Abstract
Kiwifruit is a perennial horticultural crop species of the Actinidiaceae family and has high nutritional value. For a species with a long generation time, traditional breeding and genetic improvement is predicted to take more than 20 years to obtain superior cultivars. Thus, marker-assisted selection (MAS) should be used to accelerate the breeding process. Development of a genetic linkage map and molecular markers are pre-requisites for MAS of crop species. Here, we report a genome-wide SNP-based genetic map of kiwifruit by analysing next-generation restriction-site-associated DNA sequencing (RADseq) reads. To construct a genetic linkage map, a 102 F1 line mapping population of Actinidia chinensis (2n = 58) was derived by combining parents that had contrasting phenotypic traits. The maternal map contained 4112 SNP loci and spanned a distance of 3821 cM, with an average adjacent-marker interval length of 0.929 cM. The map length of the 29 linkage groups ranged from 78.3 to 169.9 cM, with an average length of 131.8 cM. High levels of collinearity between the 29 genetic maps with the kiwifruit reference genome were found. The genetic map developed in this study can serve as an important platform to improve kiwifruit research, including anchoring unmapped scaffolds of the kiwifruit genome sequence and mapping QTLs (quantitative trait loci) that control economically important traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a successful example of plant domestication in the twentieth century, kiwifruit (Actinidia chinensis) is a popular horticultural crop with a delicious flavour and high nutritional value, with an annual production of 1.8 million tons (Belrose, Inc., 2015). The Actinidia genus is the second largest genus after Saurauia Willd. in the Actinidiaceae and comprises 54 species and 21 botanical varieties (Li et al. 2007). It is widely distributed in Asia, with a main centre of diversity in southwestern China. Actinidia chinensis possesses economic importance, as it is the species from which most commercial kiwifruit varieties (e.g. ‘Hayward’, ‘Hort16A’ and ‘Jintao’) have been developed (Ferguson and Huang 2007). There is increasing interest in developing new varieties with disease resistance and superior commercial traits through intraspecific or interspecific hybridization. For most fruit trees, traditional breeding is time-consuming and inefficient due to a long juvenile period and high heterozygosity. In addition, dioecy and abundant intraspecific ploidy variation presents great challenges in kiwifruit breeding programs (Liu et al. 2016). Marker-assisted selection (MAS) is more efficient and cost-effective than traditional selection. Hence, MAS holds great promise for breeding kiwifruit cultivars by speeding up progeny screening. To implement MAS for traits of interest, it is necessary to first develop a genetic linkage map. Genetic linkage maps provide an important genomic resource for studying the genetic architecture of quantitative traits (QTLs, Doganlar et al. 2002; Wu et al. 2014). With relatively high-resolution maps, it can also be used for map-based gene cloning (Watanabe et al. 2011) and genome assembly (Huang et al. 2013).
The genome of kiwifruit is relatively small (750 Mb, Huang et al. 2013), consisting of 29 chromosomes. In the past two decades, great efforts have been made to construct several genetic maps for kiwifruit. Testolin et al. (2001) reported the first genetic map of kiwifruit based on an interspecific mapping population consisting of 91 F1 hybrids derived from an interspecific cross between the diploid species A. chinensis (♀) and A. callosa var. henry (♂). The map contained molecular markers of amplified fragment length polymorphisms (AFLPs) and simple sequence repeats (SSRs), but the markers did not cover the predicted 29 kiwifruit chromosomes. Fraser et al. (2009) constructed a map based on an intraspecific cross population of 272 F1 individuals using 644 microsatellite markers. Traditional genotyping methods are low throughput, expensive and labour intensive. Hence, the traditional molecular markers cannot satisfy the needs for constructing high-density genetic linkage maps for crop species with high heterozygosity.
Recent advances in next-generation sequencing technologies allow detection of thousands of single nucleotide polymorphisms (SNPs) in a rapid and cost-effective manner (Li et al. 2009a). Restriction-site-associated DNA (RAD) sequencing, a genotyping-by-sequencing (GBS) method that reduces representation of individual genomes with restriction enzymes, can produce a large number of SNPs for the species without reference genome sequences. RADseq has been widely used to construct a SNP-based genetic map (Baird et al. 2008; Wang et al. 2012; Zhang et al. 2015) and ecological genomics (Laporte et al. 2016). To date, two high-density SNP-based linkage maps containing more than 4000 markers were successfully constructed using SNP arrays in kiwifruit (Scaglione et al. 2015; Zhang et al. 2015).
