Abstract
The emergence and spread of the Ug99 race group of the stem rust pathogen (Puccinia graminis Pers. f. sp. tritici) in the past decade have exposed the vulnerability of wheat (Triticum aestivum L.) to this disease. Discovery of novel and effective sources of resistance is vital for breeding resistant varieties to avert losses. The experimental breeding line MN06113-8 and cultivar RB07 developed by the University of Minnesota wheat breeding program exhibited adult plant resistance (APR) to the Ug99 race group in field tests in Kenya and Ethiopia. Both lines were found to be susceptible at the seedling stage to isolates of the race TTKSK, TTKST, and TTTSK. To dissect the genetic mechanism of resistance present in these lines, MN06113-8 was crossed to RB07 to generate 141 F6 recombinant inbred lines (RILs). The RIL population was evaluated for APR to Ug99 in Kenya and Ethiopia over three seasons and for resistance to North American stem rust pathogen races in St. Paul, MN, in one season. The population was genotyped using high-throughput SNP genotyping assays. Composite interval mapping detected six quantitative trait loci (QTL) involved in APR to African stem rust races and three QTLs involved in stem rust resistance to North American stem rust races. One QTL located on chromosome 2B was associated with APR to stem rust races in all environments. Development of diagnostic markers linked to this gene will facilitate marker-assisted selection of resistant lines to develop varieties with enhanced levels of stem rust resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
This study reports the detection of QTL associated with resistance to the Ug99 race group of stem rust of wheat, in US spring wheat breeding germplasm.
Introduction
One of the primary objectives of resistance breeding is the effective deployment of resistance sources that ensure durability. Constantly evolving pathogen populations challenge the effectiveness and durability of deployed resistance genes. Stem rust of wheat, caused by the fungal pathogen Puccinia graminis Pers. f. sp. tritici (Pgt), is one such example where the pathogen is persistently evolving to overcome host resistance. One of the oldest plant diseases known to mankind (Kislev 1982), stem rust of wheat is highly destructive and bears potential to completely destroy small-scale farm plots to millions of hectares of susceptible varieties (Roelfs 1985).
The severe threat that stem rust has historically posed to global wheat production has been magnified in recent decades by the evolution of a highly virulent race of Pgt typed as TTKS. The isolate of this pathogen race was first observed in Uganda in 1998 and was named Ug99 for the country of origin and the year it was evaluated (Pretorius et al. 2000). Ug99 was found to overcome lines with Sr31, a widely deployed stem rust resistance gene that provided effective resistance at the time. Within a few years of its discovery, this race spread toward North Africa, West Africa, and the Middle East and has potential to travel to West and/or South Asia as global wind patterns may transport the fungal spores over long distance (Hodson et al. 2011; Singh et al. 2008). Race TTKS, later named TTKSK after characterization using an expanded North American stem rust differential set (Jin et al. 2008), along with its six other related races are virulent to 85–95 % of breeding materials worldwide (Wanyera et al. 2006; Singh et al. 2011). The rapid evolution of race TTKSK and related races, the Ug99 race group, means that the pathogen is capable of defeating multiple important stem rust resistance genes, as evident by the breakdown of genes such as Sr31, Sr24, Sr36, and Sr9h (Jin et al. 2008, 2009; Singh et al. 2008; Pretorius et al. 2012; Rouse et al. 2014a). Furthermore, many of the effective stem rust resistance genes in bread wheat and its wild relatives were either ineffective against these races or unusable for practical breeding because of linkage drag associated with large translocations carrying the stem rust resistance genes linked to reduced grain quality, yield, and other desired traits (Singh et al. 2011). While this evolution of the Ug99 race group exposed the high vulnerability of wheat varieties grown worldwide, it also drew attention to the fact that the efforts in global rust monitoring and resistance breeding were not adequate to protect wheat from stem rust, making a search for durable resistance even more urgent.
Durability of rust resistance in wheat is expected to be enhanced by adult plant resistance (APR) genes (Singh 2012). APR is generally observed to condition nonspecific resistance and is characterized by low infection frequency, reduced size of urediniospores, and overall diminished urediniospore production (Stuthman et al. 2007). In the case of wheat stem rust, APR has been further described as expressed in mature plants, mostly associated with the absence of a hypersensitive response to the pathogen (Hare and McIntosh 1979), and quantitatively inherited (Knott 1982). Singh et al. (2005) discuss that pyramiding four to five APR genes can confer near immunity against diseases but may be difficult to accomplish due to large population sizes required to select transgressive segregants, and a lack of diagnostic markers associated with the resistance alleles (Singh 2012). Combining multiple seedling genes, also known as all-stage resistance genes, with or without APR genes has also been proposed and utilized to obtain durable resistance against the disease (Mago et al. 2011; Ayliffe et al. 2008; Evanega et al. 2014; Kolmer et al. 1991). Only a few stem rust APR genes have been discovered in wheat, namely Sr2 (Knott 1968), Sr55 (Lr67/Yr46/Pm46; Herrera-Foessel et al. 2014), Sr56 (Bansal et al. 2014), Sr57 (Lr34/Yr18/Pm38; Lagudah et al. 2006), and Sr58 (Lr46/Yr29/Pm39; Singh et al. 2013c). Continual discovery of new genes that confer APR is vital for the protection of the wheat crop against stem rust.
