assisted breeding.
In previous reports, several genes associated with rice TGW have been isolated, including GW2, GW5/qSW5, GS6, TGW6, OsCKX2, GS3, and RGB1; these genes control grain weight indirectly by regulating cell division and/or cell expansion in specific grain tissues (Zuo and Li 2014). In recent years, genes related to grain weight in wheat, such as TaGW2-A1 (Su et al. 2011; Yang et al. 2012), TaTGW6 (Hanif et al. 2016; Hu et al. 2016), TaCwi (Jiang et al. 2015; Ma et al. 2012), TaGS5-3A (Ma et al. 2016), TaGS1a (Guo et al. 2013), TaGASR7-A1 (Dong et al. 2014), TaCYP78A3 (Ma et al. 2015), and TaTPP-6AL1 (Zhang et al. 2017), have been isolated, which has improved our understanding of grain weight determination in wheat.
However, all of the abovementioned genes were isolated via a homology-based approach. To date, no genes correlated with wheat grain weight have been isolated via map-based cloning. The molecular basis of QTL/genes in the regulation of grain weight is still largely unknown. Owing to a narrow genetic background of the biparental population or a lack of tight linkage to functional genes, some markers are not efficient or are ineffective for marker-assisted selection (MAS), which limits their range of application in selection or molecular breeding design.
The purpose of the present study is to identify QTL associated with TGW in wheat, locate high-confidence candidate genes, develop molecular markers to track the identified genetic effects or QTL, and finally verify the effects of the developed markers for breeding.
Materials and methods
Plant materials
A recombinant inbred line (RIL) population (F8:9) was used in the current study that consists of 173 lines derived from a cross between the common winter wheat lines Shannong 01-35 and Gaocheng 9411. Shannong 01-35 was bred by our laboratory at Shandong Agriculture University and has a relatively high TGW of approximately 55–60 g. Gaocheng 9411 is a winter variety with good baking quality but a low TGW of 30–34 g.
Field trials
The RIL population was evaluated in four cropping seasons in Tai’an (116° 36′ E, 36° 57′ N) in 2007–2008, 2008–2009, and 2009–2010 (E1, E2, and E3, respectively) and in Suzhou (116° 58′ E, 33° 38′ N) in 2009–2010 (E4). In addition, a panel of 205 varieties and the RIL population were grown in Tai’an, China, in 2014–2015 (E5) and 2015–2016 (E6) to test the efficiency of the developed marker. Trial management was performed in accordance with local cultivation practices. The materials were planted in a randomized complete block design with two replications in each environment. Each plot consisted of three rows, and each row was 2 m long and spaced 21 cm apart.
Measurement of kernel traits
TGW was evaluated by weighing three samples of 1000 kernels from each plot with a precision of 0.01 g. Kernel width (KW), kernel length (KL), and kernel thickness (KT) were determined using Vernier callipers with precision of 0.1 mm. Three samples comprising 30 intact kernels were randomly selected from each plot, lined up according to length to measure KL, and then arranged by width and depth to measure KW and KT, respectively.
Genotyping and linkage map construction
The linkage map used in the current study was described previously by Liu et al. (2018). A total of 9576 polymorphic molecular markers, including 9072 SNP, 442 DArTs, and 59 simple sequence repeat (SSR) markers, were used for genotyping the RIL population.
Statistical analyses and QTL mapping
Statistical analyses were conducted with the SPSS 17.0 (SPSS, Chicago, IL, USA). Differences between the mean values of kernel traits over the environments were estimated via the least significant difference (LSD), and the significance criterion was set at P < 0.05.
Analysis of variance (ANOVA) was performed for kernel traits over the factors of environment and genotype. Broad-sense heritability (hB2) for each trait was estimated by the equation hB2 = σg2/(σg2 + σge2/n + σe2/nr), where σg2 is the genetic variance, σge2 is the interaction between the genotype and environment, σe2 is the environmental variance, n is the number of environments, and r is the number of replications per environment (Wyman et al. 1991).
QTL analyses were performed using the QTL Network 2.0 (Yang and Zhu 2005; Yang et al. 2007) based on the mixed linear model approach (Wang et al. 1999). The significance thresholds for QTL detection were calculated with 1000 permutations. An LOD score of 3.0 was used to validate the presence of putative QTL. Phenotypic values obtained from each environment (E1, E2, E3, and E4) and the pooled data detected from the average of the four environments (AE) were used for the QTL analyses. QTL were named as described previously (Liu et al. 2017).
