Abstract
Vitamin E refers to eight distinct compounds collectively known as tocochromanols and can be further divided into two classes, tocotrienols and tocopherols. Tocochromanols are the major lipid-soluble antioxidants in maize (Zea mays L.) grain. Enhancing vitamin E content of maize through plant breeding has important implications for human and animal nutrition. Four inbred lines exhibiting unique variation for tocochromanol compounds were chosen from the Goodman maize diversity panel to construct two biparental mapping populations (N6xNC296 and E2558xCo125). The N6xNC296 population was developed to analyze segregation for α-tocopherol and α-tocotrienol content. The E2558WxCo125 population was developed to analyze segregation for the ratio of total tocotrienols to tocopherols. The tocochromanol variation in two replicates of each population was quantified using liquid chromatography-diode array detection. Using high-density linkage mapping, novel quantitative trait loci (QTL) in the N6xNC296 population were mapped using tocopherol ratio traits. These QTL contain the candidate gene homogentisate phytyltransferase (ZmVTE2) within the respective support intervals. This locus was not mapped in a previous genome-wide association study that analyzed tocochromanols in the Goodman diversity panel. Transgressive segregation was observed for γ- and α-tocochromanols in these populations, which facilitated QTL identification. These QTL and transgressive segregant families can be used in selection programs for vitamin E enhancement in maize. This work illustrates the complementary nature of biparental mapping populations and genome-wide association studies to further characterize genetic variation of tocochromanol content in maize grain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tocochromanols represent eight distinct compounds that are commonly referred to as vitamin E. These eight compounds are grouped into two classes, tocotrienols (α-, β-, γ-, and δ-tocotrienol) and tocopherols (α-, β-, γ-, and δ-tocopherol) (Kamal-Eldin and Appelqvist 1996). The role of tocochromanols in plants as potent antioxidants has been well-documented (Falk and Munné-Bosch 2010; Fryer 1992; Munné-Bosch and Alegre 2002). Studies in Arabidopsis demonstrated that the primary role of tocochromanols in plants is to protect the seed from lipid peroxidation, which can result in a gradual loss of seed viability (Sattler et al. 2004). There is also evidence that tocochromanols also have a role in quenching singlet oxygen in adult plants thereby reducing photo-oxidative damage to photosystem II and increasing overall photosynthetic efficiency (Havaux et al. 2005).
Tocochromanols are an essential nutrient in the human diet. Vitamin E deficiency in humans is rare but does occur when an individual is severely malnourished or has a genetic condition involving the dysfunction of α-tocopherol transferase. In these instances, a neurodegenerative disease known as ataxia with vitamin E deficiency (AVED) can develop (Ouahchi et al. 1995). Some tocochromanols have been shown to prevent oxidative damage to cells and are also associated with a reduction in deaths from cancer (Borek 2004; Kline et al. 2007; Wright et al. 2006) and coronary heart disease (Emmert and Kirchner 1999). Tocochromanol structure influences vitamin E activity, with α-tocopherol having the highest activity (Eitenmiller 1997) because of its preferential retention during digestion (DellaPenna 2005).
Maize is a staple food crop of global importance. Current maize varieties that produce grain for human consumption do not provide sufficient levels of vitamin E to meet recommended dietary requirements (Fitzpatrick et al. 2012). However, maize germplasm exhibits a substantial amount of tocochromanol variation (Chander et al. 2008; Li et al. 2012; Wong et al. 2003; Lipka et al. 2013) that can be exploited to improve the vitamin E content of this staple crop through biofortification (Bouis and Welch 2010). Genetic analysis of tocochromanol variability in maize grain will facilitate the identification of chromosomal targets for marker-assisted selection, which could ultimately expedite the production of maize grain with improved vitamin E status.
The first quantitative trait loci (QTL) study to analyze the natural variation of tocopherols in maize utilized composite interval mapping (CIM) in a F2:4 biparental mapping population with a genetic map containing 163 markers (Wong et al. 2003). Numerous QTL were identified, most notably those with the largest effects residing on chromosome 1 and 5, where maize γ-tocopherol methyl transferase (ZmVTE4) and p-hydroxyphenylpyruvic dioxygenase (ZmHPPD) were later located. A subsequent study (Chander et al. 2008) used a biparental recombinant inbred line (RIL) population and a genetic map with 203 markers, including tocochromanol biosynthetic gene-targeted markers. Of the 31 QTL identified in this study, ten contained ZmVTE4, ZmVTE5 (phytol kinase), or ZmHPPD within their support intervals (Chander et al. 2008). More recently, a QTL study using two F2:3 biparental mapping populations that shared a common parent and a high-density linkage map constructed from 1536 single-nucleotide polymorphism (SNP) markers located 30 QTL associated with various tocopherols, some of which contained ZmVTE4 and ZmHPPD in the support intervals (Shutu et al. 2012).
