Abstract
Changing climatic conditions with warming winters and shifts in the frequencies of drought, intense rainfall and cold spells together with associated changes in the geographical distribution of arable crops increase the challenges for selecting new varieties. In this context, we aim to contribute to a better understanding of the determinants of barley (Hordeum vulgare) frost tolerance (FRT) and consequent improvements to marker-assisted selection (MAS). Freezing injury in a diversity panel of 121 barley genotypes with different growth habits and origins was assessed using phenotyping based on chlorophyll fluorescence (Fv/Fm) measurements to screen genetic diversity in plants at an early growth stage. The haplotypes of vernalisation and photoperiod genes were determined with PCR, and correlation analyses were done using data from 12 laboratory and field-laboratory FRT tests. Previous results of allelic combinations of VRN-H1/VRN-H2 for FRT were confirmed with these experiments using a larger set of genotypes. The predictive power of polymorphisms in VRN-H1 intron 1 region for FRT was significantly higher than that of the VRN-H1 promoter polymorphism. The vrn-H1/vrn-H2 facultative genotypes had similar or higher FRT than vrn-H1/Vrn-H2 winter genotypes under suboptimal hardening conditions. Genes regulating long-day and short-day photoperiodic responses were significantly correlated with FRT. The most parsimonious model for prediction of FRT was based on polymorphisms in the VRN-H1 intron 1 region, VRN-H2 and PPD-H2 and explained 69% of the variation in FRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of genetic diversity in cultivated germplasm by evaluating genotypes selected in different environments is valuable for improving crop yield and quality. However, the identification of critical factors for crop adaptation in different geographical regions is a challenge now and in the future because of the effects of climate change on growing conditions.
In small grain crops, the long growing cycle of autumn-sown compared with spring-sown cereals has a significant impact on increasing yield, especially in modern cultivars. For this reason and because of their superior winter hardiness (WH), winter cultivars are cultivated in regions subject to severe winters, but also in milder areas where occasional severe frosts can occur.
We define WH herein as the capacity of plants to survive winter stresses and frost tolerance (FRT) as the ability to survive episodes of sub-zero temperatures by tolerating the presence of ice in the apoplastic (extracellular freezing) spaces (Levitt 1980; Fuchigama et al. 1996). While WH is commonly assessed in the field at the end of winter by visual scoring, FRT is usually evaluated under controlled conditions during vegetative growth, when accurate determination of injury after exposure to freezing temperatures is possible (Prášil et al. 2007).
Winter cultivars require vernalisation, i.e. a minimum exposure to low non-freezing temperatures to induce flowering (Sasani et al. 2009) or to reduce the time to flowering (Karsai et al. 2001; Casao et al. 2011a). The satisfaction of these requirements in plants can be limiting in some geographical regions, such as the Mediterranean, especially when climate change is taken into account. In regions similar to the Po Valley (Northern Italy), delayed sowing has become more frequent in the last few years since recurring adverse meteorological conditions, such as prolonged droughts followed by persistent rains, cause excessive soil moisture that limits the access of field machinery. These problems, which will become commoner in future, already cause yield reductions in winter cultivars (Rizza et al. 2010).
Hence, there is increasing interest of breeders and seed companies for identifying vernalisation-insensitive cultivars. One solution is the use of cultivars with facultative growth habit, i.e. tolerant to low temperature, but not requiring vernalisation. Facultative genotypes with high FRT and WH have been identified in barley cultivars originating from the USA (Karsai et al. 2001; von Zitzewitz et al. 2011; Fisk et al. 2013), Turkey (Akar et al. 2009) and Central and Eastern Europe (Prášil et al. 2007; Rizza et al. 2011). Facultative genotypes, traditionally referred to as “alternative”, can be cultivated in environments subject to severe frosts and occasional severe winters, resulting in an increased flexibility for managing sowing date according to seasonal conditions. The characterisation of facultative barley germplasm is, however, still ambiguous (Cockram et al. 2015).
Tolerance and susceptibility to sub-zero temperatures have been traditionally associated with winter and spring growth habit, respectively (Fowler and Carles 1979; Limin and Fowler 2002; Galiba et al. 2009). Reported FRT variability within winter, spring and facultative types indicates that the distinction between the groups is more complex than traditionally assumed. Rather, a continuous range of variation can be expected for FRT as already found for flowering time (Takahashi and Yasuda, 1971; Szűcs et al. 2007; Hemming et al. 2009; Saisho et al. 2011).
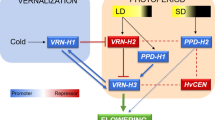
Cold acclimation and vernalisation have a similar optimum temperature range for induction and are controlled by interrelated genetic systems (Dhillon et al. 2010). FRT, photoperiod and vernalisation sensitivity are the main plant internal factors determinant for WH (Pan et al. 1994). Furthermore, vernalisation and photoperiod response have been identified as the main factors regulating the expression level of the genes determining flowering time (Mahfoozi et al. 2001; Szűcs et al. 2006).
The complex interactions between the key genes determining flowering time have been determined and models for the interactions of these factors proposed (Karsai et al. 2005; Cockram et al. 2007a; Trevaskis et al. 2007; Distelfeld et al. 2009; Sasani et al. 2009; Deng et al. 2015). Some of these studies dealt with genetic variation of FRT or WH with respect to genes governing flowering time (Fisk et al. 2013). Nevertheless, the nature of the interactions in different genetic material is still under investigation (Karsai et al. 2008; Casao et al. 2011b; Deng et al. 2015). Genetic analysis of FRT in barley has identified two major quantitative trait loci (QTL), FR-H1 and FR-H2, located on chromosome 5H (Francia et al. 2004). Candidates for FR-H1 and FR-H2 are VRN-H1 (FR-H1 overlaps the VRN-H1 vernalisation response) and a cluster of CBF family members, respectively (Stockinger et al. 2007; Francia et al. 2007; Francia et al. 2016). In this model, the genetic basis of vernalisation response in barley was initially based on phenotypic data in terms of a three-locus epistatic model (Takahashi and Yasuda 1971). The vernalisation-responsive haplotype is vrn-H1/Vrn-H2/vrn-H3 whereas all other allelic combinations at the three loci confer spring habit. Allelic variants at the VRN-H3 locus were reported only in exotic barley genotypes (Takahashi and Yasuda 1971). Though various sequence and copy number variations of the candidate gene, HvFT1, have been identified recently at the VRN-H3 locus, these variations have no effects on the vernalisation requirement (Loscos Aranda et al. 2014). Therefore, Yasuda et al. (1993) proposed a two-gene epistatic model based on the VRN-H1 and VRN-H2 alleles and their interaction in cultivated barley to define the major growth habits. They described these as winter, facultative and spring types, and the proposal was supported by extensive phenotypic data in wheat and barley populations segregating for growth habit.
According to this model, first studied in T. monococcum (Yan et al. 2003; Yan et al. 2004a) and validated in barley (von Zitzewitz et al. 2005; Szűcs et al. 2007), VRN2 encodes a dominant repressor of generative development (Trevaskis et al. 2003). Flowering is repressed by high levels of VRN2 transcripts that suppress the expression of VRN3 before winter. During the winter months, vernalisation-activated VRN1 transcription down-regulates VRN2 expression and after vernalisation up-regulates the expression of VRN3 when the photoperiod is longer than 12 h (Distelfeld et al. 2009; Deng et al. 2015).
The presence of the dominant Vrn-H1 allele in the barley genome results in a spring growth habit regardless of the allelic constitution of VRN-H2. The VRN-H2 locus is present in winter genotypes and deleted in facultative and spring genotypes (von Zitzewitz et al. 2005). In winter cultivars, vernalisation requirement is conferred by the presence of the recessive vrn-H1 allele along with the dominant Vrn-H2 allele. In the facultative type, a physical deletion associated with the VRN-H2 gene results in loss of repression of the winter allele vrn-H1 and, therefore, in the pseudo-spring facultative genotype, the vernalisation requirement has been eliminated (Karsai et al. 2005). In barley and wheat, allelic variation at VRN1 is ascribed to a number of mutations identified in the promoter and/or first intron (Yan et al. 2003, 2004b; von Zitzewitz et al. 2005). With respect to the ancestral winter vrn-H1 allele, a loss of vernalisation requirement occurred with the evolution of the spring Vrn-H1 allele through mutation in its regulatory sites—the CarG box—in the promoter region (Yan et al. 2004b) and the ‘vernalisation critical region’ in the first intron (Fu et al. 2005). In determining growth habit, the promoter mutations are more important in the case of VRN-A1 in wheat (Yan et al. 2004b; von Zitzewitz et al. 2005), while in barley, a large variation in the length of intron 1 is critical (Fu et al. 2005; von Zitzewitz et al. 2005; Cockram et al. 2007b; Szűcs et al. 2007; Hemming et al. 2009; Zhang et al. 2015).