Primer design, PCR, and sequence analysis
The markers flanking the confidence interval were positioned on the reference genome sequence of Chinese Spring by querying their sequences against the International Wheat Genome Sequencing Consortium (IWGSC) RefSeq 1.0 (https://urgi.versailles.inra.fr/blast_iwgsc/blast.php) sequence via BLAST. The wheat sequence of the flanking marker of the QTL region was used to perform a BLAST search against the genomic sequences of brachypodium, rice, and Aegilops tauschii to identify orthologous genomic regions, as described by Zhai et al. (2016). The genes within the corresponding regions were used to query the IWGSC survey sequences of the hexaploid wheat cultivar Chinese Spring (http://www.wheatgenome.org) to identify homologous contig sequences for marker development (Lu et al. 2016). The sequence variation between chromosomes was used for primer design.
PCR was performed in a total volume of 20 μL, which comprised 0.8 μL of each primer (10–15 pmol), 3 μL of genomic DNA (0.1–2 μg/L), 10 μL of Trans PCR SuperMix (TransGen Biotech), and 5.4 μL of ddH2O. The PCR amplification procedure was as follows: 94 °C denaturation for 5 min, followed by 35 cycles of 94 °C denaturation for 40 s, 59 °C annealing for 45 s, and 72 °C extension for 50 s, with a final extension of 72 °C for 10 min.
The unique F/R amplification primers used for the cleaved amplified polymorphic sequence (CAPS) TaGW-4B markers were as follows:
-
F: 5′-CACTTGTGAAATGATTGGCATTGTTC-3′
-
R: 5′-GATTCTTTTCGACATTTGTTTGCTGTGG-3′
The F/R primers used for wild type Rht1 (Rht-B1a) were as follows:
-
NH-BF.2: 5′-TCTCCTCCCTCCCCACCCCAAC-3′
-
WR1.2: 5′-CCATGGCCATCTCGAGCTGC-3′
The F/R primers used for the mutant Rht1 (Rht-B1b) were as follows:
-
NH-BF.2: 5′-TCTCCTCCCTCCCCACCCCAAC-3′
-
MR1: 5′-CATCCCCATGGCCATCTCGAGCTA-3′
The PCR products amplified from the genomic DNA templates were sequenced using Sanger technology by Shenzhen Huada Gene Technology Co., Ltd. (China).
Restriction endonuclease digestion and agarose gel electrophoresis
According to the polymorphisms within the sequencing results, endonucleases were selected using http://helix.wustl.edu/dcaps/dcaps.html. BsmAI restriction enzyme digestion was performed in a total volume of 10 μL, which comprised 3 μL of PCR products, 0.3 μL of restriction enzymes (10 U/μL), 0.7 μL of 10× buffer, and 6 μL of ddH2O. The digestion involved immersion in a 37 °C water bath for 1 h, immersion in a 65 °C water bath, and inactivation for 5 min. Then, the PCR-amplified products and restriction fragments were analysed by electrophoresis in 8% nondenaturing PAGE gels and visualized with silver staining.
Bioinformatics analysis of high-confidence genes in the target region
The mapped markers were used to identify the high-confidence (HC) genes in the target region using the wheat reference genome assembly RefSeq v1.0 via https://urgi.versailles.inra.fr/jbrowseiwgsc/gmod_jbrowse/. The gene expression profiles were obtained via http://www.wheat-expression.com, and the functional annotations of the HC genes were checked using the RefSeq v1.0 functional annotation via https://blast.ncbi.nlm.nih.gov/Blast.cgi.
Result
Phenotypic variation and analysis of grain-related traits
The mean phenotypic values of the grain traits of the RIL and parental lines in the four environments are presented in Supplementary Table 1. Compared with Gaocheng 9411, Shannong 01-35 had a higher TGW and larger kernel size in all environments. Each kernel trait segregated continuously in the RIL population, and the skewness values for all traits were less than 1.0 (absolute value), indicating normal distributions for these traits. The ANOVA results showed that the genotype and environmental variances for all kernel traits were highly significant at P < 0.001 (Supplementary Table 2). The HB2 of the investigated kernel traits ranged from 63.1 (KW) to 71.2% (TGW), indicating that both genetic and environmental factors played roles in the phenotypes of these traits.