The threat from the Ug99 race group has been addressed, at least partly, by wheat research teams throughout the world by screening for resistance and using the identified resistant lines in their breeding programs. Many of the genes that have recently been demonstrated to be effective to Ug99 (Jin et al. 2007) were originally identified in non-bread wheat species. Examples include Sr32 which was identified in Aegilops speltoides (McIntosh et al. 1995), Sr37 in Triticum timopheevi (McIntosh and Gyarfas 1971), Sr39 in Aegilops speltoides (Kerber and Dyck 1990), Sr40 in Triticum araraticum (Dyck 1992), Sr44 in Thinopyrum intermedium, and Sr53 in Aegilops geniculata (Liu et al. 2011). While wild relatives of bread wheat are excellent sources of resistance genes, the issue of linkage drag, which occurs from introgression of genes derived from non-elite germplasm to elite breeding material, is a challenge because of the linkage of sometimes large alien chromatin blocks with the introgressed resistance genes. Breeders may hesitate to utilize such wild sources of resistance in their materials because of the time and effort it takes to select for lines with desired agronomic traits. Hence, discovery of resistant material in existing breeding programs would be a clear advantage, as crossing advanced lines with resistance to other elite lines would incorporate the resistance while also preserving desired agronomic qualities.
The wheat breeding program at the University of Minnesota develops hard red spring wheat varieties with superior agronomic performance and disease resistance. To safeguard the released varieties against a potential threat of Ug99, the program has routinely contributed dozens of advanced experimental lines each year since 2005 for resistance screening in a stem rust nursery coordinated by USDA-ARS, the International Center for the Improvement of Maize and Wheat (CIMMYT), and the Kenya Agriculture and Livestock Research Organization (KALRO) in Njoro, Kenya. The advanced experimental line MN06113-8, despite being susceptible to Ug99 races at the seedling stage, was found to exhibit APR to Ug99 races in the Njoro stem rust nursery in Kenya. The University of Minnesota wheat cultivar RB07 also displayed APR to Ug99 races in the field, but is susceptible at the seedling stage. In order to understand the genetic mechanism of stem rust resistance in these lines, they were crossed (RB07/MN06113-8) to generate a biparental recombinant inbred line (RIL) population segregating for APR to Ug99 races.
In this study, we map the Ug99 race group resistance segregating in the RB07/MN06113-8 RIL population. We also estimate the genetic effects of the detected loci and trace their origin. We hypothesize that some of the QTL regions detected in this study have not been previously identified.
Materials and methods
Plant materials
A mapping population of 141 recombinant inbred lines (RILs) was developed via advancing the F2 genotypes using the single seed descent method by crossing ‘MN06113-8’ and ‘RB07,’ both hard red wheat lines with spring growth habit. The line RB07 has the pedigree Norlander (PI 591623)/HJ98 (Busch et al. 2000) and was developed by the University of Minnesota Agricultural Experiment Station. RB07 was released as a cultivar in 2007 on the basis of its high and consistent grain yield, earliness, resistance to wheat leaf rust (caused by Puccinia triticina Eriks.), moderate resistance to Fusarium head blight (caused primarily by Fusarium graminearum Schwabe), and good grain end-use quality (Anderson et al. 2009). The F6-derived line MN06113-8 has the pedigree MN97695-Lr52/HJ98-Fhb1 and is a breeding line from the University of Minnesota Wheat Breeding Program that was advanced to second year yield trials before being discontinued for consideration as a new cultivar candidate. Seed increases in the F6 lines were done in a greenhouse to obtain enough seeds for field phenotyping. Seedling tests for reaction of RB07 and MN06113-8 to races TTKSK (isolate 04KEN156/04), TTKST (isolate 06KEN19v3), and TTTSK (isolate 07KEN24-4) were carried out by following Rouse and Jin (2011) at the United States Department of Agriculture, Agricultural Research Service (USDA-ARS) Cereal Disease Laboratory. Infection types (ITs) were recorded on a 0–4 scale according to Stakman et al. (1962).