Two candidate genes of photoperiod sensitivity have been identified in barley, namely HvPRR7 for PPD-H1 on chromosome 2H and HvFT3 for PPD-H2 on chromosome 1H (Laurie et al. 1994, 1995). In the general model of flowering, PPD-H1 affects flowering time under long-day conditions whereas PPD-H2 is important in short photoperiods. In the case of PPD-H1, an SNP mutation in exon 7 of the HvPRR7 gene results in recessive allele insensitive to long photoperiods (Turner et al. 2005). For PPD-H2, a non-functional truncated version of HvFT3 leads to the formation of a recessive mutant allele and delayed plant development (Faure et al. 2007).
In a previous experiment, a set of 54 European barley genotypes were compared using a combined phenotyping-genotyping approach to improve prediction of WH as a complex trait that involves FRT and developmental traits (Rizza et al. 2011). The association of FRT with haplotypes of the vernalisation loci VRN-H1/VRN-H2 was studied using a polymorphism in the promoter region of VRN-H1 (von Zitzewitz et al. 2005) to identify the growth habit of the genotypes. Field screenings and physiological laboratory tests based on measurements of leaf chlorophyll fluorescence monitored the effects of cold acclimation and freezing stress on FRT. A significant correlation was found among chlorophyll fluorescence data on plants at first-leaf stage, LT50 (lethal temperature for 50% of plants) measured in plants at the three-leaf stage and winter survival in a 4-year field experiment in Poland (Rizza et al. 2011). Chlorophyll fluorescence measurement as an indicator of freezing stress is a well-established method for evaluating FRT (Clement and van Hasselt 1996). In particular, measurements of Fv/Fm provide an indirect assessment of damage. This ratio of variable (Fv) to maximum (Fm) fluorescence yield measures the maximum yield of photosystem II (PSII) in a dark-adapted state (Butler and Kitajima 1975). Unlike studies of the direct effects of low temperatures on the efficiency of various components of the photosynthetic apparatus, assessment of frost damage measuring Fv/Fm takes this ratio as an indicator of the breakdown of compartmentalisation and the subsequent decline in maximum quantum yield of the photosynthetic apparatus (Dai et al. 2007). Two QTLs for FRT were identified in the Nure x Tremois population in two experiments conducted in a growth chamber at Fiorenzuola (Fv/Fm analysis in plants at first-leaf stage) and in phytotrons at Martonvásár (plant survival at three-leaf growth stage). Results were compared with the visual score in field experiments (Francia et al. 2004). The same major QTLs, FR-H1 (VRN-H1) and FR-H2, were mapped on chromosome 5H for all the experiments.
The combined phenotyping-genotyping approach has been extended herein to a panel of 121 barley genotypes with different geographical origins. FRT was tested by simulating various levels of freezing stress both in growth chambers and in a field-laboratory experiment. The previous study (Rizza et al. 2011) revealed significant differences in FRT of genotypes carrying diverse allelic combinations of VRN-H1/VRN-H2. However, substantial residual variance remained within each group.
The recent findings on the importance of the VRN-H1 intron 1 variation strongly suggest the need to improve germplasm characterisation. Thus, in this report, we extend the set of molecular markers to study potential interactions with genes regulating the response to photoperiod and insertion/deletion within intron 1 of VRN-H1. Results were analysed in relation to the allelic combinations of a panel of functional markers designed from the main candidate genes for VRN-H1, VRN-H2, PPD-H1 and PPD-H2.
In the current study, the physiological approach based on Fv/Fm analysis was used to confirm the relevance of the VRN-H1/VRN-H2 haplotypes on a larger scale and the evidence of high FRT in the vrn-H1/Vrn-H2 (winter) and vrn-H1/vrn-H2 (facultative) genotypes.
In addition, a molecular study based on DNA polymorphism was introduced to test the following hypotheses: (1) allele types of the VRN-H1 intron 1 are correlated with FRT in the same way as they are with flowering time; (2) genes regulating the vernalisation response (VRN-H2), long-day (PPD-H1) and short-day (PPD-H2) photoperiod sensitivities are correlated with FRT.
Materials and methods
A diversity panel of 121 barley genotypes including 62 six-row and 59 two-row genotypes with different growth habit and origin was analysed (Supplementary Table 1). The panel consisted mainly of cultivars originated from 26 different countries—16 European and 10 non-European countries (see Supplementary Table 1 for more information).
Thirty genotypes, most of them winter cultivars, were derived from the breeding programme at CREA—Genomic Research Centre since 1974.
Molecular marker analysis
DNA was extracted from young leaf samples according to Nazari et al. (2014).
Haplotype analysis for VRN-H1, VRN-H2, PPD-H1 and PPD-H2 loci was carried out in the diversity panel.
Markers for vernalisation requirement
The germplasm was characterised with the CAPS (cleaved amplified polymorphic sequence) marker targeting HvBM5A (VRN-H1) and with the STS (sequence-tagged site) marker for Zcct-H (VRN-H2) (von Zitzewitz et al. 2005). The marker for identifying the polymorphism at the promoter region of HvBM5A was previously mapped in Nure x Tremois as an NlaIII-based CAPS marker using the primers HvBM5A-F and HvBM5A-R (von Zitzewitz et al. 2005).
Marker polymorphisms among the genotypes were scored using A (Nure) and B (Tremois) alleles (Supplementary Table 1). AA and BB indicate the vrn-H1/Vrn-H2 and Vrn-H1/vrn-H2 haplotypes of Nure and Tremois, respectively.
“VRN-H1promo/VRN-H2” haplotype is used with reference to this approach, based on the polymorphism at the promoter region for VRN-H1 .
VRN-H1 allelic diversity in the intron 1 structure was recorded, based on the methods of von Zitzewitz et al. (2005), Szűcs et al. (2007) and Hemming et al. (2009). The extent of deletion/insertion in the first intron was analysed with the diagnostic 8 marker-pair sets (88/85, 55/56, 55/85, 55/73, 72/67, 42/43, 42/56, 72/67) (von Zitzewitz et al. 2005; Szűcs et al. 2007). Their locations and nucleotide sequence information are presented in von Zitzewitz et al. (2005).
In this study, “VRN-H1intron/VRN-H2” is used to designate the haplotype obtained on the basis of the VRN-H1 intron 1 structure.
Markers for photoperiod sensitivity
For PPD-H1, the CAPS assay (Turner et al. 2005) differentiated the two functional alleles at the candidate gene HvPRR7. A CAPS marker was developed on the ‘Morex’ BAC clone Hv347D22, which harbours HvFT3, the candidate gene for PPD-H2 (Faure et al. 2007). The identification of the recessive ppd-H2 (truncated, non-functional gene) and the dominant Ppd-H2 (complete, functional gene) alleles was done using a primer pair designed to detect deletions of HvFT3 (Faure et al. 2007).
Assessment of FRT by chlorophyll fluorescence analysis in growth chamber experiments
Seeds were directly germinated in polystyrene containers filled with peat.
The following experiments (E) were carried out in this study (Supplementary Table 2):
Four weeks hardening at 3/1 °C (E1–E4)
Plantlets were grown for 1 week at 20/15 °C (day/night) and 10 h photoperiod, 200 μmol m−2 s−1 photosynthetic photon flux density (PPFD) at leaf level, supplied by cool white fluorescence lamps, then acclimated for 4 weeks at hardening temperatures 3/1 °C day/night and the same light conditions. Subsequently, plants at the first-leaf stage were subjected to a freezing treatment at the minimum temperature levels of −13 or −14 °C at which the plants were kept for 16 h.
