Abstract
Flowering time of wheat cultivars contributes greatly to the adaptability to environmental conditions and it is largely controlled by vernalization genes. In this study, 262 Chinese mini-core wheat cultivars were used to identify the allelic variation at VRN-B1 locus. A novel dominant allele Vrn-B1d was found in Chinese spring wheat landrace cultivar Hongchunmai. This allele contained several genetic divergence within the first intron comparing to the recessive allele vrn-B1, including one large 6850-bp deletion (670–7519 bp), one small 187-bp deletion (7851–8037 bp), one unique SNP (T to C, 7845 bp), and one 4-bp mutation (TTTT to ACAA, 7847–7850 bp). Meanwhile, it was also different from the three known dominant alleles at VRN-B1 locus. Two pairs of primers were designed to identify the novel allele Vrn-B1d and other four known alleles of VRN-B1. A multiplex PCR was established to discriminate all five alleles simultaneously. The greenhouse experiment with high temperature (non-vernalizing condition) and long light showed that F2 plants containing Vrn-B1d allele headed significantly earlier than those with recessive vrn-B1 allele, suggesting that Vrn-B1d is a dominant allele conferring the spring growth habit. This study provides a useful germplasm and molecular markers for wheat breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flowering time influences the adaptability of wheat to a large range of environments and is mainly controlled by three types of genes, vernalization (VRN), photoperiod (PPD), and earliness per se (ESP) genes (Yasuda and Shimoyama 1965; Kato and Yamagata 1988; Hanocq et al. 2004). The VRN genes in wheat greatly contribute to flowering time and growth habits, dividing wheat into winter and spring types. To date, four VRN genes (VRN1, VRN2, VRN3, and VRN4) have been cloned and characterized in wheat (Yan et al. 2003, 2004b, 2006; Kippes et al. 2015).
The VRN1 gene, a flowering promoter that has an indispensable role in the floral transition pathway of wheat (Loukoianov et al. 2005; Shitsukawa et al. 2007), encodes a MADS-box transcription factor closely related to the Arabidopsis AP1/FRUITFULL family (Yan et al. 2003; Moon et al. 2003). Vernalization treatment can up-regulate the expression of VRN1 conferring early flowering time (Yan et al. 2003). Insertions or deletions within the first intron and the promoter of VRN1 genes are associated with dominant alleles for spring growth habit (Yan et al. 2004b; Fu et al. 2005). The VRN2 gene, a dominant repressor of flowering, encodes two linked and related proteins designated ZCCT1 and ZCCT2 (zinc finger-CCT domain genes), featured by the presence of a putative zinc finger and a CCT (for CONSTANS (CO), CONSTANS-LIKE (CO-Like), and TIMING OF CAB EXPRESSION1 (TOC1) domain (Yan et al. 2004b). The VRN2 is down-regulated by vernalization treatment and VRN1 expression. The VRN2 is up-regulated to block flowering by repressing FT (FLOWERING LOCUS T gene) under long day condition (Trevaskis et al. 2007). Deletion and mutation of both ZCCT1 and ZCCT2 genes are associated with recessive alleles for spring growth habit (Fu et al. 2005; Hemming et al. 2009; Yan et al. 2004a). Due to the dominant allele of VRN2 conferring the winter growth habit, at least one functional copy of VRN2 combined with homozygous recessive alleles at all three VRN1 are required to confer winter growth habit in hexaploid wheat. VRN3 is a homologous gene of the Arabidopsis FT (Yan et al. 2006). The dominant allele is associated with the insertion of a retroelement in VRN3 promoter (Yan et al. 2006). High expression level of VRN3 promotes the transcription of VRN1 and accelerates flowering (Li and Dubcovsky 2008; Yan et al. 2006). The molecular model for VRN1/VRN2/VRN3 interactions suggests that VRN3 is repressed by VRN2 without vernalization treatment (Hemming et al. 2008; Yan et al. 2006). The low-temperature treatment caused the up-regulation of VRN1 and the down-regulation of VRN2 (Chen and Dubcovsky 2012); meanwhile, the low-level expression of VRN2 also resulted in higher transcription level of VRN3 (Distelfeld et al. 2009; Trevaskis et al. 2007). Therefore, low-temperature treatment advanced the flowering by directly up-regulating VRN1 and down-regulating VRN2 and indirectly up-regulating VRN3. VRN-D4 gene originated by the insertion of an approximate 290-kb region from chromosome arm 5AL into the proximal region of chromosome arm 5DS. The inserted 5AL region included a copy of VRN-A1 that carried distinctive mutations in its coding and regulatory regions. These shared mutations can be used to modulate vernalization requirements and to develop wheat varieties better adapted to different or changing environments (Kippes et al. 2015).