Analysis of the additive effects of TGW and kernel size
As the results showed, eight additive QTL for TGW were identified, mainly on chromosomes 4B and 6A (Table 1, Fig. 1). Of these QTL, QGW4B.4-17 and QGW4B.4-2 each explained more than 13% of the variation. The maximum net effect of QGW4B.4-17 in E4 caused a TGW increase of 3.06 g with a PVE of 36.3%. QGW4B.4-5 (E1), QGW6A.2-232 (E1), and QGW6A.2-137 (E3) explained 8.2%, 7%, and 8.9% of the phenotypic variation, respectively. Four KL QTL, QKL1B.1-3, QKL1B.1-8, QKL1D-25, and QKL4B.4-17, located on chromosomes 1B, 1D, and 4B, had PVE of 13.5%, 15.7%, 9.5%, and 13.2%, respectively. In addition, QKL4B.4-17 was repeatedly identified in E2 and in additive-by-environment (A×E) interactions, with net increased effects for KL of 0.1 mm and 0.09 mm.
Comparative mapping and location of candidate genes in the target region in wheat. (a–c) Comparative analysis of homology between chromosome 3 of rice (a), chromosome 1 of brachypodium (b), and chromosome 4B of wheat (c) within the genetic map. (d) High-confidence (HC) gene information contained in the target segment on the physical map of chromosome 4B
Seven QTL for KW on chromosomes 4B, 5B, and 6A were identified; three of them, QKW4B.4-17 (E2/E4), QKW4B.4-1, and QKW4B.4-5, explained 24.7%, 7.6%, and 15.4% of the phenotypic variation with net effects on KW of 0.11 mm, 0.05 mm, and 0.06 mm, respectively. All the identified QTL presented positive additive effects, indicating that favourable alleles were contributed by Shannong 01-35. A total of 3 QTL for KT were identified on chromosomes 4B and 6B, with PVE values ranging from 5.0 to 19.4%. QKT4B.4-5 was detected in both E3 and A×E with PVE values of 19.4% and 15.8%, respectively. These results demonstrated that locus 4B.4-17 was a major and stable QTL with a pleiotropic effect on various TGW-related traits.
To determine the role of genotype by environment (G×E) interactions in the adaptation to the environment of the studied genotypes, investigations were performed with the same set of genotypes within the multienvironment (ME) interactions that were observed for all investigated kernel traits except KT (Table 1). QGW3B.1-18 presented the maximum additive × environment interaction, with a PVE of 1.3%. With respect to KW, in the ME, QKW4B.4-5 and QKW5B.5-469 were identified as contributors to A×E interactions, with PVE of 0.5% and 0.7%, respectively. Epistatic interactions (AAs) and epistasis-by-environment (AAE) interactions are also important factors for understanding the genetic mechanisms that underlie complex quantitative traits. In our study, three epistatic QTL controlling TGW and KL were detected in the ME (Supplementary Table 3). Among these, the AAs of QGW3B.1-18-QGW6A.2-167 explained 1.2% of the phenotypic variance, and the favourable alleles were contributed by Gaocheng 9411. However, no AAE interactive effects of this QTL were identified. Two epistatic QTL controlling KL were detected, QKL4B.4-17-QKL5A.5-7 and QKL1B.1-3-QGW6A.2-337, with PVE of 1.5% and 2.3%, respectively. The AAE effect of QKL4B.4-17-QKL5A.5-7 contributed 0.2%, with a net positive effect of 0.04 mm. These results suggested that AAs occurred between the same or different chromosomes. Epistasis also contributed to the variation in kernel traits, and more attention should be paid to this phenomenon.