Shorter hardening at 3/1 °C (E5)
The effect of a shorter hardening treatment of 2 weeks and 1 week under the same conditions described above was tested. The freezing temperature was −11 °C.
Suboptimal hardening temperature (E6–E10)
Plants were grown for 1 week at 20/15 °C, 200 μmol m−2 s−1 PPFD then acclimated 3 or 4 weeks at 12/7 °C (day/night) under the same light conditions. Plants were subjected to freezing at minimum temperatures lethal for non-hardened barley plants (Crosatti et al. 2008). Plants with two unfolded leaves and a third one in expansion were exposed to minimum temperatures ranging from −7 to −13 °C.
The freezing treatments were simulated in the dark in a test cabinet as described in Rizza et al. (2011) at ±1 °C for the prescribed minimum temperature.
FRT was quantified by measuring chlorophyll fluorescence with a PAM-2000 fluorometer (Walz, Effeltrich, Germany) using the last fully expanded leaf. The ratio of variable (Fv) to maximal (Fm) fluorescence in the dark-adapted state, Fv/Fm (Butler and Kitajima 1975) was measured before, immediately after the freezing treatment and after 24 h of recovery at the same conditions as those employed for growth.
Each experiment was arranged as a randomised complete block design with 6 replications except the experiments E9, E10 with 4 and 2 replications, respectively.
Assessment of FRT by chlorophyll fluorescence in field-laboratory experiments (EF1, EF2, Supplementary Table 2)
The germplasm was tested by measuring Fv/Fm in leaf samples collected from the field at Fiorenzuola d’Arda (PC) on December 16, 2013 and January 21, 2014. The validity of FRT tests on field sampled leaves was tested by Badeck and Rizza (2015). FRT tests at −14 °C were done on five leaves for each genotype and sampling date.
Vernalisation response in the diversity panel
The vernalisation response of a smaller part of the germplasm (85 genotypes) was evaluated in the field at Martonvásár in 2011. Heading dates (DEV49) of the genotypes were recorded using two treatments. Seeds were germinated in Jiffy pots at room temperature for 1 week before planting in the field on April 7th either after a 35-day low temperature vernalisation (DEV49_35v) or without vernalisation (DEV49_0v). There were four plants (replications) of each genotype for each treatment. Heading date was recorded and a value of 150 days for statistical purposes was assigned to those plants that did not head. The vernalisation response was then characterised by the difference of DEV49_0v and DEV49_35v.
Statistical analysis
Fv/Fm as an indicator of frost damage does not always have a normal distribution and its distribution may be skewed and bimodal. Hence, the statistical analyses were done with non-parametric tests. The Kruskal-Wallis test identified differences among genotypes, with subsequent non-parametric multiple comparisons (mctp algorithm in R package nparcomp). Two-factor ANOVA on the rank-transformed data was used to test for genotype x treatment interactions.
Differences among groups of genotypes with dissimilar combinations of molecular markers for vernalisation and photoperiod genes were tested with the Kruskal-Wallis rank sum test with subsequent non-parametric multiple comparisons (R, package nparcomp).
Generalised linear models
Generalised linear models for the mean relative ranks of genotype (n = 121) and experiment used the R glm function (R Core Team 2014) with alleles and haplotypes as independent variables and a binomial link. The models were compared based on the fraction of deviance explained relative to a model using the genotypes as independent variable and the Akaike information criterion (AIC; Akaike 1973). The AIC compares models based on the goodness of fit and by a term penalising more complex models. The model with the lowest value of AIC is regarded as the most parsimonious solution.
Results
VRN-H1promo/VRN-H2 haplotype and FRT
The 121 genotypes were assigned to the four VRN-H1promo/VRN-H2 haplotypes as follows (Supplementary Table 1): 60 winter AA (vrn-H1/Vrn-H2), 18 facultative AB (vrn-H1/vrn-H2) and 43 spring, including 30 BA (Vrn-H1/Vrn-H2) and 13 BB (Vrn-H1/vrn-H2), where A and B refer to the winter Nure and spring Tremois alleles, respectively, for the two vernalisation genes.
FRT was assessed with Fv/Fm in the 10 growth chamber experiments (E1–E10) and were ranked for every experiment. Genotypic differences were significant when measured directly after stress and after recovery. However, larger variability in the response of the genotypes was observed after 24 h recovery because of the greater range of Fv/Fm. The median genotype relative ranking across all experiments varied between 0.0 and 0.9 (Fig. 1). The relative rank based on Fv/Fm combines results for experiments with different levels of stress severity because of different hardening and frost stress.
Relative ranks of FRT for all individual measurements evaluated within the single experiments E1 through E10. Colours code VRN-H1promo/VRN-H2 allele combinations. Blue indicates AA, green indicates AB, orange indicates BA, yellow indicates BB. The four haplotypes were sorted by the alleles of the CAPS marker HvBM5A (VRN-H1, promoter region) and the STS marker for Zcct-H (VRN-H2). The “A” and “B” indicate the winter Nure and the spring Tremois alleles, respectively. Box and whisker plots were used to visualise distributions of results where the line within the box stands for the median. The box range includes the second and third quartile and the whiskers are located at the maximum and minimum values or at 1.5 times the interquartile range from the box. If more extreme values are present, these are then shown with circles
Most of the facultative genotypes (AB) were among the most frost tolerant (Fig. 1, right-hand side). The winter genotypes (AA) were widely distributed, ranging from high to fairly low tolerance. The lowest FRT levels were mostly found among the spring genotypes (BA and BB), but some had medium or high FRT especially in the BA group.
The AA and AB haplotypes on average showed superior FRT in all experiments (E1–E10, Fig. 2). The absolute value was always highest for the AB group and, within the spring types, for the BA rather than the BB haplotype. AB showed the highest FRT especially in experiments with plants subjected to suboptimal hardening treatments and confirms previous data (Rizza et al. 2011). The differences between AA and AB were statistically significant in 5 out of 11 growth chamber experiments (Supplementary Table 2).
Fv/Fm recorded after 24 h recovery, means and standard error for the growth chamber experiments (E1 through E10) carried out to evaluate FRT of the 121 genotypes. The effect of the VRN-H1promo/VRN-H2 allele combinations is shown for each experiment. Haplotype colour codes as defined in Fig. 1
VRN-H1 intron 1 polymorphisms
The results obtained with the VRN-H1promo/VRN-H2 haplotype analyses were compared with those based on the VRN-H1 intron 1 polymorphisms, subsequently referred to as VRN-H1intron/VRN-H2.
The 121 genotypes were assigned to nine groups on the basis of the extent of deletion/insertion in intron 1. Details of the primer pair analysis and the haplotype are presented in Supplementary Table 3. With the exception of a one-cultivar group (named as 1190), most of these groups could be assigned to the allele types described by Hemming et al. (2009). VRN1-1, VRN1-2, VRN1-3, VRN1-4, VRN1-6, VRN1-7 alleles and the wild type allele of vrn-H1 varied in their frequencies. In the case of vrn-H1, two subgroups could be distinguished differing only in a short deletion of about 100 base-pairs located before the regulation element important for vernalisation requirement. The deleted version was designated as vrn-H1(5200) and the full version as vrn-H1(5300). In this genotype set, the two versions of the vrn-H1 allele type were the most frequent; 40.5% belonged to the vrn-H1(5200) and 23.1% to the vrn-H1(5300) subgroup. The next most frequent allele was VRN1-6 allele (15.7%), whereas the frequencies of all the other allele groups were less than 10% and consisted of one to eight cultivars.
When the vernalisation requirements of these allele groups were examined, only the VRN1-6 and vrn-H1 allele groups showed responses in the presence of the VRN-H2 gene (Supplementary Table 3). The average responses of vrnH1(5200) and vrnH1(5300) were the largest and statistically similar (97.0 and 96.9 days, respectively), while VRN1-6 combined with VRN-H2 showed on average a significantly lower level of response compared to the previous two groups (60.3 days). Thus, these three allele groups were considered to be winter alleles while all the others were spring.
Effect of the VRN-H1 intron 1 structure on FRT
The nine VRN-H1 alleles significantly discriminated FRT of the 121 genotypes (Fig. 3a).