Genome-wide association studies have been conducted to analyze tocochromanol content in maize. The first reported tocochromanol genome-wide association study (GWAS) in maize analyzed tocopherols in 500 diverse maize inbred lines and identified three polymorphisms associated with α-tocopherol variation, two indels within ZmVTE4, and another SNP that is approximately 85 kb upstream of ZmVTE4 (Li et al. 2012). A more recent GWAS (Lipka et al. 2013) provided a comprehensive analysis of tocochromanol genetic architecture in maize by analyzing six tocochromanol compounds and 14 derived sums, ratios, and proportions in the Goodman diversity panel, which consists 281 lines (Flint-Garcia et al. 2005). This study reported the first association for tocotrienols in maize and strengthened the evidence for the association between ZmVTE4 and α-tocopherol.
The objective of this research was to locate QTL responsible for tocochromanol variation in maize. Two biparental mapping populations were created specifically for this purpose, with parents of the respective populations representing contrasting phenotypes for two different tocochromanol traits. Both populations were phenotyped using liquid chromatography for tocopherol and tocotrienol content of the maize grain and a de novo genetic map was constructed for each population from genotyping-by-sequencing (GBS)-produced sequence data (Elshire et al. 2011). To our knowledge, this research represents the first QTL mapping study in maize where all tocochromanols were included in the analysis, rather than just tocopherols. We hypothesized that these methods would allow (1) associations with additional loci to be detected through the inclusion of measurement of additional tocochromanol compounds and calculated sums and ratios of those compounds, and (2) the use of GBS-derived higher marker density genotypic data would allow for narrower QTL intervals than was previously possible thus allowing for finer resolution of the genetic architecture.
Materials and methods
Germplasm
The tocochromanol phenotypic data (Lipka et al. 2013) collected on the Goodman diversity panel and the founder parents of the maize nested association mapping population (NAM) (McMullen et al. 2009) were used to identify four lines that exhibited variation for tocochromanol compounds of interest. These lines (N6, NC296, E2558W, and Co126) were selected for unique tocochromanol variation that was not present in the NAM founders and was used to create two biparental mapping populations. The parents of the N6xNC296 population exhibited extremely low and high levels of α-tocopherol and α-tocotrienol in the grain, whereas the parents of the E2558WxCo125 population exhibited extremely high and low ratios of total tocopherols to total tocotrienols in the grain, relative to the other lines measured.
For each population, F2:3 families were produced by crossing the two respective parents to generate F1 seed. The F1 seed was planted and self-pollinated to produce F2 seed which was planted and self-pollinated to produce F2:3 progeny. The planted population size was 231 F2:3 families for N6xNC296 and E2558WxCo125. After filtering for missing genetic and phenotypic measurements, the final dataset of the N6xNC296 population consisted of 213 F2:3 families and for E2558WxCo125 consisted of 197 F2:3 families.
Field experimental design
One replicate of the N6xNC296 population and two replicates of the E2558WxCo125 population were grown during the summer of 2012 at the Purdue Agronomy Center for Research Education (ACRE) in West Lafayette, Indiana, USA. The second replicate of the N6xNC296 F2:3 was grown during the summer of 2013 at ACRE in West Lafayette, Indiana, USA. The experiment was planted in an augmented incomplete block design (Federer and Raghavarao 1975) with each block containing both of the respective population parents. Each replicate of the population consisted of 11 blocks. Within each block, there were 21 individuals and four checks, representing two checks for each of the respective parents. This design resulted in 231 individuals and 44 checks per replicate.