Comparative mapping
The QTL results showed that QGW4B.4-17 was a stable and major QTL related to several kernel sizes with high PVE in multiple environments. According to the preliminary positioning results of the flanking marker of QGW4B.4-17 (RAC875_C27536_611-EX_C101685_705), the genetic distance was approximately 2.6 cm. When the marker sequences were compared with the rice and brachypodium genome sequences, RAC875_C27536_611 was homologous to OS10G0709000 on chromosome 3 of rice and to BRADI1G11220 on chromosome 1 of brachypodium; the sequence corresponding to EX_C101685_705 was homologous to that of the BRADI1G11027 gene on chromosome 1 of brachypodium and to that of OS03G0704700 on chromosome 3 of rice (Fig. 1). Additional analyses of the linear segments of the rice and brachypodium genome information revealed that the corresponding physical chromosomal segment of rice included 24 genes, and the corresponding chromosomal segment of brachypodium contained 20 genes. Among these genes, 18 homologous genes were detected in rice, brachypodium, and wheat. This detection further verified that the wheat target gene segment exhibits a good linear relationship with the partial regions of both chromosome 1 of brachypodium and chromosome 3 of rice. Comparison of the results showed that the linear segment of rice contained two cloned genes, SLR1, and PLA2-III. SLR1 is highly homologous to Rht1 in wheat, which consists of two alleles, a wild type (Rht1a) and a mutant type (Rht1b) (Dowla et al. 2018; Yang et al. 2006). Based on this, allele-specific markers for the Rht1 locus were developed. The genotypic analysis of both parents (Fig. 2) revealed that Shannong 01-35 is the wild type (Rht1a) and that Gaocheng 9411 is the mutant type (Rht1b). Adding the Rht1 marker to the existing map of the RIL population, which is located between RAC875_c27536_611 and EX_C101685_705, elucidated the relationships between Rht1a and Rht1b and grain size and grain weight (Table 2). It showed that Rht1a was related to high grain weight, while Rht1b was related to low TGW. The grain size, KW, and KT of the two genotypes were highly different in both years (P < 0.001). The KL of the two types was significantly different (P < 0.01 or P < 0.05) in both years. Nevertheless, Rht1’s role in the QGW4B.4-17 QTL remains unknown.
Narrowing the interval of TaGW4B.4-17 using Rht1 and the developed marker TaGW-4B
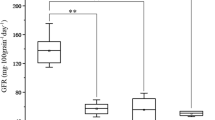
According to the polymorphism of Rht1 and TaGW-4B in the RIL population, the TGW and grain-related traits were reanalysed across the four environments (Table 3, Supplementary Table 5). The results showed that a major QTL-controlling grain weight was located in the interval Rht1-EX_C101685_705 in all four environments and explained 22.93 to 34.38% of the phenotypic variation. In addition, the genetic region controlling KL, KW, and KT was also located within this interval, and these explained 8.38–12.77%, 12.10–19.74%, and 10.05–22.63% of the phenotypic variation, respectively.
To develop markers for accurately and efficiently tracking the genetic effects of the QTL, specific primers were designed to amplify and sequence the target regions (Fig. 3). The results showed two SNP within the sequence of the PCR product at the − 812 and − 950 sites between the two parents. The SNP types in Shannong 01-35 were -812T and -950C, while those in Gaocheng 9411 were -812C and -950T. The -812 SNP was selected for CAPS marker development. Owing to the difference in the T/C base at -812, the PCR products of Shannong 01-35 contain three BsmAI GTCTC restriction enzyme recognition sites, while those of Gaocheng 9411 contain only two restriction sites.
The 1243 bp amplified from Shannong 01-35 was digested into four fragments that were 576, 239, 136, and 292 bp in length by BsmAI restriction enzymes, while the amplicon of Gaocheng 9411 was digested into three fragments that were 576, 375, and 292 bp in length (Fig. 4). Based on the size differences of the digested fragments, the two parental types of allelic variations could be reliably distinguished.
The 173 lines of the RIL population were scanned with the developed CAPS marker to verify its accuracy. Eighty-four were Shannong 01-35 or Hap-T type, and 89 were Gaocheng 9411 or Hap-C type. Adding the CAPS marker to the linkage map showed that it is positioned between Rht1 and EX_C101685_705 with an interval of 0.8 cm to Rht1 and 0.2 cm to EX_C101685_705 (Fig. 1).
Verification of the effectiveness of the TaGW-4B CAPS marker
The effectiveness of the CAPS marker TaGW-4B was verified using a natural population in E5 and E6 (Tables 4). The differences in grain size and KT between Hap-T and Hap-C showed that 130 cultivars were Hap-T, and 75 cultivars were Hap-C. The differences for all traits were significant between the two genotypes for both environments. TGW had P < 0.01, while KL and KT had P < 0.05. Varieties containing haplotype Hap-T possess a larger kernel size, indicating that Hap-T is a positive allele. This demonstrated that the TaGW-4B CAPS marker can be used as a marker for TGW and related grain traits in breeding.