The objective of the present study was to construct a SNP-based high-density genetic map for kiwifruit using RADseq markers in the F1 segregating populations crossed between two highly heterozygous diploid A. chinensis with contrasting phenotypic traits. The collinearity between the linkage maps and the reference genome was evaluated. The dense and reliable linkage map produced will be beneficial for identifying QTLs that control economically important traits.
Materials and methods
The mapping population and DNA extraction
An intraspecific cross in the diploid species A. chinensis was conducted to create a F1 mapping population of 102 individuals. Both parents were collected from the wild populations. The female parent, coded ‘QS1A’, was collected from Hunan province, Central China; the male parent, coded ‘CG1A’, was from a seed accession from Jiangxi province, eastern China. Intraspecific variations in fruit attributes were observed in the female and male parent (Fig. S1). The fruit of the female parent is short, cylindrical, round in cross section and uniform in size and shape. Fruits are small, with a mean weight of 45–50 g, maximum 60 g. The mean soluble solid content (SSC) is 17.5–19% when fruit are ripe, making it an important breeding resource to improve kiwifruit cultivars. The fruit of male plants inferred from the attributes of female siblings from the same wild population are long and ellipsoidal, with yellow spots on the skin. Fruit are an average of 75–85 g, maximum 116 g. The mean soluble solids content (SSC) is 10–12%. The mapping population was grown in the Wuhan Botanical Garden research orchard in Dawu, Hubei, China. Unfortunately, the male parent was dead due to damage to the root nematodes. Thus, in this study, we only constructed a maternal genetic linkage map.
Young leaves from each individual in the mapping population and the female parent were collected for DNA extraction using the DNeasy Plant Mini Kit (Qiagen™) according to the manufacturer’s instructions. The concentration and quality of the extracted DNA were detected using a NanoDrop™ Lite Spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA).
RAD library preparation and sequencing
The RAD library was constructed following the protocol described by Zhang et al. (2015). Briefly, genomic DNA from the female parent and each of 102 progenies were digested for 15 min at 37 °C in a 50-μL reaction with 20 units (U) of EcoR I (NEB, USA). P1 adapter, a modified Illumina adapter, was ligated to the samples. After adapter ligation, sample were pooled and randomly sheared with a Bioruptor (Diagenode, Belgium) to an average size of 500 bp. DNA fragments of 300–500 bp were purified using the MinElute Gel Extraction Kit (Qiagen). The dsDNA ends were repaired using the Quick Blunting kit Enzyme Mix (NEB). Then, a modified Solexa P2 adapter was ligated to the obtained DNA fragments. Finally, purified and quantified DNA products were PCR-amplified using Phusion Master Mix (NEB, USA). PCR amplification was performed with the following cycle profile: 98 °C for 2 min, followed by 13 cycles at 98 °C for 30 s, 60 °C for 30 s and 72 °C for 15 s, and a final extension at 72 °C for 5 min. The prepared DNA libraries were sequenced using Illumina Hiseq2000 instrumentation.
SNP identification and genotyping
To ensure high-quality genotype calling, raw sequence reads were filtered using the following criteria: (1) reads lacking correct barcodes were discarded; (2) reads without unique barcodes were removed; (3) reads containing > 50% low-quality bases (quality value of ≤ 5) were filtered out. The retained reads from each individual were mapped onto the kiwifruit repeat masked genome (Huang et al. 2013) using software SOAP2.25 (Li et al. 2009b). RAD-based SNP detection was conducted using SOAPsnp.26. To minimize errors in assigning markers, consensus alignments with less than 4 or more than 200 reads were discarded.