Relative rank of FRT for the 9 VRN-H1 allele types identified among the 121 barley genotypes assessed in 10 independent experiments (a) and 2 field-laboratory experiments carried out at Fiorenzuola, Northern Italy (b). Five leaves per genotype were sampled in the field and Fv/Fm measured after a freeze stress test and 24 h of recovery. Significant differences in FRT are shown by the letters at the bottom of the boxes. Same letters indicate means that were not significantly different. Use of box and whisker plots as defined with caption of Fig. 1
Based on analyses of all individual growth chamber experiments, three groups were identified. Genotypes carrying alleles mainly associated with high frost damage (1190, VRN1-1, VRN1-2, VRN1-3, VRN1-4) did not significantly differ from each other and were grouped as “VRN1low”. The genotypes with alleles associated with intermediate FRT, VRN1-6, VRN1-7, vrn-H1(5200), did not significantly differ from each other and were grouped as “VRN1medium”. Alleles 1190 and VRN1-7 were detected in one genotype only. Their group association was assigned based on the mean FRT but could not be tested statistically. The group of genotypes with the highest FRT carrying the vrn-H1(5300) allele differed significantly from all others and was called “VRN1high”. Only winter and facultative genotypes belonged to this group and among them were the most tolerant (Supplementary Tables 3). There were significant between genotype differences for FRT. The relative rank of FRT assessed across all experiments was as follows: VRN1low < VRN1medium < VRN1high (Supplementary Fig. 1). The relative rank of FRT of the groups and the significance of differences were the same when “full” hardening at 3/1 °C (E1–E4) or suboptimal hardening (E5, E6–E10) experiments were analysed separately (Supplementary Fig. 2).
VRN-H1intron/VRN-H2 haplotypes and FRT
FRT differed significantly among the VRN-H1intron/VRN-H2 haplotypes. The range of variation of FRT for each genotype across all experiments (E1–E10) is presented in Fig. 4. Comparing the distribution of relative ranks for FRT by genotypes based on these VRN-H1intron/VRN-H2 haplotypes (Fig. 3a) with the VRN-H1promo/VRN-H2 haplotypes (Fig. 1) shows that changes in the assignment of the growth habit of some genotypes can lead to more homogeneous blocks of FRT for the different haplotypes. The variability among the spring genotypes, especially of the BA group, based on VRN-H1promo was reduced with the VRN-H1intron model and most genotypes with higher FRT were re-assigned to the winter and facultative growth habit (Supplementary Fig. 3).
Relative ranks of FRT for all individual measurements evaluated within the single experiments E1 through E10. The four genotypes classes were sorted by the alleles of the intron 1 marker HvBM5A (Vrn-H1, intron structure) and STS marker for Zcct-H (Vrn-H2). Colour codes for the VrnH1intron/VrnH2 haplotypes are blue for vrn-H1Vrn-H2, green for vrn-H1vrn-H2, orange for Vrn-H1Vrn-H2 and yellow for Vrn-H1vrn-H2. The genotypes identified in orange, BA, or in yellow, BB, on the right-hand side of Fig.1, thus were not spring types with high FRT, but winter and facultative genotypes. Use of box and whisker plots as defined with caption of Fig. 1
The 19 genotypes carrying the VRN1-6 allele (Supplementary Table 3) were formerly classified as spring (17 BA and 1 BB in Supplementary Table 1) and only one as winter based on the promoter polymorphism. This explains the variability among spring genotypes, especially in the BA group. VRN1-6 is characterised by a short deletion in intron 1, which retains the vernalisation requirement and renders it a winter type. Thus, within the 19 genotypes carrying the VRN1-6 allele, 18 are winter and one facultative, and they were re-assigned accordingly.
After the re-assignment, the resulting haplotypes for growth habit were distributed as follows: 77 winter (vrn-H1/Vrn-H2), 19 facultative (vrn-H1/vrn-H2), 25 spring (13 Vrn-H1/Vrn-H2, 12 Vrn-H1/vrn-H2).
The analysis of the single FRT experiments (E1–E10) presented in Fig. 2 and Supplementary Table 2 was revised after the re-assignment of the winter growth habit for the 19 genotypes with the VRN1-6 allele. The main effect of the re-assignment is a decrease in the mean FRT within the spring group as evidenced by a significant difference in the relative rank across all experiments (Fig. 4).
The limited changes in the significance of differences among haplotypes are reported in the Supplementary materials (Supplementary Table 4). The differences between winter and facultative types were statistically significant in 4 out of the 10 growth chamber experiments. Within the spring phenotypes, Vrn-H1/vrn-H2 and Vrn-H1/Vrn-H2 did not differ significantly in any of the experiments.
Model-significance testing for FRT in the group of 121 genotypes: VRN-H1
In generalised linear models (GLM) of relative ranks across all experiments, we compared each of VRN-H1promo, VRN-H1intron, VRN-H2, PPD-H1 and PPD-H2 as independent variables. The “full set” VRN-H1intron is a variable with nine levels (alleles), the “grouped” VRN-H1intron has three levels (low, medium, high FRT), while the others have variables with two levels, (dominant or recessive allele). Based on AIC as a selection criterion, the “grouped” VRN-H1intron alleles yielded the best fit. The next best fitting model was the one with the “full set” of nine VRN-H1intron alleles (AIC = 5159 versus AIC = 5150 for the grouped haplotypes). The explained deviance among genotypes was 56.5% for the “grouped” haplotypes and 56.8% for the full intron allele set and confirms that grouping the VRN-H1intron types does not lead to a substantial loss of information. Nevertheless, the slightly higher fraction of deviance explained by the full set of VRN-H1intron alleles comes at the cost of many additional parameters. The third best model that used the “dominant or recessive” VRN-H1intron as independent variables explained only 44.0% of the deviance and yielded an AIC of 5245. Using the VRN-H1promo led to an additional reduction in explained deviance (28.5%) and was penalised with a further increase in AIC to 5355. In conclusion, the GLMs demonstrate that VRN-H1 alone explains a high fraction of the between genotype variability in FRT. Analyses of the intron 1 structure, therefore, turns out to be superior in predicting FRT relative to the use of the haplotypes determined on the basis of the markers for the promoter alone. The most parsimonious model is the one that groups the VRN-H1intron alleles in three groups. VRN-H2, PPD-H1 and PPD-H2 individually always explained a lower fraction of the deviance than VRN-H1. The independent variables in GLMs in order of decreasing explanatory power (increasing AIC) were “grouped” VRN-H1intron > “full set” VRN-H1intron > “dominant or recessive” VRN-H1intron > VRN-H1promo > PPD-H2 > PPD-H1 > VRN-H2.
Combined actions of VRN-H1 and VRN-H2, PPD-H1, PPD-H2
Models based on “grouped” VRN-H1 types plus an additional gene led to improved fits in decreasing order for VRN-H2 (AIC = 5001, explained deviance 64.8%) > PPD-H2 (AIC = 5137, explained deviance 59.8%) > PPD-H1 (AIC = 5153, explained deviance 57.0%). An additional improvement of fit was obtained by combining the “grouped” VRN-H1 haplotypes with PPD-H2 and VRN-H2 as independent variables (AIC = 5085, explained deviance 68.5%). Combining the grouped VRN-H1 haplotypes with PPD-H1, PPD-H2 and VRN-H2 as independent variables led to a further small increase in explained deviance but was offset by an increase in AIC. Thus, the model analyses for the diversity set used for the current study allowed the identification of a combined action of VRN-H1, VRN-H2 and PPD-H2 and explained more than two-thirds of the inter-genotype variability in FRT.
Diversity for photoperiod sensitivity
In order to simplify the presentation, the results reported in this section relate to the growth habit assignment based on the VRN1intron polymorphisms, which better explained FRT than VRN-H1promo (see above).
DNA polymorphisms in the genes for photoperiod sensitivity showed a preponderance of the dominant Ppd-H1 allele in the vrn-H1/Vrn-H2 (68%) and in vrn-H1/vrn-H2 (84%) groups (Supplementary Tables 1, 3, 5). In the spring haplotypes, the dominant allele was more frequent in Vrn-H1/Vrn-H2 (54%) while the recessive was commonest in the Vrn-H1/vrnH2 (75%) groups.