Vernalization requirement in common wheat is primarily controlled by three orthologous VRN1 genes located in the middle of the long arms of chromosomes 5A, 5B, and 5D (Pugsley 1971; Pugsley 1972; Law et al. 1975; Stelmakh 1993; Galiba et al. 1995; Dubcovsky et al. 1998; Kato et al. 1999; Barrett et al. 2002; Iwaki et al. 2002; Tóth et al. 2003). Multiple alleles have been described within the VRN-A1, VRN-B1, and VRN-D1, based on their special structural characterizations and vernalization requirement as well as effect on flowering time. The different dominant alleles (Vrn-A1a and Vrn-A1b) of VRN-A1 in common wheat were mostly caused by promoter mutations (Yan et al. 2004a; Fu et al. 2005). The most abundant one in common wheat, Vrn-A1a, has an insertion of a foldback repetitive element and a duplicated region in the promoter, resulting in the complete elimination of the vernalization requirement (Yan et al. 2004a). Dominant alleles (Vrn-B1a, Vrn-B1b, and Vrn-B1c) of VRN-B1 commonly contain large deletions in the first intron which are responsible for a spring habit (Fu et al. 2005; Santra et al. 2009; Milec et al. 2012; Shcherban et al. 2012; Wang et al. 2014). Dominant allele Vrn-B1c possesses a higher level of transcripts and an earlier heading date compared with Vrn-B1a (Shcherban et al. 2013). Dominant alleles (Vrn-D1a, Vrn-D1b, and Vrn-D1c) of VRN-D1 are main results of large deletion in the first intron and promoter mutation in common wheat (Yan et al. 2004a; Fu et al. 2005; Zhang et al. 2015). The dominant Vrn-D1a allele headed earlier than Vrn-D1b allele (Zhang et al. 2012). Zhang et al. (2015) indicated that a 174-bp insertion in Vrn-D1c promoter region contributed to the increasement in VRN-D1 gene expression leading to early heading and flowering. Therefore, various alleles of VRN1 genes contribute differences in vernalization requirements, growth habits, and heading time of wheat.
Cloning of wheat VRN genes has facilitated the development of a series of molecular markers for improving efficiency in identifying different vernalization response alleles (Yan et al. 2003, 2004a, 2006; Fu et al. 2005; Milec et al. 2012; Chen et al. 2013; Chu et al. 2008). The characteristic distributions of allelic combinations of VRN in some countries have been identified by the molecular markers (Fu et al. 2005; Zhang et al. 2008; Sun et al. 2009; Milec et al. 2013; Guo et al. 2015). Allelic variations at VRN genes in diverse eco-geography regions were different. Those results suggested that different combinations of VRN genes have an adaptive value to various environmental conditions (Iwaki et al. 2000, 2001). Therefore, further identification of new VRN alleles in wheat can enrich genetic diversity resources and guarantee the maximize grain production of wheat under different or changing environments.
Chinese wheats are mainly planted in 10 agro-ecological zones, which are further divided into 26 sub-zones based on wheat reaction to temperature, types of growth habit, and wheat growing seasons (He et al. 2001; Zhuang 2003). This distribution condition shows that Chinese wheat cultivars are greatly diversified in vernalization response to various environments, and alleles of VRN genes are abundant in Chinese wheat cultivars (Zhuang, 2003). Recently, several new alleles of VRN genes have been found in Chinese wheat cultivars (Chen et al. 2013; Zhang et al. 2015). In the present study, a novel dominant allele Vrn-B1d from Chinese landrace cultivar Hongchunmai was cloned and characterized. Functional markers were developed to identify novel allele Vrn-B1d. The effect of this allele on heading time was revealed. Additionally, a multiplex PCR was developed to distinguish all five alleles at VRN-B1 locus simultaneously. These results would provide useful germplasm resources and method for marker-assisted selection (MAS) in wheat breeding for adaptation of wheat to different and changing environments.
Materials and methods
Plant material
Hongchunmai, one of Chinese mini-core germplasms, is a spring landrace wheat cultivar originating from Manasi, Xinjiang, China. The combination of four VRN genotypes of Hongchunmai is vrn-A1, Vrn-B1d, Vrn-D1b, and vrn-B3. The 262 Chinese mini-core germplasms were obtained from Dr. Chenyang Hao at Chinese Academy of Agricultural Sciences. To reveal the effect of novel allele Vrn-B1d on heading date, 238 F2 plants derived from a cross between Jing 411 (a Chinese winter cultivar with vrn-A1, vrn-B1, vrn-D1, and vrn-B3) and Hongchunmai were used for evaluation of heading time in the greenhouse under non-vernalizing (20–25 °C) and long day (16 h light) conditions. Chinese Spring nulli-tetrasomic lines N5AT5D, N5BT5D, and N5DT5B were used to validate the genome specificity of the designed primers. Cultivars Longmai 32 (vrn-B1), Ganmai 8 (Vrn-B1a), Longmai 34 (Vrn-B1b), and Baiyoumai (Vrn-B1c) were used as controls to confirm the accuracy and efficiency of the newly developed markers.