Tocochromanol analysis
The two mapping populations, including experimental checks, were analyzed by liquid chromatography (LC) for α-tocopherol (αT), δ-tocopherol (δT), γ-tocopherol (γT), α-tocotrienol (αT3), δ-tocotrienol (δT3), and γ-tocotrienol (γT3). The high-throughput extraction method and subsequent LC analysis were conducted as previously described (Lipka et al. 2013) with modifications detailed here. Approximately 20 g of maize grain samples was ground from a bulk of each F2:3 family using a commercial Foss Cyclotec 1093 sample mill (ThermoFisher Scientific, Waltham, MA). The ground samples were stored in cryogenic Poly-Con containers for long-term storage at − 80 °C until ready for LC analysis (US Plastic, Lima, Ohio). Extraction and LC solvents (Sigma-Aldrich, St. Louis, MO) used were certified HPLC grade. For extraction of the tocochromonal compounds, 15–20 mg of ground maize seed was transferred into a 1.4-ml U-bottom bar-coded extraction tube (Micronic USA, Aston, PA) containing two glass beads (5 mm size). Four hundred microliters of extraction solution was added to each tube. The extraction solution is composed of 60:40 v:v acetone:ethyl acetate containing 1 mg/mL of butylated hydroxytoluene (Sigma-Aldrich, St Louis, MO). In addition, 150 μL of HPLC grade water (Sigma-Aldrich, MO) was added to each tube. The 96-well plate of samples was capped with strip caps and shaken on a TissueLyser at 20 Hz for 10 min. Samples were centrifuged for 10 min at 250g in a Sorvall Legend RT centrifuge (Kendro Laboratory Products, Newtown, CT). After centrifugation, 200 μL of the upper organic phase was transferred into a 750-μL tube and dried in a Speedvac (ThermoFisher Scientific, Waltham, MA) at room temperature for approximately 30 min. The dried samples were re-suspended by shaking on a microplate shaker for 15 min at 2000 rpm in 100 μL of re-suspension solution which consisted of 3:1 v:v methanol:methyl tert-butyl ether. The plates were then centrifuged for 5 min at 2500g and the supernatant transferred to a microtiter plate.
For LC analysis, 10 μL of the re-suspended extraction was injected into a Shimadzu HPLC LC-20AD (Shimadzu, Kyoto, Japan). The tocochromonal compounds were separated on a YMC 3.0 × 100 mm C30 reverse-phase column with a 3-μm particle size (YMC, Kyoto, Japan). The column oven temperature during separation was 30 °C. Mobile phase A consisted of methyl tert-buthyl ether. Mobile phase B consisted of 90:10 (v:v) of methanol:ammonium acetate (1 M, pH 4.6). The mobile phases were pumped at a rate of 0.8 mL/min using the following gradient: 0 to 12 min at 0% B to 60% B, 12 to 17.5 min at 60% B to 22.5% B, 17.5 to 19.5 min at 22.5% B to 100% B, and 19.5 to 21 min at 100% B, followed by re-equilibration of mobile phase A. The tocochromanol compounds were detected using a Shimadzu (Kyoto, Japan) RF-535 fluorescence detector with 290-nm excitation and 325-nm emission wavelengths. The tocochromanols were quantified using external standard curves constructed with authentic standards of αT, δT, γT, αT3, δT3 (Sigma-Aldrich, St Louis, MO), and γT3 (Cayman Chemical, Ann Arbor MI). The reverse-phase LC conditions applied in this study could not completely resolve β-tocochromanols and γ-tocochromanols, and therefore, the β-tocochromanols were not measured. β-Tocochromanols are considered a minor component in maize, and the γ-tocochromanols present as the primary components in the maize tocochromonal profile and the exclusion of β-tocochromanols in our analysis is consistent with previous studies on maize tocochromanols (Lipka et al. 2013; Shutu et al. 2012; Li et al. 2012; Chander et al. 2008). This procedure was used to analyze one sample from each field plot of the two replicates of each population.
Phenotypic data analysis
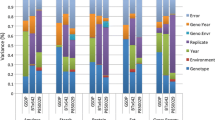
A total of 20 phenotypic traits were analyzed, including the six primary phenotypes (αT, δT, γT, αT3, δT3, and γT3) and 14 derived sums, ratios, and proportions. The sums included total tocopherols (TT), total tocotrienols (TT3), and total tocochromanols (TT + TT3). The ratios of biological relevance for tocochromanol analysis are αT/γT, αT3/γT3, γT/(γT + αT), γT3/(γT3 + αT3), δT/(γT + αT), δT/αT, δT/γT, δT3/(γT3 + αT3), δT3/αT3, δT3/γT3, and TT/TT3 (Lipka et al. 2013). In individuals where the δT and δT3 levels were below detection limits, a uniformly distributed random number was assigned that was between zero and the minimum level of detection (Lubin et al. 2004). Studentized deleted residuals (Kutner et al. 1996) were obtained by fitting a mixed linear model using SAS version 9.3 (SAS Institute 2012) with the genotypes and blocks set as random effects, and the checks set as fixed effects. The studentized deleted residuals were used to identify outliers in the final dataset. The heritability (h2) of each trait was calculated on a line-mean basis using the Gibbs sampling algorithm (Casella and George 1992; Sorensen et al. 1994; Yang et al. 2007).
Best linear unbiased predictors (BLUPs) of each trait for each line were predicted from a random effects model analysis across environments in ASReml version 3.0 (Gilmour et al. 2009) using the following model:
where Y ijk is an individual observation, μ is the overall mean, gen i is the main effect of the ith genotype, env is the main effect of the jth environment, (gen × env) ij is the two-way interaction effect between the ith genotype and jth environment, and ε ijk denotes the random error term. All terms in the model except for the intercept are random.