The recessive ppd-H2 of Hv-FT3 (Faure et al. 2007) was prevalent in the vrn-H1/Vrn-H2 winter (73%) haplotype, while the spring groups more frequently carried the dominant Ppd-H2 85% in Vrn-H1/Vrn-H2 and 100% in Vrn-H1/vrn-H2 (Supplementary Table 6). The dominant and recessive alleles were balanced in the vrn-H1/vrn-H2 facultative genotypes (47 and 53% for Ppd-H2 and ppd-H2, respectively).
Fv/Fm (means) at recovery are reported for each VRN-H1/VRN-H2 haplotype combined with the dominant or recessive PPD-H1 (Supplementary Table 5) or PPD-H2 (Supplementary Table 6) alleles. The relative rank analysis of all experiments taken together is shown with Figs. 5a, b.
Effect of the allele phase in PPD-H1 in the VRN-H1/VRN-H2/PPD-H1 haplotypes (a) and the allele phase in PPD-H2 in the VRN-H1/VRN-H2/PPD-H2 haplotypes (b) on relative rank of Fv/Fm evaluated after 24 h of recovery for all growth chamber experiments to evaluate FRT in the 121 barley genotypes. Same letters indicate means that were not significantly different. Use of box and whisker plots as defined with caption of Fig. 1
For the combination with PPD-H1 (VRN-H1/VRN-H2/PPD-H1 haplotype) no significant differences in FRT were found in the winter group comparing the means of genotypes with the dominant (vrn-H1/Vrn-H2/Ppd-H1) or recessive (vrn-H1/Vrn-H2/ppd-H1) haplotype (Fig. 5a). The major effect was observed for the facultative type where a higher FRT was found in the presence of the dominant Ppd-H1 with respect to the recessive ppd-H1 allele. No differences were found within the Vrn-H1/Vrn-H2 and Vrn-H1/vrn-H2 spring haplotypes.
Unlike PPD-H1, the combination of the vernalisation genes with PPD-H2 (VRN-H1/VRN-H2/PPD-H2 haplotype) showed that the recessive ppd-H2 allele was associated with significantly higher levels of FRT in the facultative haplotype, a close to significant increase in the winter haplotype while no differences were found in the spring groups (Fig. 5b). Only dominant PPD-H2 alleles were found in the Vrn-H1/vrn-H2 spring group.
The trends described for the relative rank of all the experiments were confirmed for the single experiments (see Supplementary Table 5 and Table 6 for VRN-H1/VRN-H2/PPD-H1 and VRN-H1/VRN-H2/PPD-H2 haplotypes, respectively). However, due to the low number of replicates for some haplotypes, the differences between haplotypes carrying recessive or dominant alleles in PPD-H1 and PPD-H2 were not statistically significant in single experiments (with two exceptions in E4 and E6, Supplementary Table 5).
Field-laboratory phenotyping for FRT
Results obtained with FRT tests sampled from plants grown and hardened in the field (see Supplementary Table 7 for climatic conditions at the sampling dates) were consistent with the growth chamber experiment results (see lines for EF1 and EF2, Supplementary tables 4, 5, 6). The ranking of FRT between the allele types of VRN-H1intron was also very similar to the ranking resulting from the laboratory tests (compare Fig. 3b with Fig. 3a and Supplementary Table 2) with the only exception being a small advantage for VRN1-6 with respect to vrn-H1(5200) compared with vrn-H1(5300). The differences between winter and facultative groups were confirmed in the field-laboratory tests under conditions of mild stress that was predominant at Fiorenzuola (Northern Italy).
Discussion
In the present research, we combined physiological phenotyping with gene-specific genotyping in a set of 121 barley cultivars in order to characterise the associations between developmental gene alleles and FRT and to develop a diagnostic method for selecting frost-tolerant genotypes for barley breeding. The physiological phenotyping consisted in evaluating chlorophyll fluorescence of seedlings subjected to different types of hardening and subsequent freezing stresses and of plants grown under natural winter conditions.
The results confirm the suitability of the VRN-H1/VRN-H2 haplotypes for characterising the action of regulators on VRN-H1 and significantly correlated with FRT as described in the following sections.
The gene-specific genotyping targeting the vernalisation response genes VRN-H1 and VRN-H2 was extended to establish the allele compositions in VRN-H1 and the photoperiod sensitivity genes PPD-H1 and PPD-H2. The results obtained with this approach are discussed along the two working hypotheses.
Relevance of the promoter and intron 1 polymorphisms of VRN-H1 in determining FRT
In the barley VRN-H1 gene, several small polymorphisms have previously been identified in addition to large intron 1 variations (von Zitzewitz et al. 2005; Cockram et al. 2007b, 2015). Of all the polymorphic regions, however, intron 1 is the major mutational hotspot with strong functional importance. Until now, 10 allele variants of the wild type VRN-H1 with various lengths of deletion and one allele with a small insertion in intron 1 have been identified (Cockram et al. 2007b; Hemming et al. 2009; Zhang et al. 2015). It was shown that the structure of intron 1 strongly correlates with the activity of the VRN-H1 gene and has, therefore, significant effects on the vernalisation response and flowering time (Hemming et al. 2009). Nevertheless, there has been no systematic research on studying the effect of promoter vs. intron 1 polymorphisms on FRT.
In the Nure x Tremois barley mapping population, VRN-H1 was mapped using a CAPS marker in the promoter region and was identified as a major source of growth habit and FRT (Francia et al. 2004). This promoter marker also explained a significant fraction of the variance in the FRTs of 54 barley cultivars and thus it was considered to be a good predictor (Rizza et al. 2011). Tremois, however, possesses a unique VRN-H1 allele with a small insertion at the beginning of an otherwise full-length (recessive) intron 1. This small insertion leads to a complete loss of vernalisation requirement, rendering Tremois, a spring barley. In order to clarify the effects of the promoter and intron 1 polymorphisms on FRT, both types were assayed in this set of 121 barley cultivars of mostly European origin. The generalised linear models across the 10 freezing tests demonstrated that VRN-H1 alone explains a high fraction of the between genotype variability in FRT. The GLMs also revealed that the promoter polymorphism explained only 28.6% of the variance as compared to 56.5% in the case of the full intron 1 set, but functional effects of the promoter region cannot completely be ruled out. To resolve the role of promoter polymorphisms and the possible associations with the intron 1 allele type in determining FRT, further studies are necessary on selected genetic materials—for example among genotypes segregating for the promoter allele but having the same intron 1.
In the present study, nine VRN-H1 intron 1 allele types were identified: the recessive wild type allele variants, vrn-H1(5300) and vrn-H1(5200); the allele type characteristic to Spanish barley landraces (1190) and six allele variants already described (from VRN1-1 to VRN1-4, VRN1-6 and VRN1-7; von Zitzewitz et al. 2005; Cockram et al. 2007b; Hemming et al. 2009; Zhang et al. 2015). The commonest of the three winter alleles were vrn-H1(5200), the wild type allele vrn-H1(5300) and VRN1-6 in decreasing order. The vrn-H1(5200) allele was among the first to be characterised with a deletion of a region that included a MITE (miniature inverted-repeat transposable element) smaller than 100 bp just before the 436-bp conserved regulatory element of intron 1 (von Zitzewitz et al. 2005; Cockram et al. 2007b). It was, however, considered to belong to the wild type vrn-H1(5300) carrying the full intron 1 length (Cockram et al. 2007b) and its frequency as well as its effect on phenotypic traits have not yet been studied separately (Cockram et al. 2007b; Hemming et al. 2009; Zhang et al. 2015). In this germplasm collection, vrn-H1(5200) is the most frequent allele. 40.5% of the genotypes carried it while the frequency of the wild type allele was only 23.1% and that of VRN1-6 was 15.7%. The latter allele was identified in Spanish winter landraces, where it appeared in two-thirds of the genotypes (Casao et al. 2011a). VRN1-6 was the only intron 1 allele which was in opposite association with the promoter allele type (winter/spring type), leading to the weaker predictive power of the promoter polymorphism.