Sequencing and structural analysis of VRN-B1 gene
Three primer pairs Ex1/B/F3 (gaagcggatcgagaacaaga) and Ex2/B/R3 (tagcgctcataccgttcaag), Ex2/B/F1 (tcttgaacggtatgagcgctactt) and Ex3/B/R1 (tgtctcaaccttcgccttcagtttcc), and Ex3/B/F2 (ggaaactgaaggcgaaggttgaga) and Ex8/B/R2 (tgcactgccgcatcctctgc) designed by Milec et al. (2012) were used to clone the complete VRN-B1 sequence of Hongchunmai. The number next to “Ex” in the primers refers to the numerical order of exons. PCR was performed following Milec et al. (2012). The cloned PCR products were excised from agarose gel and purified using Quick DNA Extraction Kit. Purified products were ligated into the pGEM-T Easy vector and transformed into competent cells of the Escherichia coli DH-5α strain. More than three clones detected by each colony PCR for new allele were sequenced by Augct Company (http://www.augct.com). Sequence assembly of the new allele and multiple alignments with the known alleles vrn-B1 (AY747604.1), Vrn-B1a (AY747603.1), Vrn-B1b (FJ766015), and Vrn-B1c (HQ130482) were performed by DNAMAN Version 8.0 software (https://www.lynnon.com/) to reveal the specific structural characterization of new allele.
Primer design and analysis of genotypes
Based on sequence alignments of the new allele and four known alleles at the VRN-B1 locus, two forward primers (B/F-1 and B/F-2) and a common reverse primer (B/R) were designed by Primer 5 software to discriminate the new allele and other known alleles (Supplement 1). PCR reactions were conducted in the volume 20 μL containing 100 ng genomic DNA, 4 pmol of each primer (Supplement 1) and 10 μL 2 × ES Taq Master Mix (CWBIO, Beijing, China) (200 μM of each dNTP and 1.5 mM MgCl2) using BIO-RAD T100 Thermal Cycler. The cycling conditions were as follows: 94 °C for 5 min followed by 30 cycles of 94 °C for 45 s, 58 °C for 45 s, and 72 °C for 1.5 min, and a final 8-min extension at 72 °C. PCR products were separated by electrophoresis on a 1.5% agarose gel stained with ethidium bromide and visualized using UV light.
The multiplex PCR was developed with two forward primers (B/F-1 and B/F-2) and a common reverse primer (B/R). The PCR conditions were as described above.
VRN-B1-specific primers described by Fu et al. (2005) and Milec et al. (2012) were used to detect genotypes of 262 Chinese mini-core germplasms. VRN-D1-specific primers described by Fu et al. (2005) and Zhang et al. (2012) were used to screen the genotypes of the 238 F2 plants.
Greenhouse experiment
In order to reveal the effect of novel allele Vrn-B1d on heading time, the F2 plants from the Jing411/Hongchunmai cross and parents were grown in the greenhouse with the temperature between 20 and 25 °C (non-vernalizing condition) and a photoperiod condition for 16 h light (long day). The light resource in the day was natural daylight and incandescent lamps were used at night as supplementary light to extend photoperiod. Two hundred thirty-eight germinated F2 seeds were sown in soil-filled pots with five plants each pot. Heading date was recorded at the time when the first ear of each plant half emerged from the flag leaf. Heading time was the number of days from sowing to heading date. The mean value and standard deviation analysis were used to reveal the average level and fluctuation of the heading time by Microsoft Excel software. The greenhouse data was analyzed by SPSS software to reveal the effect of new allele Vrn-B1d on the heading time of wheat.
Results
Molecular characterization of a novel allele Vrn-B1d
Two hundred sixty-two Chinese mini-core collections were tested with the PCR primers designed by Fu et al. (2005) and Milec et al. (2012) for VRN-B1 gene (Supplement 1). Among these cultivars, 222 (84.7%), 29 (11.1%), 9 (3.4%), and 1 (0.4%) carried alleles vrn-B1, Vrn-B1a, Vrn-B1b, and Vrn-B1c, respectively. However, the spring landrace wheat cultivar Hongchunmai did not produce any PCR products using the primers. This indicated that Hongchunmai did not have an intact first intron sequence or the Vrn-B1a/-B1b/-B1c first intron deletion, suggesting the presence of a new allele at VRN-B1 locus.