Genotyping and genetic map construction
The genotypes representing the F2 generation of the N6xNC296 and E2558WxCo125 populations were derived from bulking tissue samples from ten F2:3 plants. The genotypes were determined using the genotyping-by-sequencing (GBS) procedure (Elshire et al. 2011) performed at The Institute for Genomic Diversity at Cornell University, in addition to the bioinformatics analysis for calling single-nucleotide polymorphism (SNP) markers. The original genotypic dataset for N6xNC296 and E2558WxCo125 consisted of 955,690 SNPs for each population.
The Full-Sib Family Haplotype (FSFHap) imputation method was used to correct for the high degree of missing data and undercalled heterozygotes that are prevalent in GBS data (Swarts et al. 2014). The imputation and subsequent filtering of SNPs were conducted in Trait Analysis by Association, Evolution and Linkage (TASSEL) version 5.0 (Bradbury et al. 2007). Before imputation was conducted, any SNP or individual that had more than 20% missing data was filtered out. Imputation and subsequent filtering for minor allele frequencies (MAF) less than 0.30 resulted in 89,125 SNPs for the N6xNC296 population and 76,081 SNPs for the E2558WxCO125 population that was polymorphic between the parents of the respective mapping population.
A subset of SNPs was selected that gave high-density coverage of the genome for each population. Redundant SNP markers were identified and removed using a customized R script (R Development Core Team 2011). OneMap was used to calculate recombination fractions, form linkage groups, test for segregation distortion, order the markers, and estimate genetic maps (Margarido et al. 2007). The recombination fractions were converted to map distance using the Kosambi mapping function (Vinod 2011). Markers that tested significant for segregation distortion using a Bonferroni correction were removed. The COMPARE and TRY algorithms (Lander and Kruglyak 1995) were used in OneMap to order the markers in their respective linkage groups.
Quantitative trait loci analysis
Quantitative trait loci mapping was performed to identify chromosomal regions that contribute to tocochromanol variation in maize. The QTL analyses were conducted using the standard model (6) in QTL-Cartographer (Basten et al. 1994) to conduct composite interval mapping (CIM) with a walking speed of one centimorgan (cM) (Zeng 1993, 1994). The model included up to five marker covariates, which entered and exited the model based on forward and backward regression model fitting, to account for variation within a three cM window. The significance thresholds were determined for each trait individually using 1000 permutation tests in each of the populations (Churchill and Doerge 1994). The QTL analysis was conducted on the BLUPs that represent a combined data point of the two replications of each population. The QTL that exceeded the permutation thresholds were reported, and for each QTL, the one logarithm of odds (LOD) support interval, additive effect, dominance effect, and cM position were estimated. The markers immediately flanking the support interval were manually identified. The physical position of each SNP marker, according to the B73 reference genome v2 (Lai et al. 2010), was used to identify a physical position interval. The physical positions of the tocochromanol biosynthetic genes were determined from the Maize Genomic Database (Maize GDB) (Andorf et al. 2010). The presence of putative tocochromanol biosynthetic genes in the one LOD support intervals was reported.
Results
Phenotypic variability
The N6xNC296 population was created to analyze variation for α-T and α-T3. The α-T content for the parental checks (n = 22) was 5.97 and 51.89 μg/g for N6 and NC296, respectively. The α-T content for the entire N6xNC296 F2:3 population was 33.08 μg/g, and ranged from 9.4 to 90.91 μg/g for individual families. The α-T3 content was 23.54 and 66.55 μg/g for N6 and NC296, respectively. The α-T3 content for this population was 46.34 μg/g and ranged from 28.86 to 76.27 μg/g. Thus, α-T and α-T3 both showed transgressive segregation to the high end of their phenotypic distributions, but not to the low end. This pattern of transgressive segregation was also observed for αT, γT, and αT3 in the N6xNC296 population (Table 1).
The E2558WxCo125 population was created to study variation in the ratio of total tocotrienols to total tocopherols (TT/TT3). The observed TT/TT3 was 0.92 and 8.29 for E2558W and Co125 parental checks (n = 22), respectively. The TT/TT3 for the population was 5.51 and ranged from 5.39 to 9.26, which indicates transgressive segregation at the high end of the distribution (Table 2).
Genetic linkage map
A de novo high-density genetic map was constructed for each population. The original genotypic dataset for the N6xNC296 population had 57% missing data and only 1% heterozygous SNPs. After filtration and imputation with FSFHap, there were 0% missing data and 58% heterozygous SNPs. The genetic map for the N6xNC296 population (Fig. 1) consists of 1280 SNP markers, with an average marker spacing of 1.08 cM. Similarly, the original genotypic dataset for the E2558WxCo125 population had 55% missing data and 1% heterozygous SNPs. After filtration and imputation with FSFHap, there was 0% missing data and 56% heterozygous SNPs. The genetic map for the E2558WxCo125 population (Fig. 2) consists of 1249 markers, with an average spacing of 1.1 cM between markers (Table 3).