Discussion
Important QTL and HC genes involved in wheat grain weight on chromosome 4B
In recent years, several important genetic loci controlling grain weight have been detected on chromosome 4B (Zhang et al. 2013; Patil et al. 2013; Guan et al. 2018). In the present study, QGW4B.4-17 (Rht1-TaGW-4B) was found to be a major and stable QTL. It was detected in multiple environments and accounted for 36.3% of the phenotypic variation. In addition to grain weight, this locus was also strongly correlated with several other kernel characteristics (i.e. KT and KW). The QTL is located near the centromeric region, and the Rht1 gene appears as a flanking locus (Liu et al. 2006; Deng et al. 2011). Chromosome 4BS, especially genetic regions around Rht1, has been widely reported to be associated with yield-related traits. In 2005, Quarrie et al. reported three yield-related QTL clusters coincident with the dwarfing gene Rht1 on 4BS. In this common region, marker Xgwm113 was found to be correlated with plant height, spike length, and grain number per spike (Liu et al. 2006), and Qgw4B-15 (gwm192a-gwm192b) was revealed to control grain weight (Zhang et al. 2013). In the same region, the QTL QGW.macs-4B.2 (XRht1-Xgwm368) associated with TGW was also reported previously (Patil et al. 2013). Nevertheless, our study developed a CAPS marker for efficiently tracking the genetic effects of the QTL. It is worth noting that the Rht1 gene is associated with plant height. Since there are numerous Rht genes in the genome that also affect plant height (Dowla et al. 2018), plant height value cannot be utilized to track the Rht1 gene variation or the TGW QTL. Although PCR markers are available for Rht1 alleles (Dowla et al. 2018), our CAPS marker serves as an alternative marker that is suitable for high-throughput screening in modern wheat breeding.
The potential candidate gene for the identified QTL was investigated. The genetic association between Rht1 alleles and yield-related traits has been previously reported. Rht1 has two domains, a DELLA and a GRAS domain; DELLA proteins are transcriptional regulators of growth (Tyler et al. 2004). Li et al. (2018) reported that the interval of 15.5–32.3 cm around the Rht-B1 locus on chromosome 4BS harbours a major QTL determining grain yield (GY). They found that the allele from Doumai at QTKW.caas-4BS significantly increased TKW and kernel number per spike and conferred semi-dwarf traits. Xu et al. (2019) further developed eight new markers and narrowed QTKW.caas-4BS to a genetic interval of 1.5 cm. However, there are insufficient data to confirm the molecular relationship between yield traits and Rht1. In the current study, in addition to the Rht1 gene, the QTL region contains three potential candidate genes (Supplementary Table 4). The first is TraesCS4B01G042700.1, which encodes a TCP family transcription factor that is involved in multiple developmental regulatory pathways, such as regulating cell differentiation and growth (Parapunova et al. 2014) and inflorescence architecture and development in bread wheat (Dixon et al. 2018). The second candidate gene is TraesCS4B01G043000.1, which encodes a DMT superfamily factor that can be used to predict the transport and metabolism of carbohydrates and amino acids (Tsuchiya et al. 2016). The third potential candidate gene is TraesCS4B01G043100.1, which is highly expressed in spikes (Supplementary Fig. 1).
In members of the grass family (Gramineae), such as rice, corn, and barley, fully annotated and ordered genome sequences have promoted more time-efficient approaches for the selection and understanding of important traits. However, the identification of functional genes related to grain weight in wheat is difficult because of its large and complex genome and polyploid nature. Many studies have focused on gene cloning, but there have been no reports of successfully cloning major QTL for grain weight via map-based cloning approaches in wheat. Combining the newly annotated reference sequence of wheat with comparative genomics approaches represents an effective way to develop tightly linked markers.
Gupta et al. (2006) suggested that the chromosome of the fourth homologous group of wheat had a good linear relationship with both chromosome 3 of rice and chromosome 1 of brachypodium. In the present study, this collinearity was also confirmed for the target region of QGW4B.4-17, the major QTL for TGW (RAC875_C27536_611-EX_C101685_705).
Although there is a good linear relationship between the genomes of gramineous crops, nonideal factors for comparative genome analysis still exist due to the duplication, insertion, deletion, and inversion of genes during species evolution (Bossolini et al. 2010). In this study, the 4B chromosomal segment, including TaGW4B.4-17, was inverted in the corresponding QTL homologous region in rice and brachypodium.