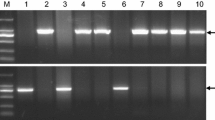
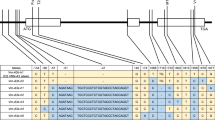
Three primer pairs designed by Milec et al. (2012) were used to clone the complete VRN-B1 gene in Hongchunmai. Amplicon sizes of two primer pairs (Ex2/B/F1 and Ex3/B/R1, Ex3/B/F2 and Ex8/B/R2) from Hongchunmai were consistent with fragment sizes of control cultivars Longmai 32 (vrn-B1), Ganmai 8 (Vrn-B1a), Longmai 34 (Vrn-B1b), and Baiyoumai (Vrn-B1c) (Fig. 1). These results suggested that there was no different existence from the second to eighth exon region of Hongchunmai. However, amplicon sizes of primers Ex1/B/F3 and Ex2/B/R3 in Hongchunmai were different from those of control cultivars (Fig. 1). Further DNA sequencing indicated that the fragment amplified in Hongchunmai was 2828 bp. Multiple alignments (Supplement 2) showed that compared with recessive vrn-B1, there were differences existing in the first intron region of Hongchunmai including a large 6850-bp deletion (from 670 to 7519 bp, counting from the start of the intron 1 in the recessive vrn-B1 allele), a small 187-bp deletion (from 7851 to 8037 bp), a unique SNP (T to C, 7845 bp), and a 4-bp mutation (TTTT to ACAA, from 7847 to 7850 bp). Meanwhile, the unique intron 1 region of Hongchunmai was also significantly different from the corresponding part of the other known dominant alleles (Fig. 2). It was clear to highlight the special composition of Hongchunmai compared with Vrn-B1a: a small 187-bp deletion (from 7851 to 8037 bp), a unique SNP (T to C, 7845 bp), and a 4-bp mutation (TTTT to ACAA, from 7847 to 7850 bp). There were still differences between Vrn-B1d and Vrn-B1b,Vrn-B1c. Compared with Vrn-B1b, novel allele contained a small 176-bp deletion (from 7862 to 8037 bp), a unique SNP (T to C, 7845 bp), and a 4-bp mutation (TTTT to ACAA, from 7847 to 7850 bp). Additionally, the structural differences between Vrn-B1d and Vrn-B1c were even more intricate: 544-bp deletion (from 127 to 670 bp), 432-bp replication fragment (from 8195–8626 to 7325–7756 bp), SNP (T to C, 8225 bp) of Vrn-B1c and a small 187-bp deletion (from 7851 to 8037 bp), a unique SNP (T to C, 7845 bp), and a 4-bp mutation (TTTT to ACAA, from 7847 to 7850 bp) of Vrn-B1d. Therefore, the new allele was designated as Vrn-B1d in Hongchunmai (submitted to NCBI no. MG242339). Because the new allele Vrn-B1d contained a deletion from 7851 to 8037 bp where the binding site of primer Intr1/B/R3 was located, no PCR products was generated using the primer pair Intr1/B/F and Intr1/B/R3 (Fu et al. 2005).
The complete Vrn-B1 sequences were amplified by the three primers in wheat cultivars. 1–5 PCR products amplified with Ex2/B/F1 and Ex3/B/R1, 6–10 Ex3/B/F2 and Ex8/B/R2, and 11–14 Ex1/B/F3 and Ex2/B/R3. M: DNA ladder DL 10000, 1, 6:Longmai32 (vrn-B1); 2, 7, 11:Ganmai 8 (Vrn-B1a (3015 bp)); 3, 8, 12:Longmai 34 (Vrn-B1b (2979 bp)); 4, 9, 13:Baiyoumai (Vrn-B1c (2692 bp)); 5,10, 14:Hongchunmai (Vrn-B1d(2828 bp))
Schematic comparison of the first intron variation of Vrn-B1 alleles. Light and dark gray rectangles indicate exon 1 and intron 1, respectively. The dotted horizontal line indicates deletion. The dotted vertical line indicates the position of nucleotide from the start of intron 1. The filled triangles indicate the position of mutations. The Vrn-B1d allele in Hongchunmai contains a large 6850-bp deletion (from 670 to 7519 bp), a small 187-bp deletion (from 7851 to 8037 bp), a unique SNP (7845 bp) and a 4-bp mutation (from 7847 to 7850 bp) compared with the recessive allele vrn-B1. The black rectangle represents a region duplicated in allele Vrn-B1c. There is a 1-bp mutation (8225 bp, T to C) in allele Vrn-B1c compared with other four alleles, and a unique 432-bp duplicated region (from 7325 to 7756 bp). There is a 1-bp deletion (7843 bp) in allele Vrn-B1a compared with the recessive allele vrn-B1.The black arrow shows positions of forward primers B/F-1 and B/F-2, respectively. The gray arrow shows the position of reverse primer B/R. The primer B/R combines the 432-bp duplicated region (from 7325 to 7756 bp) in the allele Vrn-B1c
Molecular markers of Vrn-B1d allele
Firstly, two pairs of new primers (B/F-1 and B/R, B/F-2 and B/R) were designed by Primer 5.0 software (http://www.premierbiosoft.com/primerdesign/) for identification of the novel allele Vrn-B1d and other four known alleles at VRN-B1 locus. Secondly, Chinese Spring nulli-tetrasomic lines N5AT5D, N5BT5D, and N5DT5B were used to validate the genome specificity of the designed primers. Generally, we have to make sure whether the primers can specifically recognize the 5B chromosome on which the VRN-B1 located. Afterwards, we should find out if the fragments were the same as the expected. Screening of Chinese Spring N5AT5D, N5BT5D, and N5DT5B with the primers B/F-2 and B/R showed a consistent 870-bp fragment in Chinese Spring N5AT5D and N5DT5B (Fig. 3b), while no PCR product in N5BT5D, which meaned primers could specifically recognize the 5B chromosome where the VRN-B1 is located. Meanwhile, screening the control cultivars Ganmai 8, Longmai 34, Baiyoumai, and the landrace cultivar Hongchunmai with the primers B/F-1 and B/R correspondingly showed the expected fragments of 1541 bp, 1505 bp, 318 bp, and 1354 bp. These results meaned that the four cultivars contained Vrn-B1a, Vrn-B1b, Vrn-B1c, and Vrn-B1d, respectively (Fig. 3a). Therefore, the amplified fragments were the same as expected and the genotype data were consistent with the known genotypes for control cultivars, confirming the accuracy and efficiency of the designed molecular markers.