Quantitative trait loci analysis
For the N6xNC296 population (Table 4), a total of 31 QTL were detected that contained a tocochromanol biosynthetic gene or explained substantial phenotypic variation for the primary traits. Two QTL were detected for the ratio trait, δT3/(γT3 + αT3). One QTL explained 25% of the phenotypic variation for δT3/(γT3 + αT3) and contained homogentisic acid geranylgeranyl transferase (ZmHGGT1) in the 5.1 cM support interval on chromosome 9. For TT3, a QTL on chromosome 9 was mapped that explained 22% of the variation and contained homogentisate phytyltransferase (ZmVTE2) in the 2.8 cM support interval. For γT/(γT + α)T, a QTL on chromosome 5 explained 53% of the phenotypic variation and contained γ-T methyl transferase (ZmVTE4) within the support interval. A QTL was mapped on chromosome 5 that explained 5% of variation for αT but did not contain a known tocochromanol biosynthetic gene in the 7.7 cM support interval. Another QTL was detected on chromosome 5 that explained 14% of the αT3 variation and also did not contain a known tocochromanol biosynthetic gene in the 4.1 cM support interval.
For the E2558WxCo125 population (Table 5), a total of 58 QTL were detected, with those that contained a tocochromanol biosynthetic gene or explained substantial phenotypic variation for primary traits presented here. A QTL for TT3 on chromosome 5 contained hydroxyphenylpyruvate dioxygenase (ZmHPPD1) in the 12 cM support interval. A QTL for TT/TT3 on chromosome 5 explained 10% of the phenotypic variation but did not contain a known tocochromanol biosynthetic gene. One QTL for TT was mapped on chromosome 2 and explained 4% of the phenotypic variation. Two QTL for TT3 were mapped on chromosomes 5 and 8 and explained 7 and 5% of the variation, respectively.
Discussion
The approach for this study originated from review of tocochromanol content data on the Goodman diversity panel and founders of the maize NAM population. These data revealed maize lines in the Goodman diversity panel that exhibited unique variation for tocochromanol compounds of interest. A GWAS was conducted on the Goodman diversity panel for tocochromanol compounds and the respective sums, ratios, and proportions to explore the genetic architecture of these traits (Lipka et al. 2013). A limitation of this GWAS was that it used an underpowered population of 252 inbreds, which limits ability to detect rare causative alleles. Therefore, to complement the GWAS and gain a better understanding of the genetic architecture of tocochromonal accumulation in maize, the N6xNC296 and E2558WxCo125 populations were developed and analyzed.
Genetic architecture of tocochromanols in maize
The populations analyzed both displayed transgressive segregation for traits of interest. This transgressive segregation facilitated mapping of the genetic architecture of tocochromanols in maize. The analysis of the N6xNC296 mapping populations revealed QTL for T3 and TT/TT3 that contained a putative homogentisate phytyltransferase (ZmVTE2) gene in the support interval that previously has not been associated with these traits in maize. The ZmVTE2 and ZmHGGT1 genes lie within 15 megabases from each other on chromosome 9, and previously, it may not have been possible to resolve the positions of two distinct QTL without the use of a high-density linkage map. In the tocochromanol biosynthetic pathway, ZmVTE2 condenses homogentisic acid with phytyl diphosphate to yield 2-methyl-6-phytylbenzoquinol (MPBG), which is the precursor for tocopherols. Given that the ZmVTE2 was associated with a QTL for total tocotrienols and the ratio of total tocotrienols to total tocopherols, the gene mapped here may indicate that one parent has a weaker allele that sends more flux into the tocotrienol branch of the pathway. Notably, the associated SNP positions relative to the reference genome indicate ZmHGGT1 is outside of the T3 and TT/TT3 QTL intervals, providing some support for ZmVTE2 as a candidate gene. However, further work needs to be performed to be able to conclude that polymorphic regions in ZmVTE2 segregate with the phenotype.
Results from the two mapping populations support some associations previously reported in GWAS and QTL studies of tocochromanol variation in maize grain. Results from the N6xNC296 population further strengthened previous associations of tocotrienol traits with ZmVTE1, ZMVTE4, and ZmHGGT1, and from the E2558WxCo125 population further strengthened previous associations of tocotrienol traits with ZmVTE1, ZmVTE4, and ZmHPPD (Wong et al. 2003; Chander et al. 2008; Shutu et al. 2012; Lipka et al. 2013).