A comparison of the wheat, rice, and brachypodium genomes showed that 24 genes were included in the corresponding TaGW4B.4-17 region of the rice genome, and 20 genes were included in brachypodium. Among these genes, 18 homologous genes were detected. The homologous relationship between the wheat genome and the brachypodium genome is stronger than that between the wheat genome and the rice genome in the target area, according to the number of homologous genes. These results were consistent with those of Griffiths et al. (2006) and Bossolini et al. (2010).
Conclusion
In the present study, a stable and major QTL QGW4B.4-17 controlling grain weight was detected and mapped to a 2.0-Mb physical region based on the Chinese Spring reference sequence. The region contains nine HC genes, three of which are potential candidate genes. A CAPS marker (TaGW-4B) for grain weight was developed and verified using a natural population, demonstrating a direct application in breeding.
Abbreviations
- CAPS:
-
Cleaved amplified polymorphic sequence
- GWAS:
-
Genome-wide association study
- TGW:
-
Thousand grain weight
- KL:
-
Kernel length
- KW:
-
Kernel width
- KT:
-
Kernel thickness
- MTAs:
-
Significant marker-trait associations
- PVE:
-
Phenotypic variation explained
- QTL:
-
Quantitative trait locus
- RIL:
-
Recombinant inbred line
- SNP:
-
Single-nucleotide polymorphism
- HC:
-
High-confidence
- EST:
-
Expressed sequence tag
- MAS:
-
Marker-assisted selection
- SSR:
-
Simple sequence repeat
- GY:
-
Grain yield
- AE:
-
Average environment
- A×E:
-
Additive-by-environment interactions
References
Acreche MM, Slafer GA (2006) Grain weight response to increases in number of grains inwheatin a Mediterranean area. Field Crop Res 98:52–59
Bolot S, Abrouk M, Masood-Quraishi U, Stein N, Messing J, Feuillet C, Salse J (2009) The ‘inner circle’ of the cereal genomes. Curr Opin Plant Biol 12:119–125
Bossolini E, Wicker T, Knobel PA, Keller B (2010) Comparison of orthologous loci from small grass genomes brachypodium and rice: implications for wheat genomics and grass genome annotation. Plant J 49:704–717
Chastain TG, Ward KJ, Wysocki DJ (1995) Stand establishment responses of soft white winter wheat to seedbed residue and seed size. Crop Sci 35:213–218
Deng SM, Wu XR, Wu YY, Zhou RH, Wang HG, Jia JZ, Liu SB (2011) Characterization and precise mapping of a QTL increasing spike number with pleiotropic effects in wheat. Theor Appl Genet 122:281–289
Dixon LE, Greenwood JR, Bencivenga S, Zhang P, Cockram J, Mellers G, Ramm K, Cavanagh C, Swain SM, Boden SA (2018) Teosinte branched1 regulates inflorescence architecture and development in bread wheat (triticum aestivum L.). Plant Cell 30:563–581
Dong LL, Wang FM, Liu T, Dong ZY, Li AL, Jing RL, Mao L, Li YW, Liu X, Zhang KP, Wang DW (2014) Natural variation of TaGASR7-A1 affects grain length in common wheat under multiple cultivation conditions. Mol Breed 34:937–947
Dowla NU, Edwards I, O’Hara G, Islam S, Ma W (2018) Developing wheat for improved yield and adaptation under a changing climate: optimization of a few key genes. Engineering 4:514–522
Girin T, David LC, Chardin C, Sibout R, Krapp A, Ferrario-Méry S, Daniel-Vedele F (2014) Brachypodium: a promising hub between model species and cereals. J Exp Bot 65:5683–5696
Griffiths S, Sharp R, Foote TN, Bertin I, Wanous M, Reader S, Colas I, Moore G (2006) Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature 439:749–752
Guan PP, Lu L, Jia LJ, Peng HR (2018) Global QTL analysis identifies genomic regions on chromosomes 4A and 4B harboring stable loci for yield-related traits across different environments in wheat (Triticum aestivum L.). Front Plant Sci 9:529