Identification of VRN-B1 genotypes by newly developed primers in some wheat cultivars. A: identification of dominant alleles of VRN-B1 by the primers B/F-1 and B/R. B: identification of recessive allele of VRN-B1 by the primers B/F-2 and B/R. M: DNA Ladder DL2000; 1: Chinese Spring (vrn-B1, 870 bp); 2: N5AT5D (vrn-B1, 870 bp); 3: N5BT5D (−, −); 4: N5DT5B (vrn-B1, 870 bp); 5: Longmai 32 (vrn-B1, 870 bp); 6: Hongchunmai (Vrn-B1d, 1354 bp); 7: Baiyoumai (Vrn-B1c, 318 bp); 8: Longmai 34 (Vrn-B1b, 1505 bp); 9:Ganmai 8 (Vrn-B1a, 1541 bp)
With the purpose to identify all the five alleles of VRN-B1 gene in one PCR reaction, a multiplex PCR was developed based on the newly developed primers. One common reverse B/R and two forward primers B/F-1 and B/F-2 were applied to characterize all five alleles via successful amplification. The reaction components, amplification program, and electrophoresis condition of the multiplex PCR assays were shown in the “Materials and Method.” Five alleles of VRN-B1 gene could be discriminated by the multiplex PCR, according to the different sizes of fragments: 1541 bp (Ganmai 8; Vrn-B1a), 1505 bp (Longmai 34; Vrn-B1b), 318 bp (Baiyoumai; Vrn-B1c), 1354 bp (Hongchunmai; Vrn-B1d), and 870 bp (Jing 411; vrn-B1) (Fig. 4). These results were consistent with the single PCR amplifications, indicating that the multiplex PCR was accurate and reliable.
Effect of novel allele Vrn-B1d on heading time
To precisely reveal the effect of new allele Vrn-B1d on heading time and the association between vernalizaition genotypes and heading time, the F2 population from the cross of Hongchunmai (vrn-A1, Vrn-B1d, Vrn-D1b, vrn-B3) and Jing 411 (vrn-A1, vrn-B1, vrn-D1, vrn-B3) were analyzed. Considering the different composition of the parental genotypes, there were nine possible vernalization genotypes in the F2 population which were tested by PCR primers (Supplement 3). Meanwhile, the possible genotypes and the phenotypes of the F2 population were shown (Supplement 3 and Table 1).
Among the F2 plants carrying homozygous recessive allele (vrn-D1vrn-D1) at VRN-D1 locus, plants with Vrn-B1dVrn-B1d genotype (69 d) headed significantly earlier than those with Vrn-B1dvrn-B1 (79 d) (p < 0.05); plants with vrn-B1vrn-B1 genotype did not head before 140 d (when the experiment was stopped) (Table 1). As for the other F2 plants carrying dominant allele of VRN-D1 in both heterozygous (Vrn-D1bvrn-D1) and homozygous (Vrn-D1bVrn-D1b) stages, a similar result that the heading time significantly advanced with the gradually increasing number of new dominant Vrn-B1d allele (from vrn-B1vrn-B1, Vrn-B1dvrn-B1 to Vrn-B1dVrn-B1d) still existed, suggesting that new allele Vrn-B1d apparently advanced the heading time of wheat.