High-density linkage mapping with GBS produced SNPs
The F2:3 generation of each population was sequenced using GBS that was used to produce two high-density genetic maps. The GBS procedure has commonly been used in maize to genotype individuals for GWAS, and due to the low cost, it is gaining popularity for genotyping biparental mapping populations for QTL analysis. A limitation of the low coverage multiplexing GBS procedure is that it results in a high degree of missing genotypic information and heterozygous loci in individuals that are undercalled (Nielsen et al. 2012). This occurs because in order to call heterozygotes correctly, the sample must be covered by at least two reads which should come from sister chromatids. With next-generation sequencing methods like GBS, there is a greater chance that only one of the two sister chromatids of a diploid individual is sampled at a specific base pair position (Nielsen et al. 2011). Specifically, when using GBS in maize, it is expected that only 12% of the genome will be sampled two or more times and this introduces error when identifying heterozygous individuals (Swarts et al. 2014). To overcome these limitations of GBS, imputation was used. The research presented here used an imputation method called FSFHap that utilizes the hidden Markov model and the Viterbi algorithm to impute missing data and improve heterozygote calling (Swarts et al. 2014). The low-cost sequencing information available from GBS was improved with this imputation approach so that accurate high-density genetic maps could be constructed.
Increased QTL resolution with high-density linkage mapping
High-density genetic maps with an average interval spacing of one cM or less have been reported to provide increased QTL resolution, improved effect estimates for QTL, and increased power to resolve closely linked QTL (Stange et al. 2013). In the N6xNC296 population, using 213 F2:3 families and 1280 markers, we detected a QTL associated with γT/(γT + αT) that contained ZmVTE4 in a support interval of 1.4 cM. An earlier study on a biparental population of 233 recombinant inbred lines with 201 markers reported a QTL associated with α-T that contained ZmVTE4 within a support interval of 10 cM (Chander et al. 2008). Another linkage mapping study detected QTL associated with αT, γT, and αT /γT that contained ZmVTE4 in a 14.6-cM interval (Shutu et al. 2012). This study used two populations of 237 and 218 F2:3 families with a common parent and 468 and 357 markers on each population, respectively. Our study provides results consistent with these earlier reports, and our higher-resolution mapping provides more precise support of ZmVTE4 as a candidate gene for tocochromanol traits involving αT and γT.
The complementary nature of GWAS and linkage mapping
Previous GWAS in maize has provided useful information on genetic architecture of tocochromanols (Li et al. 2012; Lipka et al. 2013). Yet notably, there are QTL detected in experimental biparental crosses that are not detected in diverse association panels used in GWAS (Gibson 2012). A main limitation of GWAS is that in the case of small sample size, there is not enough statistical power to detect certain QTL. This is especially true when causal variants have a low MAF (Yang et al. 2010). This limitation may be overcome by utilizing traditional composite interval mapping in biparental populations. Biparental populations by design increase the probability of detecting rare alleles (Gibson 2012). The two parents of biparental mapping populations are usually chosen because they show considerable differences in the trait of interest. If one of the parents carries a rare causative allele, that allele will be at a much higher frequency in the biparental mapping population in comparison to an association panel of the same number of individuals where the MAF for a rare causative allele would be very low. If one parent carries the rare allele, the frequency of that allele is approximately 0.5 in a biparental population, which should increase the ability to detect the QTL containing that allele. The parental lines in this study were previously part of a panel used in a GWAS for tocochromanol variation in maize, where an association with ZmVTE2 was not detected. This illustrates the complementary nature of using high-density QTL mapping as a complementary analysis to GWAS to better characterize and refine the genetic architecture of a trait.
Parental lines with contrasting phenotypes were chosen to create the biparental mapping populations used in this study. In addition to the detection of a novel association that was made with ZmVTE2, QTL intervals containing the genes for all of the major branch points in the tocochromanol pathway were detected. The allelic variation in these five genes, and some families in this population with extreme transgressive segregation for tocol traits, could be used in a breeding program to improve tocochromanol levels in maize grain resulting in improved grain products that could directly impact human and animal nutrition.