Hemming et al. (2009) determined that deletions and insertions in the VRN-H1 intron 1 are associated with different gene expression levels in non-vernalised plants and with variation in flowering time. Our results also establish that the structure of intron 1 is strongly correlated not only with the vernalisation response but also with FRT. Genotypes with the wild type vrn-H1(5300) allele possessed both the largest vernalisation requirement when it was associated with the VRN-H2 winter allele (57.1%), and the highest level of FRT. The small deletion within vrn-H1(5200) did not influence the vernalisation response, but it significantly decreased FRT. The genotypes carrying vrn-H1(5200) were winter (87.7%) or facultative types with a range of FRT from high to low, but on average the group showed intermediate tolerance. The phenotypic functionality of the small deletion in vrn-H1(5200) was also indicated by the results on ambient temperature sensing in barley. In this case, the group of cultivars whose development was most delayed by 25 °C had the vrn-H1(5200) allele (Karsai et al. 2008). Thus, vrn-H1(5200) should be considered as a different allele from vrn-H1(5300). It requires further studies to determine the exact nature of this allele. In the case of VRN1-6, the 0.5 kb deletion in the wheat-barley conserved region resulted in a significant decrease in vernalisation response, which was already apparent through the increased gene expression of VRN-H1 after partial vernalisation (Hemming et al. 2009; Casao et al. 2011b). We have shown in this report that this decreased vernalisation response was also accompanied by a significant decrease in FRT. Both vernalisation requirement and FRT, however, remained significantly higher in VRN1-6 than in all the dominant Vrn-H1 alleles with larger intron 1 deletions. The lowest FRT levels were found in the five spring groups (1190, VRN1-1, VRN1-2, VRN1-3, VRN1-4) including most of the Vrn-H1/vrn-H2 and some of the Vrn-H1/Vrn-H2 haplotypes.
Based on these results, it can be concluded that FRT of barley genotypes is strongly correlated with the VRN-H1 intron 1 structure and the effects identified at the promoter site were probably mainly due to linkage drag.
Although further investigation is necessary to clarify the role of the VRN-H1 promoter polymorphism with respect to the intron 1, the application of markers described herein for marker-assisted selection can identify a range of variation in genotype responses not only for flowering time but also for FRT and greatly aids WH prediction.
Relevance of VRN-H2 and the photoperiod sensitivity genes in modulating FRT
The level and duration of FRT is determined by the rate of phenological development and by the activation of transcriptional pathways regulating the stress response. The latter is in turn closely associated with the transition of shoot apices from the vegetative to the reproductive phase and subsequent inflorescence development (Galiba et al. 2013; Fowler et al. 2014). Apex development is primarily a dual function of vernalisation requirement and photoperiod sensitivity, and our results indicate that the major genes of vernalisation response and photoperiod sensitivity are significantly correlated with FRT already in plants at the first-leaf stage. Although VRN-H2 individually contributed to only a small part of the variance of FRT, the VRN-H1/VRN-H2 haplotype combinations explained more of the deviance in FRT than single VRN-H1 alleles with different deletions/insertion at the intron 1 (56.5% for VRN-H1 versus 65.9% for VRN-H1/VRN-H2). VRN-H2 exercises its role as a major determinant of FRT because it is the crucial element in defining vernalisation requirement (Kóti et al. 2006; Szűcs et al. 2007). Nevertheless, its role in determining FRT may not be so important since facultative genotypes lacking this gene may possess similar or higher FRT than winter genotypes. Ten growth chamber experiments carried out under diverse cold hardening and freezing stress conditions consistently showed that most of the genotypes with facultative growth habit were among the most frost tolerant with levels comparable or superior to the winter types. As a group, the facultative haplotype did not differ significantly from the winter haplotype and in some cases (suboptimal hardening conditions) performed better. These results confirm other studies that reported high levels of survival after frost in facultative genotypes tested under different conditions from those used herein (Karsai et al. 2001; Von Zitzewitz et al. 2011; Rizza et al. 2011; Fisk et al. 2013, Cuesta-Marcos et al. 2015). Also, the results obtained in the present study with the field-laboratory test were highly consistent with the outcome of the laboratory experiments.
In winter genotypes, photoperiod sensitivity influences FRT gene expression even before vernalisation saturation (Mahfoozi et al. 2001). In genotypes lacking vernalisation requirements, photoperiod sensitivity to short and/or long photoperiods is a major regulating source of plant development that delays the vegetative-generative transition under non-inductive conditions (Karsai et al. 2008). A strong photoperiod requirement is more important in climates with large temperature fluctuations during winter comprising longer periods of non-freezing temperatures, which would otherwise allow for periods of active growth (Fowler et al. 2014). Thus, it is essential to study the major genes involved in the vernalisation and photoperiod pathways as a whole to understand the interactions and functions of these genes in determining FRT. Our results also confirm previously published data, which highlight the central role of VRN-H1 and VRN-H2 in determining FRT. The findings in this large set of barley cultivars, however, are among the first to establish that the level of FRT correlates with alleles present at the photoperiod loci PPD-H1 and, most importantly, PPD-H2. Model results obtained for the combinations of the grouped intron VRN-H1 types with other genes show a significant improvement from 56.9 to 68.5% of explained deviance when VRN-H2 and PPD-H2 were added as predictors.
HvFT3, the candidate gene of the PPD-H2 locus was identified originally as an active gene influencing heading date under short-day conditions (Faure et al. 2007). It was later deduced that PPD-H2 is an active regulator of plant development when VRN-H2 is absent or not active irrespective of the photoperiod (Karsai et al. 2008; Casao et al. 2011b). The presence of a functional complete gene is the dominant ancestral allele, which results in earlier flowering under short photoperiod, while the non-functional truncated gene of the recessive allele strongly delays plant development. PPD-H2 plays a significant adaptive role, associated with a strong latitudinal cline across the European winter barley cultivars. The PPD-H2 dominant allele was predominant in winter cultivars from southern latitudes, whereas the proportion of cultivars with the recessive (null) allele ppd-H2 was greater at higher latitudes (Casao et al. 2011a; Cockram et al. 2015). The presence of the dominant PPD-H2 allele promotes flowering of winter cultivars under all non-inductive conditions, i.e. under short days or long days in plants that have not had their vernalisation requirement satisfied. This promotion of flowering is accompanied by a strong decrease in FRT. As all the freezing experiments were carried out on young plants vernalised for up to 4 weeks, the phenotypic effect of the PPD-H2 allele composition on FRT could already be detected at the seedling stage and before the vegetative-generative transition. In the present work, the recessive ppd-H2 was prevalent in the group of winter and facultative barley genotypes with the highest FRT level. The most recent high yielding cultivars selected at Fiorenzuola (Northern Italy) carry the recessive ppd-H2 and the VRN1-6 allele and were selected without using molecular markers for VRN-H1intron and PPD-H2.
The effect of the PPD-H1 allele type on FRT was smaller compared to PPD-H2 in this group of barley genotypes. In general, the dominant allele increased FRT in the facultative and winter barleys. But since more than two-thirds of the most frost-tolerant genotypes with the vrn-H1(5300) allele contain the recessive PPD-H2 allele together with the dominant PPD-H1 allele, it is not possible to dissect the exact effect of PPD-H1 on FRT. To elucidate this association, it would be necessary to study FRT in a facultative population segregating for the two PPD-H genes while carrying vrn-H1(5300).
The results obtained with the current study confirm the hypothesis that genes regulating long-day and short-day photoperiodic responses correlate with FRT in a significant manner, while the role of VRN-H2 is only marginal.
Conclusions and perspectives
This work demonstrated that the integration of phenotype analysis through physiological evaluation with a genotyping study based on a few selected molecular markers can contribute to improvements in WH prediction. This combined approach is cost-effective and has the potential to characterise and identify many genotypes for breeding, genetics and biodiversity studies.
Phenotyping
Our method to assess FRT by Fv/Fm measured in seedlings at the first-leaf stage after recovery has been validated in comparison with other methods under laboratory and field conditions and in QTL studies (see discussion in Rizza et al. 2011). Despite the early vegetative stage of the plants tested in the laboratory, the roles of alleles for the vernalisation and photoperiod genes with FRT were consistent with other studies. Tests of low temperature tolerance under both natural and controlled conditions have been recommended whenever possible (Saulescu and Braun 2001) and makes it possible to extrapolate the effects of FRT in the complex of variables involved in WH. However, there is a need to clarify the effects of vernalisation and photoperiod in response to environmental cues from other stressors that determine WH, e.g. frost heaving, anoxy or snow moulds. Multiple interrelated internal and external factors contribute to the plant’s capacity for fast acclimation to environmental changes and/or the ability to acquire maximum FRT and maintain the acquired WH. Physiological and biochemical studies are still needed to investigate the mechanisms in different environments.