Discussion
Molecular characterization, effect on heading time, and the contribution to the breeding of the novel allele Vrn-B1d
The previous studies revealed that promoter mutations and large deletions within the first intron of the VRN-1 gene were associated with a spring growth habit (Yan et al. 2004a; Fu et al. 2005). Allelic variations of VRN-B1 gene were only reported to occur in the first intron due to a large deletion in the dominant allele in spring wheat (Fu et al. 2005; Santra et al. 2009; Milec et al. 2012). In this study, we found a novel allele Vrn-B1d containing large and small deletion, a unique SNP, and a 4-bp mutation within the first intron in Chinese spring wheat landrace cultivar Hongchunmai (Fig. 2). As for the effect of the novel allele, Vrn-B1d had a stronger effect on the acceleration of heading under non-vernalizing condition than the recessive allele vrn-B1 (Table 1). The first intron contains a region required to maintain repression of VRN1 (von Zitzewitz et al. 2005; Fu et al. 2005; Cockram et al. 2007; Hemming et al. 2009). Fu et al. (2005) defined a 4.2-kb region of VRN1 in the first intron as the vernalization “critical region.” It was assumed that the “critical region” contained a binding site for a putative repressor that was down-regulated by vernalization (Fu et al. 2005; von Zitzewitz et al. 2005). The repressor could not bind the functional area, resulting in loss of function of the suppressing factor in the presence of large deletion within the first intron. The elimination of a repressor recognition site from the first intron might provide a simple explanation for early heading caused by the novel allele Vrn-B1d. The large deletion within the first intron was responsible for the spring wheat and the deletion fragments of each dominant allele were not the same. Therefore, there might be a potential difference in the heading time among the four dominant alleles. Additionally, the other phenotypes (plant height, ear length, leaf number, and so on) of these four dominant alleles might also be different. Another key point is that the novel first intron sequence of Vrn-B1d may be helpful for the further research of the first intron influence on the heading time. In the next stage, we would use qRT-PCR to determine the effect of this novel allele on wheat heading time in gene expression level.
Apart from the benefit of the scientific research, the novel allele still makes great contribution to the wheat breeding in Xinjiang. The temperature of Xinjiang is persistently low in the early stage of spring and drastically fluctuates in the late spring. Protecting wheat from the frost damage in the spring is an urgent problem in Xinjiang spring wheat production. Spring wheat cultivar Hongchunmai with the new allele Vrn-B1d was widely planted in this region, indicating that the Vrn-B1d genotype would be better adapted to Xinjiang. Therefore, the new allele Vrn-B1d may supply the beneficial germplasm resource for wheat breeding in similar climate regions as Xinjiang.
Development of gene-specific primers and effectiveness of the multiplex PCR
PCR-based assays could be used to directly identify genotypes by molecular markers without any interference from the environment during any period of wheat growth stages. Compared with the recessive allele vrn-B1, all the four dominant alleles contain large deletion in the first intron which would be applied to differentiate them from the recessive allele vrn-B1 (Fig. 2, Supplement 2). For the four dominant alleles, the specific structural changes in the Vrn-B1c with 543-bp deletion (127–669 bp), 432-bp duplicated region (7325–7756 bp), and 1-bp mutation (8225 bp, T to C) can be used to separate this allele from the other three dominant alleles. Based on the different deletion positions in the Vrn-B1b (7827–7862 bp) and Vrn-B1d (7851–8037 bp) compared with Vrn-B1a, the three dominant alleles Vrn-B1a, Vrn-B1b, and Vrn-B1d can be distinguished. Therefore, reverse primer B/F-2 located in the 6850-bp deletion region was designed to specifically amplify the recessive allele vrn-B1 with 870-bp in combination with reverse primer B/R located behind a 187-bp deletion region in Vrn-B1d (Fig. 2, Supplement 2). Since the Vrn-B1c allele contained a deletion from 127 to 669 bp, the first exon-based forward primer B/F-1 was designed to detect four dominant alleles of VRN-B1 combined with common reverse primer B/R, which only combined the 432-bp duplicated region (from 7325 to 7756 bp) because of 1-bp mutation (8225 bp, T to C) in the allele Vrn-B1c.
With the combination of two forward primers (B/F-1 and B/F-2) and a common reverse primer (B/R), a multiplex PCR system was developed to discriminate all five alleles of VRN-B1 simultaneously. By the use of the multiplex PCR system, five alleles of VRN-B1 could be simply identified by one PCR-based reaction rather than two single PCR amplifications, which could reduce half of test time and sample requirements. The results tested by multiplex PCR system were consistent with those detected by single PCR, indicating that the multiplex PCR was accurate and reliable.
In this study, we identified a novel dominant allele Vrn-B1d in common wheat, developed two pairs of primers to identify Vrn-B1d and other four known alleles of VRN-B1, and established a multiplex PCR as an effective and reliable method for discriminating alleles at VRN-B1 locus. In addition, we revealed that plants containing the Vrn-B1d allele showed obviously earlier heading date compared with those with recessive vrn-B1 alleles, suggesting that Vrn-B1d was a dominant allele conferring the spring growth habit. This study provided a new wheat germplasm resource and useful markers for improvement of adaptability of wheat cultivars in breeding.