Genetic map construction
Due to a lack of male genotype data, male parent data were inferred by comparing the genotype patterns of the F1 population to that of the female parent. As the number of putative loci on the male parent is very small, it is difficult to form its linkage map. Thus, in the present study, we only constructed a maternal high-density genetic map. The double pseudo-test cross strategy was used for linkage analysis. In the present study, two types of SNP markers heterozygous in female (lm × ll and hk × hk) were used for the maternal map construction. The genetic map was constructed using JoinMap 4.0 software (Van Ooijen 2006). A “CP” model was used for data mining. Segregating markers genotyped in less than 80% of the individuals were excluded for further analysis. The ratio of marker segregation was calculated using the chi-square test. Markers displaying significant (P < 0.001) segregation distortion were excluded from map construction, as they may affect marker order or greatly alter linkage distance. Linkage groups were formed using a minimum logarithm of odds (LOD) value of 4.0 and a maximum recombination fraction of 0.4. The Kosambi mapping function (Kosambi 1944) was used to calculate map distances (centimorgans, cM). The collinear relationships between 29 linkage groups and the kiwifruit reference genome were analysed by aligning the sequence of each segregating marker with the genome sequences using CIRCOS software (Krzywinski et al. 2009).
Results and discussion
RADseq and SNP discovery
A total of 103 RADseq libraries for the female parent (A. chinensis ‘MT570553’) and 102 F1 offspring were constructed and sequenced in the two lanes of an Illumina HiSeq 2000 sequencer. After removing low-quality sequences, ambiguous barcodes and orphaned paired-end reads, 1914 million clean reads remained, representing ∼ 166 GB of sequences. The average Q20 ratio was 98.4%, indicating high-quality sequence data. The guanine-cytosine (GC) content for the female parent and the progenies was almost 36% (Table S1). The clean reads were deposited at NCBI under the bioproject accession number PRJNA391543. Approximately 54.7 million clean reads were generated for the female libraries; a total of 1859.3 million filtered reads with an average of 18.2 million reads per progeny were generated for the 102 F1 offspring. The reads were then mapped to the reference kiwifruit genome. Among sequencing reads, 80% could be exactly mapped to specific chromosome regions, and unique reads accounted for 91.6% of mapping reads.
The mean coverage of the sequenced loci was 6-fold in the maternal parent and 3-fold in the offspring, on average. After stringent selection, RAD tags from each individual were clustered and called for SNP markers. In total, 5844 high-confidence SNPs across the 102 offspring were developed, including 4363 heterozygous SNPs in the female parent.
Construction of the high-density genetic maps
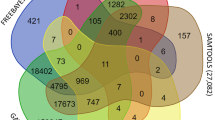
Zhang et al. (2015) published a high-density genetic map composed of 4214 SNP loci for the male parent (A. chinensis cv. ‘Guihai No4’) and 2426 loci for the female parent (A. rufa cv. ‘MT570001’). The female and male maps published in 2015 by Scaglione et al. (2015) contained 6371 and 6244 markers and had lengths of 3276 and 3614 cM, respectively (Table 2). The map produced in the present study included 4112 SNP markers with 29 linkage groups (LGs), which corresponds to the haploid number of chromosomes in A. chinensis. The number of loci mapped per LG ranged from 34 (CHR11) to 258 (CHR258), with an average of 142. The marker names and positions for all SNP loci in the 29 LGs of the maternal genetic map are listed in Supplementary Table S2.
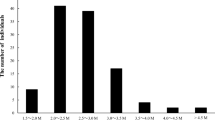
The total length of the maternal map was 3821.7 cM; the genetic map is 1555 cM larger than the previously published map of 2266 cM (Fraser et al. 2009), similar to the map length in other intraspecific SNP-based maps (Scaglione et al. 2015). Given the reference genome size of 750 Mb (Huang et al. 2013), the average ratio of genetic-to-physical distance was approximately 1 cM per 200 KB. The average distance between adjacent markers was 0.929 cM, very similar to the marker density (1.09 cM) in the genetic map of A. rufa (Zhang et al. 2015). Linkage groups widely vary in size; the map length of the 29 linkage groups ranged from 78.4 to 205 cM, with an average length of 131.8 cM (Fig. 1 and Table 1). The largest LG was CHR3, with a length of 205 cM and an average maker interval of 1.577 cM, while the smallest LG was CHR16, with a length of 78.4 cM and an average distance of 0.342 cM between adjacent markers (Table 1). Thus, the established map in the intraspecific F1 population is of high quality and resolution; it can serve as a reference linkage map for QTL mapping of traits of economic importance and MAS of Actinidia species.