References
Andorf CM, Lawrence CJ, Harper LC, Schaeffer ML, Campbell DA, Sen TZ (2010) The locus lookup tool at MaizeGDB: identification of genomic regions in maize by integrating sequence information with physical and genetic maps. Bioinformatics 26(3):434–436. https://doi.org/10.1093/bioinformatics/btp556
Basten CJ, Weir BS, Zeng Z-B. 1994 Zmap-a QTL Cartographer. In: Smith C, Gavora JS, Benkel B, Chesnais J, Fairfull W, Gibson JP, Kennedy BW, Burnside EB (ed) Proceedings of the 5th World Congress on Genetics Applied to Livestock Production: Computing Strategies and Software. Organizing Committee, 5th World Congress on Genetics Applied to Livestock Production, p 65–66
Borek C (2004) Dietary antioxidants and human cancer. Integr Cancer Ther 3(4):333–341. https://doi.org/10.1177/1534735404270578
Bouis HE, Welch RM (2010) Biofortification—a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci 50(Supplement 1):S-20–S-32. https://doi.org/10.2135/cropsci2009.09.0531
Bradbury PJ, Zhiwu Zhang DE, Kroon TM, Casstevens YR, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics (Oxford, England) 23(19):2633–2635. https://doi.org/10.1093/bioinformatics/btm308.
Casella G, George EI (1992) Explaining the Gibbs sampler. Am Stat 46(3):167–174. http://www.tandfonline.com/doi/abs/10.1080/00031305.1992.10475878
Chander S, Guo YQ, Yang XH, Yan JB, Zhang YR, Song TM, Li JS (2008) Genetic dissection of tocopherol content and composition in maize grain using quantitative trait loci analysis and the candidate gene approach. Mol Breed 22(3):353–365. https://doi.org/10.1007/s11032-008-9180-8
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138(3):963–971
DellaPenna D (2005) Progress in the dissection and manipulation of vitamin E synthesis. Trends Plant Sci 10(12):574–579
Eitenmiller RR (1997) Vitamin E content of fats and oils—nutritional implications. Food Technol 51(5):78–81
lshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 6(5):e19379. https://doi.org/10.1371/journal.pone.0019379.
Emmert DH, Kirchner JT (1999) The role of vitamin E in the prevention of heart disease. Arch Fam Med 8(6):537–542. http://www.ncbi.nlm.nih.gov/pubmed/10575394
Falk J, Munné-Bosch S (2010) Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot 61(6):1549–1566. https://doi.org/10.1093/jxb/erq030
Federer WT, Raghavarao D (1975) On augmented designs. Biometrics 31(1):29–35. https://doi.org/10.2307/2529707
Fitzpatrick TB, Basset GJC, Borel P, Carrari F, Dellapenna D, Fraser PD, Hellmann H et al (2012) Vitamin deficiencies in humans: can plant science help? Plant Cell 24:395–414. https://doi.org/10.1105/tpc.111.093120.
Flint-Garcia SA et al (2005) Maize association population: A high-resolution platform for quantitative trait locus dissection. Plant J 44(6):1054–1064
Fryer MJ (1992) The antioxidant effects of thylakoid vitamin E (alpha-tocopherol). Plant Cell Environ 15(4):381–392. https://doi.org/10.1111/j.1365-3040.1992.tb00988.x
Gibson G (2012) Rare and common variants: twenty arguments. Nat Rev Genet 13(2). Nature Publishing Group):135–145. https://doi.org/10.1038/nrg3118
Gilmour AR, Gogel BJ, Cullis BR, Thompson R (2009) ASReml User Guide Release 3.0. VSN International Ltd. http://vsni.de/downloads/asreml/release3/UserGuide.pdf%5Cnpapers3://publication/uuid/716D0761-1368-4982-AF2E-F1D8614A80DF
Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P (2005) Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17(12):3451–3469
Kamal-Eldin A, Appelqvist LA (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31(7):671–701 http://www.ncbi.nlm.nih.gov/pubmed/8827691
Kline K, Lawson KA, Yu W, Sanders BG (2007) Vitamin E and cancer. Vitam Horm 76:435–461
Kutner MH, Nachtsheim CJ, Neter J, Li W (1996) Applied linear statistical models. J R Stat Soc Ser A Gen. Vol. Fifth. Operations and Decision Sciences. McGraw-Hill/Irwin. doi:https://doi.org/10.2307/2984653
Lai J, Li R, Xu X, Jin W, Xu M, Zhao H, Xiang Z et al (2010) Genome-wide patterns of genetic variation among elite maize inbred lines. Nat Publ Group 42(11). Nature Publishing Group):1027–1030. https://doi.org/10.1038/ng.684
Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11(3):241–247. https://doi.org/10.1038/ng1195-241
Li Q, Yang X, Xu S, Cai Y, Zhang D, Han Y, Li L et al (2012) Genome-wide association studies identified three independent polymorphisms associated with tocopherol content in maize kernels. PLoS One 7(5):e36807
Lipka AE, Gore MA, Magallanes-Lundback M, Mesberg A, Lin H, Tiede T, Chen C et al (2013) Genome-wide association study and pathway-level analysis of tocochromanol levels in maize grain. G3 (Bethesda, Md.) 3(8):1287–1299. https://doi.org/10.1534/g3.113.006148
Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P (2004) Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696. https://doi.org/10.1289/ehp.7199
Margarido GRA, Souza AP, Garcia AAF (2007) OneMap: software for genetic mapping in outcrossing species. Hereditas 144(3):78–79. https://doi.org/10.1111/j.2007.0018-0661.02000.x
McMullen MD, Kresovich S, Villeda HS, Bradbury P, Li H, Sun Q, Flint-Garcia S et al (2009) Genetic properties of the maize nested association mapping population. Science (New York, N.Y.) 325(5941):737–740
Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21(1):31–57. https://doi.org/10.1080/0735-260291044179
Nielsen R, Paul JS, Albrechtsen A, Song YS (2011) Genotype and SNP calling from next-generation sequencing data. Nat Rev Genet 12(6). Nature Publishing Group):443–451. https://doi.org/10.1038/nrg2986
Nielsen R, Korneliussen T, Albrechtsen A, Li Y, Wang J (2012) SNP calling, genotype calling, and sample allele frequency estimation from new-generation sequencing data. PLoS One 7(7):e37558. https://doi.org/10.1371/journal.pone.0037558
Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R, Arai H, Inoue K, Mandel JL, Koenig M (1995) Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat Genet 9(2):141–145. https://doi.org/10.1038/ng0295-141
R Development Core Team (2011) R: A language and environment for statistical computing. In: R Development Core Team (ed). R Foundation for Statistical Computing. R Foundation for Statistical Computing. doi:https://doi.org/10.1007/978-3-540-74686-7
SAS Institute (2012) The SAS System for Windows. Release 9.3, SAS Institute, Cary, NC
Sattler SE, Cheng Z, DellaPenna D (2004) From Arabidopsis to agriculture: engineering improved vitamin E content in soybean. Trends Plant Sci 9(8):365–367. https://doi.org/10.1016/j.tplants.2004.06.002
Shutu X, Dalong Z, Ye C, Yi Z, Shah T, Ali F, Qing L, Dalong Z, Ye C, Yi Z, Shah T, Ali F, Qing L, Zhigang L, Weidong W, Jiansheng L, Xiaohong Y, Jianbing Y (2012) Dissecting tocopherols content in maize (Zea Mays L.), using two segregating populations and high-density single nucleotide polymorphism markers. BMC Plant Biol 12:201. https://doi.org/10.1186/1471-2229-12-201.
Sorensen DA, Wang CS, Jensen J, Gianola D (1994) Bayesian analysis of genetic change due to selection using Gibbs sampling. Genet Sel Evol: GSE 26(4). BioMed Central):333–360
Stange M et al (2013) High-density linkage mapping of yield components and epistatic interactions in maize with doubled haploid lines from four crosses. Mol Breed 32(3):533–546
Swarts K, Li H, Romero AJ, An D, Romay CM, Hearne S, Acharya C (2014) Novel methods to optimize genotypic imputation for low-coverage, next-generation sequence data in crop plants. Plant Genome 7(3):12
Vinod KK (2011) Kosambi and the genetic mapping function. Resonance 16(6):540–550. https://doi.org/10.1007/s12045-011-0060-x
Wong JC, Lambert RJ, Tadmor Y, Rocheford TR (2003) QTL associated with accumulation of tocopherols in maize. Crop Sci. https://doi.org/10.2135/cropsci2003.2257
Wright M, Lawson K, Weinstein S, Pietinen P (2006) Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the alpha-tocopherol, beta-carotene cancer prevention study. Am J Clin Nutr 84(5):1200–1207
Yang J, Zhu J, Williams RW (2007) Mapping the genetic architecture of complex traits in experimental populations. Bioinformatics (Oxford, England) 23(12):1527–1536. https://doi.org/10.1093/bioinformatics/btm143.
Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, Madden PA, Heath AC, Martin NG, Montgomery GW, Goddard ME, Visscher PM (2010) Common SNPs explain a large proportion of the heritability for human height. Nat Gen 42(7):565–569
Zeng ZB (1993) Theoretical basis for separation of multiple linked gene effects in mapping quantitative trait loci. Proc Natl Acad Sci U S A 90(23):10972–10976. https://doi.org/10.1073/pnas.90.23.10972
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136(4):1457–1468
Acknowledgments
The authors thank Dean DellaPenna at Michigan State University for providing training and guidance on how to perform the tocochromanol phenotyping.
Funding
This research was supported by the National Science Foundation (NSF) DBI-0922493 (D.D.P., C.R.B, E.S.B., and T.R.R.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fenton, M.E., Owens, B.F., Lipka, A.E. et al. High-density linkage mapping of vitamin E content in maize grain. Mol Breeding 38, 31 (2018). https://doi.org/10.1007/s11032-018-0780-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-018-0780-7