References
Barrett B, Bayram M, Kidwell K, Weber WE (2002) Identifying AFLP and microsatellite markers for vernalization response gene Vrn-B1 in hexaploid wheat using reciprocal mapping populations. Plant Breed 121:400–406
Chen A, Dubcovsky J (2012) Wheat TILLING mutants show that the vernalization gene VRN1 down-regulates the flowering repressor VRN2 in leaves but is not essential for flowering. PLoS Genet 8:e1003134
Chen F, Gao M, Zhang J, Zuo A, Shang X, Cui D (2013) Molecular characterization of vernalization and response genes in bread wheat from the Yellow and Huai Valley of China. BMC Plant Biol 13:199
Chu CG, Xu SS, Friesen TL, Faris JD (2008) Whole genome mapping in a wheat doubled haploid population using SSRs and TRAPs and the identification of QTL for agronomic traits. Mol Breed 22:251–266
Cockram J, Chiapparino E, Taylor SA, Stamati K, Donini P, Laurie DA, O'sullivan DM (2007) Haplotype analysis of vernalization loci in European barley germplasm reveals novel VRN-H1 alleles and a predominant winter VRN-H1/VRN-H2 multi-locus haplotype. Theor Appl Genet 115:993–1001
Distelfeld A, Li C, Dubcovsky J (2009) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12:178–184
Dubcovsky J, Lijavetzky D, Appendino L, Tranquilli G (1998) Comparative RFLP mapping of Triticum monococcum genes controlling vernalization requirement. Theor Appl Genet 97:968–975
Fu DL, Szucs P, Yan LL, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Gen Genomics 273:54–65
Galiba G, Quarrie SA, Sutka J, Morgounov A, Snape JW (1995) RFLP mapping of the vernalization (Vrn1) and frost resistance (Fr1) genes on chromosome 5A of wheat. Theor Appl Genet 90:1174–1179
Guo X, Wang Y, Meng L, Liu H, Yang L, Zhou Y, Zhang H (2015) Distribution of the Vrn-D1b allele associated with facultative growth habit in Chinese wheat accessions. Euphytica 206:1–10
Hanocq E, Niarquin M, Heumez E, Rousset M, Gouis J (2004) Detection and mapping of QTL for earliness components in a bread wheat recombinant inbred lines population. Theor Appl Genet 110:106–115
He ZH, Rajaram S, Xin ZY, Huang GZ (2001) A history of wheat breeding in China. CIMMYT, Mexico
Hemming MN, Peacock WJ, Dennis ES, Trevaskis B (2008) Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol 147:355–366
Hemming MN, Fieg S, Peacock WJ, Dennis ES, Trevaskis B (2009) Regions associated with repression of the barley (Hordeum vulgare) VERNALIZATION1 gene are not required for cold induction. Mol Gen Genomics 282:107–117
Iwaki K, Nakagawa K, Kuno H, Kato K (2000) Ecogeographical differentiation in east Asian wheat, revealed from the geographical variation of growth habit and Vrn genotype. Euphytica 111:137–143
Iwaki K, Haruna S, Niwa T, Kato K (2001) Adaptation and ecological differentiation in wheat with special reference to geographical variation of growth habit and Vrn genotype. Plant Breed 120:107–114
Iwaki K, Nishida J, Yanagisawa T, Yoshida H, Kato K (2002) Genetic analysis of Vrn-B1 for vernalization requirement by using linked dCAPS markers in bread wheat (Triticum aestivum L.). Theor Appl Genet 104:571–576
Kato K, Yamagata H (1988) Method for evaluation of chilling requirement and narrow-sense earliness of wheat cultivars. Jpn J Breed 38:172–186
Kato K, Miura H, Sawada S (1999) QTL mapping of genes controlling ear emergence time and plant height on chromosome 5A of wheat. Theor Appl Genet 98:472–477
Kippes N, Debernardi JM, Vasquez-Gross HA, Akpinar BA, Budak H, Kato K, Chao S, Akhunov E, Dubcovsky J (2015) Identification of the VERNALIZATION 4 gene reveals the origin of spring growth habit in ancient wheats from South Asia. Proc Natl Acad Sci U S A 112:5401–5410
Law CN, Worland AJ, Giorgi B (1975) The genetic control of ear-emergence time by chromosomes 5A and 5D of wheat. Heredity 36:49–584
Li C, Dubcovsky J (2008) Wheat FT protein regulates VRN1 transcription through interactions with FDL2. Plant J 55:543–554
Loukoianov A, Yan L, Blechl A, Sanchez A, Dubcovsky J (2005) Regulation of VRN-1 vernalization genes in normal and transgenic polyploid wheat. Plant Physiol 138:2364–2373
Milec Z, Tomkov L, Sumíková T, Pánková K (2012) A new multiplex PCR test for the determination of Vrn-B1 alleles in bread wheat (Triticum aestivum L.). Mol Breed 30:317–323
Milec Z, Sumíková T, Tomková L, Pánková K (2013) Distribution of different Vrn-B1 alleles in hexaploid spring wheat germplasm. Euphytica 192:371–378