Guo Y, Sun JJ, Zhang GZ, Wang YY, Kong FM, Zhao Y, Li SS (2013) Haplotype, molecular marker and phenotype effects associated with mineral nutrient and grain size traits of TaGS1 a in wheat. Field Crop Res 154:119–125
Gupta PK, Rustgi S, Kumar N (2006) Genetic and molecular basis of grain size and grain number and its relevance to grain productivity in higher plants. Genome 49:565–571
Hanif M, Gao F, Liu J, Wen W, Cao S (2016) Tatgw6 - a1, an ortholog of rice tgw6, is associated with grain weight and yield in bread wheat. Mol Breed 36:1–8
Hu MJ, Zhang HP, Cao JJ, Zhu XF, Wang SX, Jiang H, Wu ZY, Lu J, Chang C, Sun GL, Ma CX (2016) Characterization of an IAA glucose hydrolase gene TaTGW6 associated with grain weight in common wheat (Triticum aestivum L.). Mol Breed 36:25
Jiang YM, Jiang QY, Hao CY, Hou J, Wang LF, Zhang HN, Zhang SN, Chen XH, Zhang XY (2015) A yield-associated gene TaCWI, in wheat: its function, selection and evolution in global breeding revealed by haplotype analysis. Theor Appl Genet 128:131–143
Li FJ, Wen WE, He ZH, Liu JD, Jin H, Cao SH, Geng HW, Yan J, Zhang PZ, Wan YX, Xia XC (2018) Genome-wide linkage mapping of yield-related traits in three Chinese bread wheat populations using high-density SNP markers. Theor Appl Genet 131:1903–1924
Liu S, Zhou R, Dong Y, Li P, Jia J (2006) Development, utilization of introgression lines using synthetic wheat as donor. Theor Appl Genet 112:1360–1373
Liu TT, An YL, Li K, Wang FF, Xie CP, Zhang Y, Guan X, Tian JC, Chen JS (2017) A genetic analysis of the quality of northern-style Chinese steamed bread. Mol Breed 37:41
Liu K, Sun X, Ning T, Duan X, Wang QL, Liu TT, An YL, Guan X, Tian JC, Chen JS (2018) Genetic dissection of wheat panicle traits using linkage analysis and a genome-wide association study. Theor Appl Genet 131:1073–1090
Lu P, Liang Y, Li DL, Wang ZZ, Li WB, Wang GX, Wang Y, Zhou SH, Wu QH, Xie JZ, Zhang DY, Chen YX, Li MM, Zhang Y, Sun QX, Han CG, Liu ZY (2016) Fine genetic mapping of spot blotch resistance gene sb3 in wheat (triticum aestivum L.). Theor Appl Genet 129:577–589
Ma DY, Yan J, He ZH, Wu L, Xia XC (2012) Characterization of a cell wall invertase gene TaCwi-A1 on common wheat chromosome 2A and development of functional markers. Mol Breed 29:43–52
Ma M, Wang Q, Li ZJ, Cheng HH, Li ZJ, Liu XL, Song WN, Appels R, Zhao HX (2015) Expression of TaCYP78A3, a gene encoding cytochrome P450 CYP78A3 protein in wheat (Triticum aestivum L.), affects seed size. Plant J 83:312–325
Ma L, Li T, Hao CY, Wang YQ, Chen XH, Zhang XY (2016) TaGS5-3A, a grain size gene selected during wheat improvement for larger kernel and yield. Plant Biotechnol J 14:1269–1280
Nyquist WE, Baker RJ (1991) Estimation of heritability and prediction of selection response in plant populations. Crit Rev Plant Sci 10:235–322
Osborne BG, Anderssen RS (2003) Single-kernel characterization principles and applications. Cereal Chem J 80:613–622
Parapunova V, Busscher M, Busscherlange J, Lammers M, Karlova R, Bovy AG, Angenent GC, Maagd RA (2014) Identification, cloning and characterization of the tomato tcp transcription factor family. BMC Plant Biol 14:157
Patil RM, Tamhankar SA, Oak MD, Raut AL, Honrao BK, Rao VS (2013) Mapping of qtl for agronomic traits and kernel characters in durum wheat (triticum durum desf.). Euphytica 190:117–129
Salse J, Bolot S, Throude M, Jouffe V, Piegu B, Quraishi UM, Calcagno T, Cooke R, Delseny M, Feuillet C (2008) Identification and characterization of shared duplications between rice and wheat provide new insight into grass genome evolution. Plant Cell 20:11–24