In parallel, a phenotyping method suited to study the FRT component of WH in the field would be useful to complement studies under controlled conditions with the analysis of the dynamics of FRT (e.g. speed and duration of cold acclimation) under contrasting field conditions. The field-laboratory test applied in the current study can be employed to manage high numbers of genotypes with reduced costs.
Genotyping
This work reinforces the proposal by Zhang et al. (2015), namely the identification and validation of molecular markers for allelic variants important for adaptive traits. For breeding needs, all these studies could be used for the development of a “tool kit”, i.e. a minimal set of primers to track the most desirable allelic variants. A complex trait such as low temperature tolerance could, therefore, be predicted using diagnostic markers.
Our results underline the important role of VRN-H1 as the main predictor to screen genotypes for FRT. Recent works emphasise VRN1 as a major target for selection in cereal breeding not only for WH but also for other processes determinant for crop production as spike architecture and hormone metabolism (Trevaskis 2010; Deng et al. 2015). The question arises which primer pairs for VRN-H1 intron 1 to use and how additional genes can be identified that are optimised for detection of traits determinant in different environments? In the germplasm tested herein recessive vrn-H1 and recessive ppd-H2 were identified as the most important alleles for FRT. Association of these alleles with the spring vrn-H2 allele leads to a higher flexibility for sowing times of the resultant facultative growth habit. This ideotype represents a solution for environments that are characterised by prevalent moderately cold winters preceded by environmental conditions that occasionally require flexibility in sowing date and ensure FRT when unpredictable severe frost events occur.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN (ed) Second international symposium on information theory. Akademiai Kiado, Budapest, pp. 267–281
Akar T, Francia E, Tondelli A, Rizza F, Stanca AM, Pecchioni N (2009) Marker-assisted characterization of frost tolerance in barley (Hordeum vulgare L.). Plant Breed 128:381–386
Badeck FW, Rizza F (2015) A combined field/laboratory method for assessment of frost tolerance with freezing tests and chlorophyll fluorescence. Agronomy 5:71–88
Butler WL, Kitajima M (1975) Fluorescence quenching in PSII of chloroplasts. BBA-Bioenergetics 376:116–125
Casao MC, Karsai I, Igartua E, Gracia MP, Veisz O, Casas AM (2011a) Adaptation of barley to mild winters: a role for PPDH2. BMC Plant Biol. doi:10.1186/1471-2229-11-164
Casao MC, Igartua E, Karsai I, Lasa JM, Gracia MP, Casas AM (2011b) Expression analysis of vernalization and day-length response genes in barley (Hordeum vulgare L.) indicates that VRNH2 is a repressor of PPDH2 (HvFT3) under long days. J Exp Bot 62:1939–1949
Clement JMAM, van Hasselt PR (1996) Chlorophyll fluorescence as a parameter for frost hardiness in winter wheat. A comparison with other hardiness parameters. Phyton-Ann Rei Bot 36:29–41
Cockram J, Jones H, Leigh FJ, O'Sullivan D, Powell W, Laurie DA, Greenland AJ (2007a) Control of flowering time in temperate cereals: genes, domestication, and sustainable productivity. J Exp Bot 58:1231–1244
Cockram J, Chiapparino E, Taylor SA, Stamati K, Donini P, Laurie DA, O’Sullivan DM (2007b) Haplotype analysis of vernalization loci in European barley germplasm reveals novel VRN-H1 alleles and a predominant winter VRN-H1/VRN-H2 multi-locus haplotype. Theor Appl Genet 115:993–1001
Cockram J, Horsnell R, Soh E, Norris C, O’Sullivan DM (2015) Molecular and phenotypic characterization of the alternative seasonal growth habit and flowering time in barley (Hordeum vulgare ssp. vulgare L.). Mol Breeding 35:1–11
Crosatti C, Pagani D, Cattivelli L, Stanca AM, Rizza F (2008) Effects of the growth stage and hardening conditions on the association between frost resistance and the expression of the cold induced protein COR14b in barley. Environ Exp Bot 62:93–100
Cuesta-Marcos A, Munoz-Amatriain M, Filichkin T, Karsai I, Trevaskis B, Yasuda S, Hayes P, Sato K (2015) The relationships between development and low temperature tolerance in barley near isogenic lines differing for flowering behavior. Plant Cell Physiol 56:2312–2324
Dai F, Zhou M, Zhang G (2007) The changes in chlorophyll fluorescence parameters in winter barley during recovery after freezing shock and as affected by cold acclimation and irradiance. Plant Physiol Bioch 45:915–921
Deng W, Casao MC, Wang P, Sato K, Hayes PM, Finnegan EJ, Trevaskis B (2015) Direct links between the vernalization response and other key traits of cereal crops. Nat Commun 6:5882. doi:10.1038/ncomms6882
Dhillon T, Pearce SP, Stockinger EJ, Distelfeld A, Li CX, Knox AK, Vashegyi L, Vagujfalvi A, Galiba G, Dubcovsky J (2010) Regulation of freezing tolerance and flowering in temperate cereals: the VRN-1 connection. Plant Physiol 153:1846–1858
Distelfeld A, Li C, Dubcovsky J (2009) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12:178–184
Faure S, Higgins J, Turner A, Laurie DA (2007) The FLOWERING LOCUS T-like gene family in barley Hordeum vulgare. Genetics 176:599–609
Fisk SP, Cuesta-Marcos A, Cistue L, Russell J, Smith KP, Baenziger S, Bedo Z, Corey A, Filichkin T, Karsai I, Waugh R, Hayes PM (2013) FR-H3: a new QTL to assist in the development of fall-sown barley with superior low temperature tolerance. Theor Appl Genet 126:335–347
Fowler DB, Carles RJ (1979) Growth, development, and cold tolerance of fall-acclimated cereal grains. Crop Sci 19:915–922
Fowler DB, Byrns BM, Greer KJ (2014) Overwinter low-temperature responses of cereals: analyses and simulation. Crop Sci 54:2395–2405
Francia E, Rizza F, Cattivelli L, Stanca AM, Galiba G, Tóth B, Hayes PM, Skinner JS, Pecchioni N (2004) Two loci on chromosome 5H determine low temperature tolerance in a “Nure” (winter) x “Tremois” (spring) barley map. Theor Appl Genet 108:670–680
Francia E, Barabaschi D, Tondelli A, Laido G, Rizza F, Stanca AM, Busconi M, Fogher C, Stockinger EJ, Pecchioni N (2007) Fine mapping of a HvCBF gene cluster at the frost resistance locus Fr-H2 in barley. Theor Appl Genet 115:1083–1091
Francia E, Morcia C, Pasquariello M, Mazzamurro V, Milc JA, Rizza F, Terzi V, Pecchioni N (2016) Copy number variation at the HvCBF4-HvCBF2 genomic segment is a major component of frost resistance in barley. Plant MolBiol 92:161–175
Fu D, Szűcs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes MP, Dubcovsky J (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Gen Genomics 273:54–65
Fuchigama LH, Maas EV, Lyons JM, Rains DW, Raison JK, Shackel KA (1996) Stress physiology. In: Salisbury FB (ed) Units, symbols, and terminology for plant physiology: a reference for presentation of research results in the plants sciences. Oxford University Press, New York, Oxford, pp. 142–159
Galiba G, Vágújfalvi A, Li C, Soltész A, Dubcovsky J (2009) Regulatory genes involved in the determination of frost tolerance in temperate cereals. Plant Sci 176:12–19
Galiba G, Stockinger EJ, Francia E, Milc J, Kocsy G, Pecchioni N (2013) Freezing tolerance in the Triticeae. In: Varshney R, Tuberosa R (eds) Translational genomics for crop breeding: abiotic stress, yield, and quality. John Wiley and Sons, Inc., pp. 99–123
Hemming MN, Fieg S, Peacock WJ, Dennis ES, Trevaskis B (2009) Regions associated with repression of the barley (Hordeum vulgare) VERNALIZATION1 gene are not required for cold induction. Mol Gen Genomics 282:107–117