Moon J, Suh SS, Lee H, Choi KR, Hong CB, Paek NC, Kim SG, Lee I (2003) The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J 35:613–623
Pugsley AT (1971) A genetic analysis of spring-winter habit of growth in wheat. Aust J Agric Res 22:21–31
Pugsley AT (1972) Additional genes inhibiting winter habit in wheat. Euphytica 21:547–552
Santra DK, Santra M, Allan RE, Campbell KG, Kidwell KK (2009) Genetic and molecular characterization of vernalization genes Vrn-A1, Vrn-B1, and Vrn-D1 in spring wheat germplasm from the Pacific Northwest region of the USA. Plant Breed 128:576–584
Shcherban AB, Efremova TT, Salina EA (2012) Identification of a new Vrn-B1 allele using two near-isogenic wheat lines with difference in heading time. Mol Breed 29:675–685
Shcherban AB, Khlestkina EK, Efremova TT, Salina EA (2013) The effect of two differentially expressed wheat VRN-B1 alleles on the heading time is associated with structural variation in the first intron. Genetica 141:133–141
Shitsukawa N, Ikari C, Shimada S (2007) The einkorn wheat (Triticum monococcum) mutant, maintained vegetative phase, is caused by a deletion in the VRN1 gene. Genes Genet Syst 82:167–170
Stelmakh AF (1993) Genetic effects of Vrn genes on heading date and agronomic traits in bread wheat. Euphytica 65:53–60
Sun QM, Zhou RH, Gao LF, Zhao GY, Jia JZ (2009) The characterization and geographical distribution of the genes responsible for vernalization requirement in Chinese bread wheat. J Integr Plant Biol 51:423–432
Tóth B, Galiba G, Fehér E, Sutka J, Snape JW (2003) Mapping genes affecting flowering time and frost resistance on chromosome 5B of wheat. Theor Appl Genet 107:509–514
Trevaskis B, Hemming MN, Dennis ES, Peacock WJ (2007) The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci 12:352–357
von Zitzewitz J, Szucs P, Dubcovsky J, Yan L, Francia E, Pecchioni N, Casas A, Chen TH, Hayes PM, Skinner JS (2005) Molecular and structural characterization of barley vernalization genes. Plant Mol Biol 59:449–467
Wang X, Ju LP, Liu FJ, Zhang YY, Zhang F, Fu XJ, Feng Y, Zhang XK (2014) Vernalization effects of dominant alleles Vrn-B1a and Vrn-B1b and their distributions in cultivars from Yellow and Huai River Valleys facultative winter wheat zone (in Chinese). Acta Agron Sin 40(3):439–446
Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci U S A 100:6263–6268
Yan L, Helguera M, Kato K, Fukuyama S, Sherman J, Dubcovsky J (2004a) Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor Appl Genet 109:1677–1686
Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004b) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303:1640–1644
Yan L, Fu DL, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci U S A 103:19581–19586
Yasuda S, Shimoyama H (1965) Analysis of internal factors influencing the heading time of wheat varieties. Ber Ohara Inst Landw Biol Okayama U 13:23–38
Zhang XK, Xia XC, Xiao YG, Dubcovsky J, He ZH (2008) Allelic variation at the vernalization genes Vrn-A1, Vrn-B1, Vrn-D1 and Vrn-B3 in Chinese common wheat cultivars and their association with growth habit. Crop Sci 48:458–470
Zhang J, Wang Y, Wu S, Yang J, Liu H, Zhou Y (2012) A single nucleotide polymorphism at the Vrn-D1 promoter region in common wheat is associated with vernalization response. Theor Appl Genet 125:1697–1704
Zhang X, Gao M, Wang S, Chen F, Cui D (2015) Allelic variation at the vernalization and photoperiod sensitivity loci in Chinese winter wheat cultivars (Triticum aestivum L.). Front Plant Sci 6:470
Zhuang QS (2003) Wheat improvement and pedigree analysis in Chinese wheat cultivars (in Chinese). China Agriculture Press, Beijing
Acknowledgments
The authors are grateful to Prof. Xianchun Xia for kindly reviewing this manuscript.
Funding
This study was funded by the 973 projects (2014CB138102), Research and Development of Science and Technology in Shaanxi Province (2014KCT-25), National Natural Science Foundation of China (30971770 and 31671693), and a grant of Northwest A&F University for ZY Tang.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, B., Wang, X., Wang, X. et al. Molecular characterization of a novel vernalization allele Vrn-B1d and its effect on heading time in Chinese wheat (Triticum aestivum L.) landrace Hongchunmai. Mol Breeding 38, 127 (2018). https://doi.org/10.1007/s11032-018-0870-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-018-0870-6