Comparison of genetic and physical maps
To compare the genetic and physical maps, sequences surrounding markers on the genetic map were aligned to the kiwifruit reference genome. A total of 5844 SNP markers showed a match to the kiwifruit genome. Notably, most of the 29 kiwifruit linkage groups were syntenic with one of the 29 kiwifruit chromosomal pseudomolecules from the assembly by Huang et al. (2013). However, some markers mapped onto linkage groups conflicted with their positon in the assembled chromosomes of the A. chinensis cv. ‘Hongyang’ genome. For example, multiple clustered markers on Chr5 were remapped to linkage group 21 (CHR21), which was also revealed in Zhang et al. (2015). In agreement with Scaglione et al. (2015), several markers currently attributed to Chr10 remapped to Chr16. In addition, the corresponding markers on CHR12 and CHR27 were clustered into two chromosomal segments (CHR12/CHR18, CHR27/CHR22) (Fig. 2). The chromosome segment with inversion or rearrangement may result in inconsistencies among marker positions. However, preliminary synteny analyses based on the SNP-based kiwifruit map and the reference genome sequence also showed scaffold misplacement events (Scaglione et al. 2015; Zhang et al. 2015). Thus, the discrepancy may be due to assembly errors in the reference genome rather than genomic rearrangements, which are common in draft genome assemblies (Tennessen et al. 2014). In addition to assembly errors, mapping markers on unexpected linkage groups have presumably resulted from different micro-structures on chromosomes in the various species or mapping populations, or from genotyping technical errors (Han et al. 2011; Zhang et al. 2015).
Collinearity between maternal genetic maps and chromosomal pseudomolecules of the assembly by Huang et al. (2013). Linkage groups for maternal maps are colorized, and LGs for reference genetic map are in grey
Although the genome sequence of kiwifruit was released several years ago, a number of gaps and random sequences still exist in the reference genome (Huang et al. 2013). The high-quality, high-density genetic map allowed us to further improve the current genome assembly. In the present study, 441 scaffolds were anchored by 1513 markers from the maternal map (Table 2 and Table S2). Approximately 98.8 Mb previously unanchored sequences of the genome assembly (Huang et al. 2013) will assist in the assembly of the kiwifruit genome sequence by correcting misplaced scaffolds and increasing new anchored scaffolds.
In conclusion, we have constructed a high-density, high-quality genetic map of an intraspecific F1 population using RADseq technology. Comparing the sequence information to the reference genome, we found that the next-generation RADseq is a powerful strategy for developing genetic maps in a fast, cost-effective way. A highly conserved correlation between the maternal linkage map and the reference was detected by synteny and collinearity analysis. The linkage map will be useful for detecting QTLs of growth and fruit traits and assisting in assembling the kiwifruit genome sequence.
References
Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, Lewis ZA, Selker EU, Cresko WA, Johnson EA (2008) Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One 3:e3376
Belrose, Inc. (2015) World kiwifruit review 2015. Belrose, Inc., Pullman, WA
Doganlar S, Frary A, Daunay MC, Lester RN, Tanksley SD (2002) A comparative genetic linkage map of eggplant (Solanum melongena) and its implications for genome evolution in the Solanaceae. Genetics 161:1697–1711
Ferguson AR, Huang HW (2007) Genetic resources of kiwifruit: domestication and breeding. Hortic Rev 33:1–121
Fraser LG, Tsang GK, Datson PM, De Silva HN, Harvey CF, Gill GP, Crowhurst RN, McNeilage MA (2009) A gene-rich linkage map in the dioecious species Actinidia chinensis (kiwifruit) reveals putative X/Y sex-determining chromosomes. BMC Genomics 10:102
Han Y, Zheng D, Vimolmangkang S, Khan MA, Beever JE, Korban SS (2011) Integration of physical and genetic maps in apple confirms whole-genome and segmental duplications in the apple genome. J Exp Bot 62:5117–5130