Karsai I, Mészáros K, Lang L, Hayes PM, Bedö Z (2001) Multivariate analysis of traits determining adaptation in cultivated barley. Plant Breed 120:217–222
Karsai I, Szűcs P, Mészáros K, Filichkina T, Hayes PM, Skinner JS, Lang L, Bedő Z (2005) The Vrn-H2 locus is a major determinant of flowering time in a facultative winter growth habit barley (Hordeum vulgare L.) mapping population. Theor Appl Genet 110:1458–1466
Karsai I, Szűcs P, Kőszegi B, Hayes PM, Casas A, Bedő Z, Veisz O (2008) Effects of photo and thermo cycles on flowering time in barley: a genetical phenomics approach. J Exp Bot 59:2707–2715
Kóti K, Karsai I, Szűcs P, Horváth C, Mészáros K, Kiss GB, Bedő Z, Hayes PM (2006) Validation of the two-gene epistatic model for vernalization response in a winter x spring barley cross. Euphytica 152:17–24
Laurie DA, Pratchett N, Bezant JH, Snape JW (1994) Genetic analysis of a photoperiod response gene on the short arm of chromosome 2 (2H) of barley (Hordeum vulgare L.). Heredity 72:619–627
Laurie DA, Pratchett N, Snape JW, Bezant JH (1995) RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter x spring barley (Hordeum vulgare L.) cross. Genome 38:575–585
Levitt J (1980) In: Levitt J (ed) Response of plants to environmental stresses. In Vol. 1 Chilling, freezing, and high temperature stresses. Academic Press, New York, p. 497
Limin AE, Fowler DB (2002) Developmental traits affecting low-temperature tolerance response in near-isogenic lines for the vernalization locus Vrn-A1 in wheat (Triticum aestivum L. em Thell). Ann Bot-London 89:579–585
Loscos Aranda J, Igartua Arregui E, Contreras-Moreira B, Gracia Gimmeno MP, Casas Cendoya AM (2014) HvFT1 polymorphism and effect—survey of barley germplasm and expression analysis. Front Plant Sci. doi:10.3389/fpls.2014.00251
Mahfoozi SA, Limin E, Fowler DB (2001) Influence of vernalization and photoperiod responses on cold hardiness in winter cereals. Crop Sci 41:1006–1011
Nazari L, Pattori E, Terzi V, Morcia C, Rossi V (2014) Influence of temperature on infection, growth and mycotoxin in production by Fusarium langsathiae, and F. spozotrichioides in durum wheat. Food Microbiol 39:19–26
Pan A, Hayes PM, Chen F, Chen THH, Blake T, Wright S, Karsai I, Bedö Z (1994) Genetic analysis of the components of winterhardiness in barley (Hordeum vulgare). Theor Appl Genet 89:900–910
Prášil IT, Prášilová P, Maøík K (2007) Comparative study of direct and indirect evaluations of frost tolerance in barley. Field Crop Res 102:1–8
R Core Team (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. http://www.R-project.org/. Accessed 5 May 2014
Rizza F, Alberici R, Pagani D, Gianinetti A, Stanca AM, Cattivelli L (2010) Scegliere l’orzo giusto per l’epoca di semina. Inf Agrar 66(32):51
Rizza F, Pagani D, Gut M, Prasil IT, Lago C, Tondelli A, Orru L, Mazzucotelli E, Francia F, Badeck FW, Crosatti C, Terzi V, Cattivelli L (2011) Stanca. Diversity in the response to low temperature in representative barley genotypes cultivated in Europe Crop Sci 51:2759–2779
Saulescu NN, Braun HJ (2001) Cold tolerance. In: Ortiz-Monasterio JI, McNab A (eds) Reynolds MP. Application of Physiology in Wheat Breeding. CIMMYT, Mexico, D.F., Mexico, pp. 111–123
Saisho D, Ishii M, Hori K, Sato K (2011) Natural variation of barley vernalization requirements: implication of quantitative variation of winter growth habit as an adaptive trait in East Asia. Plant Cell Physiol 52:775–784
Sasani S, Hemming MN, Oliver SN, Greenup A, Tavakkol-Afshari R, Mahfoozi S, Poustini K, Sharifi H, Dennis ES, Peacock WJ, Trevaskis B (2009) The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). J Exp Bot 60:2169–2178
Stockinger EJ, Skinner JS, Gardner KG, Francia E, Pecchioni N (2007) Expression levels of barley Cbfg enes at the Frost resistance-H2 locus are dependent upon alleles at Fr-H1 and Fr-H2. Plant J 51:308–321
Szűcs P, Karsai I, von Zitzewitz J, Meszaros K, Cooper LLD, Gu YQ, Chen THH, Hayes PM, Skinner JS (2006) Positional relationships between photoperiod response QTL and photoreceptor and vernalization genes in barley. Theor Appl Genet 112:1277–1285
Szűcs P, Skinner JS, Karsai I, Cuesta-Marcos A, Haggard KG, Corey AE, Chen THH, Hayes PM (2007) Validation of the VRN-H2/VRN-H1 epistatic model in barley reveals that intron length variation in VRN-H1 may account for a continuum of vernalization sensitivity. Mol Gen Genomics 277:249–261
Takahashi R, Yasuda S (1971) Genetics of earliness and growth habit in barley. In: Nilan RA (ed) Barley Genetics II. Washington State University Press, pp 388–408
Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES (2003) MADS box genes control vernalization-induced flowering in cereals. P Natl Acad Sci USA 100:13099–13104
Trevaskis B (2010) Goldacre paper. The central role of the VERNALIZATION1 gene in the vernalization response of cereals. Funct Plant Biol 37:479–487
Trevaskis B, Hemming MN, Dennis ES, Peacock WJ (2007) The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci 12:352–357
Turner A, Beales J, Faure S, Dunford RP, Laurie DA (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310:1031–1034
von Zitzewitz J, Szûcs P, Dubcovsky J, Yan L, Francia E, Pecchioni N, Casas A, Chen THH, Hayes PM, Skinner JS (2005) Structural and functional characterization of barley vernalization genes. Plant Mol Biol 59:449–467
von Zitzewitz J, Cuesta-Marcos A, Condon F, Castro AJ, Chao S, Corey A, Filichkin T, Fisk SP, Gutierrez L, Haggard K, Karsai I, Muehlbauer GJ, Smith KP, Veisz O, Hayes PM (2011) The genetics of winterhardiness in barley: perspectives from genome-wide association mapping. Plant Genome 4:76–91
Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. P Natl Acad Sci USA 100:6263–6268
Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004a) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303:1640–1644
Yan L, Helguera M, Kato K, Fukuyama S, Sherman J, Dubcovsky J (2004b) Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor Appl Genet 109:1677–1686
Yasuda S, Hayashi J, Moriya I (1993) Genetic constitution for spring growth habit and some other characters in barley cultivars in the Mediterranean coastal regions. Euphytica 70:77–83
Zhang CH, Xu DA, Zhao CH, Yu MQ, Chen J, Qiang XL, Zhang J (2015) Identification and distribution of VERNALIZATION1 alleles in Chinese barley (Hordeum vulgare) germplasm. Mol Breeding. doi:10.1007/s11032-015-0346-x
Acknowledgments
The authors thank Richard Pickering and Paul Johnston for critical reading of the manuscript. Thanks to Ilja Tom Prášil for providing useful genetic material. We dedicate this study in memory of Giovanni Delogu to acknowledge his important role in breeding activities at Fiorenzuola. We thank the technicians and field workers for their skilful assistance.
Research was funded by the Ministero delle politiche agricole alimentari e forestali (MiPAAF) through the projects FAO-RGV “Risorse Genetiche” and AGROSCENARI “Scenari di adattamento dell’agricoltura italiana ai cambiamenti climatici”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Fulvia Rizza and Ildikó Karsai contributed equally to this work.
Rights and permissions
About this article
Cite this article
Rizza, F., Karsai, I., Morcia, C. et al. Association between the allele compositions of major plant developmental genes and frost tolerance in barley (Hordeum vulgare L.) germplasm of different origin. Mol Breeding 36, 156 (2016). https://doi.org/10.1007/s11032-016-0571-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-016-0571-y