Sorrells ME, La Rota M, Bermudez-Kandianis CE, Greene RA, Kantety R, Munkvold JD, Miftahudin MA, Ma X, Gustafson PJ, Qi LL, Echalier B, Gill BS, Matthews DE, Lazo GR, Chao S, Anderson OD, Edwards H, Linkiewicz AM, Dubcovsky J, Akhunov ED, Dvorak J, Zhang D, Nguyen HT, Peng J, Lapitan NL, Gonzalez-Hernandez JL, Anderson JA, Hossain K, Kalavacharla V, Kianian SF, Choi DW, Close TJ, Dilbirligi M, Gill KS, Steber C, Walker-Simmons MK, McGuire PE, Qualset CO (2003) Comparative DNA sequence analysis of wheat and rice genomes. Genome Res 13:1818–1827
Su ZQ, Hao CY, Wang LF, Dong YC, Zhang XY (2011) Identification and development of a functional marker of TaGW2 associated with grain weight in bread wheat (Triticum aestivum L.). Theor Appl Genet 122:211–223
Tsuchiya H, Doki S, Takemoto M, Ikuta T, Higuchi T, Fukui K, Usuda Y, Tabuchi E, Nagatoishi S, Tsumoto K, Nishizawa T, Ito K, Dohmae N, Ishitani R, Nureki O (2016) Structural basis for amino acid export by DMT superfamily transporter YddG. Nature 534:417–420
Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun TP (2004) Della proteins and gibberellin-regulated seed germination and floral development in arabidopsis. Plant Physiol 135:1008–1019
Wang DL, Zhu J, Li ZKL, Paterson AH (1999) Mapping QTL with epistatic effects and QTL×environment interactions by mixed linear model approaches. Theor Appl Genet 99:1255–1264
Xu D, Wen WW, Fu LP, Li FJ, Li JH, Xie L, Xia XC, Ni ZF, He ZH, Cao SH (2019) Genetic dissection of a major QTL for kernel weight spanning the Rht-B1 locus in bread wheat. Theor Appl Genet 132:3191–3200
Yang J, Zhu J (2005) Methods for predicting superior genotypes under multiple environments based on QTL effects. Theor Appl Genet 110:1268–1274
Yang SJ, Zhang XK, He ZH, Xia XC, Zhou Y (2006) Distribution of dwarfing genes Rht-B1b and Rht-D1b in Chinese bread wheats detected by STS marker. Sci Agric Sin 39:1680–1688
Yang J, Zhu J, Williams RW (2007) Mapping the genetic architecture of complex traits in experimental populations. Bioinformatics 23:1527–1536
Yang ZB, Bai ZY, Li XL, Wang P, Wu QX, Yang L, Li LQ, Li XJ (2012) SNP identification and allelic-specific PCR markers development for TaGW2, a gene linked to wheat kernel weight. Theor Appl Genet 125:1057–1068
Zhai HJ, Feng ZY, Li J, Liu XY, Xiao SH, Ni ZF, Sun QX (2016) QTL analysis of spike morphological traits and plant height in winter wheat (Triticum aestivum L.) using a high-density SNP and SSR-based linkage map. Front Plant Sci 7:1617
Zhang J, Dell B, Biddulph B, Drake-Brockman F, Walker E, Khan N, Wong D, Hayden M, Appels R (2013) Wild-type alleles of Rht1 and Rht-D1 as independent determinants of thousand-grain weight and kernel number per spike in wheat. Mol Breed 32:771–783
Zhang PF, He ZH, Tian XL, Gao FM, Xu DG, Liu JD, Wen WW, Fu LP, Li GY, Sui XX, Xia XC, Wang CP, Cao SH (2017) Cloning of TaTPP-6AL1 associated with grain weight in bread wheat and development of functional marker. Mol Breed 37:78
Zuo JR, Li JY (2014) Molecular genetic dissection of quantitative trait loci regulating rice grain size. Annu Rev Genet 48:99–118
Acknowledgments
The SNP analysis and the construction of the genetic maps were kindly conducted by Dr. Mingcheng Luo from the University of California, Davis, and by Dr. Jirui Wang of Sichuan Agricultural University.
Funding
This work was supported by the National Natural Science Foundation of China (31971936), the Science and Technology of Shandong project (2017GNC11105, 2019LZGC001-3, ZR2019ZD15 and 2019YQ028, 2017CXGC0308).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards
We declare that these experiments comply with the ethical standards in China.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, X., Yu, H., Ma, W. et al. A major and stable QTL controlling wheat thousand grain weight: identification, characterization, and CAPS marker development. Mol Breeding 40, 68 (2020). https://doi.org/10.1007/s11032-020-01147-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-020-01147-3