Huang HW (2014) The genus Actinidia: a world monograph. Science Press, Beijing
Huang SX, Ding J, Deng D et al (2013) Draft genome of the kiwifruit Actinidia chinensis. Nat Commun 4:2640
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugenics 12:172–175
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645
Laporte M, Pavey SA, Rougeux C, Pierron F, Lauzent M, Budzinski H, Labadie P, Geneste E, Couture P, Baudrimont M, Bernatchez L (2016) RAD sequencing reveals within-generation polygenic selection in response to anthropogenic organic and metal contamination in North Atlantic Eels. Mol Ecol 25:219–237
Liu CY, Zhang Q, Yao XH, Zhong CH, Yan CL, Huang HW (2016) Characterization of genome-wide simple sequence repeats and application in interspecific genetic map integration in kiwifruit. Tree Genet & Genomes 12:21
Li JQ, Li XW, Soejarto D (2007) Actinidiaceae. In: Wu ZY, Raven PH, Hong DY (eds) Flora of China. Science Press, Beijing; Missouri Botanical Gardens, St. Louis, Missouri, p 334–360
Li R, Li Y, Fang X, Yang HM, Wang J, Kristiansen K, Wang J (2009a) SNP detection for massively parallel whole-genome resequencing. Genome Res 19:1124–1132
Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J (2009b) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25:1966–1967
Scaglione D, Fornasiero A, Pinto C, Cattonaro F, Spadotto A, Infante R, Meneses C, Messina R, Lain O, Cipriani G, Testolin R (2015) A RAD-based linkage map of kiwifruit (Actinidia chinensis Pl.) as a tool to improve the genome assembly and to scan the genomic region of the gender determinant for the marker-assisted breeding. Tree Genet Genomes 11:115
Tennessen JA, Govindarajulu R, Ashman TL, Liston A (2014) Evolutionary origins and dynamics of octoploid strawberry subgenomes revealed by dense targeted capture linkage maps. Genome Biol Evol 6:3295–3313
Testolin R, Huang W, Lain O, Messina R, Vecchione A, Cipriani G (2001) A kiwifruit (Actinidia spp.) linkage map based on microsatellites and integrated with AFLP markers. Theor Appl Genet 103:30–36
Van Ooijen JW (2006) JoinMap® 4, software for the calculation of genetic linkage maps in experimental populations. Wageningen, Kyazma
Wang N, Fang L, Xin H, Wang L, Li S (2012) Construction of a high-density genetic map for grape using next generation restriction-site associated DNA sequencing. BMC Plant Biol 12:148
Watanabe S, Xia Z, Hideshima R, Tsubokura Y, Sato S, Yamanaka N, Takahashi R, Anai T, Tabata S, Kitamura K (2011) A map-based cloning strategy employing a residual heterozygous line reveals that the GIGANTEA gene is involved in soybean maturity and flowering. Genetics 188:395–407
Wu J, Li LT, Li M, Khan MA, Li XG, Chen H, Yin H, Zhang SL (2014) High-density genetic linkage map construction and identification of fruit-related QTLs in pear using SNP and SSR markers. J Exp Bot 65:5771–5781
Zhang Q, Liu CY, Liu YF, VanBuren R, Yao XH, Zhong CH, Huang HW (2015) High-density interspecific genetic maps of kiwifruit and the identification of sex-specific markers. DNA Res 22:367–375
Acknowledgements
We are grateful to Caihong Zhong for her assistance and advice. This work was partly supported by the Natural Scientific Foundation of China (Grant No. 31770374) and the Backbone Talent Program of the Wuhan Botanical Garden, Chinese Academy of Sciences (Y655291A04).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material

Figure S1
Morphological differences in fruits of female and male kiwifruit individuals. Fruit of male plant inferred from attributes of female siblings from the same wild population. (JPEG 2011 kb).
Table S1
Clean data statistics. (XLS 4 kb).
Table S2
Marker names and positions for all SNP loci in the 29 LGs of the maternal genetic map. (XLSX 509 kb).
Rights and permissions
About this article
Cite this article
Liu, CY., Li, DW., Zhou, JH. et al. Construction of a SNP-based genetic linkage map for kiwifruit using next-generation restriction-site-associated DNA sequencing (RADseq). Mol Breeding 37, 139 (2017). https://doi.org/10.1007/s11032-017-0729-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-017-0729-2