Field stem rust evaluation
The F6:7 and F6:8 populations, along with the parents, were evaluated for their field response to African stem rust races at two locations in East Africa over three seasons: at Njoro, Kenya, during the ‘main season’ from June to October of 2012 and the ‘off-season’ from January to April 2013 (referred to as Ken12 and Ken13 hereafter, respectively) and at Debre Zeit, Ethiopia, during the ‘off-season’ from January to June of 2013 (hereafter referred to as Eth13). The population was also evaluated in St. Paul, MN, USA, during May to August 2013 (hereafter referred as StP13) for field response to North American stem rust races.
In the Njoro nursery (Ken12 and Ken13), lines were planted in an augmented design with the susceptible check line ‘Red Bobs’ (Cltr. 6255) planted after every fifty entries. Each line was sown in double 70-cm-long rows, 20 cm apart. On each side of the plot, and in the middle of the plots, a twin row of susceptible spreader wheat cultivar ‘Cacuke’ was sown. The field was also surrounded by a border of several spreader rows comprised of susceptible wheat varieties that were artificially inoculated using a bulk inoculum of Pgt urediniospores collected at the Njoro field site. Wheat stem rust differential lines with known stem rust resistance genes indicated that the predominant, if not only, Pgt race present in the nursery since 2008 was race TTKST (avirulence/virulence formula on the wheat stem rust differential panel: Sr36, SrTmp/Sr5, Sr6, Sr7b, Sr8a, Sr9a, Sr9b, Sr9d, Sr9e, Sr9g, Sr10, Sr11, Sr17, Sr21, Sr24, Sr30, Sr31, Sr38, SrMcN; Njau et al. 2010).
In the Debre Zeit nursery (Eth13), lines were planted in 1-m-long twin rows that were flanked by spreader rows comprised of a mixture of susceptible wheat lines ‘PBW343,’ ‘Morocco,’ and ‘Local Red.’ The RILs were planted in an augmented design with the susceptible check line ‘Red Bobs’ planted after every fifty entries. To initiate the disease, spreader rows were artificially inoculated with bulk inoculum of fresh Pgt urediniospores collected locally from wheat cultivar ‘PBW343’ and urediniospores also collected from local fields. PBW343 contains the gene Sr31, and several races in the Ug99 race group are virulent to Sr31, whereas all other known Pgt isolates are avirulent.
In the St. Paul nursery, lines were planted in hill plots with 20 cm distance between the hills. The population was planted in an augmented design with four check varieties ‘Oklee’ (Anderson et al. 2005), ‘Thatcher’ (Hayes et al. 1936), ‘Tom’ (Anderson et al. 2012), and ‘Verde’ (Busch et al. 1996) planted after every 30 entries. A mixture of susceptible lines ‘Morocco’ and ‘LMPG-6’ were planted perpendicular to surround the lines on all sides. To initiate disease, spreader rows were syringe-injected with a mixture of North American Pgt races MCCFC (isolate 59KS19), QFCSC (isolate 03ND76C), QTHJC (isolate 75ND717C), RCRSC (isolate 77ND82A), RKQQC (isolate 99KS76A), and TPMKC (isolate 74MN1409) at the jointing stage. The spreader rows were also sprayed with a bulked mixture of the six Pgt races suspended in a light mineral oil suspension using an Ulva + sprayer (Micron Sprayers Ltd, Bromyard, UK) after heading.
Phenotyping and data analysis
Field reaction of the RILs to stem rust were recorded as disease severity on the 0–100 modified Cobb scale (Peterson et al. 1948), and infection response, based on the size of pustules and amount of chlorosis and necrosis visible on the stem (Roelfs et al. 1992). Phenotyping of the population segregating for resistance was carried out after the susceptible check varieties in each trial had attained maximum disease severity. Following Stubbs et al. (1986), the severity response value was multiplied with the infection response to obtain coefficient of infection values. Growth stages of the lines were used as covariates in a mixed model in lme4 (R 3.0.3, R Development Core Team, 2013) to obtain phenotypic values corrected for differences in growth stage among the lines. Growth stages of the lines were determined mainly by assessing grain development stages, such as watery, milky, soft dough, and hard dough, and also for stages of booting and flowering, as explained by Zadoks et al. (1974). Phenotypic values corrected for growth stage differences were used to perform analysis of variance (ANOVA) in SAS 9.1 (SAS Institute Inc, Cary, NC, USA). Using the function PROC GLM, genotypes were modeled as random effects and locations (Kenya, Ethiopia, St. Paul) and trials (Ken12, Ken13, Eth13, StP13) as fixed effects. Thus obtained best linear unbiased predictors (BLUPs) were used to map QTL. Replicated checks were used to calculate the pooled error mean square value. Pearson correlation coefficients among the trials were calculated using the function PROC CORR in SAS 9.1.
Molecular marker assay
Genomic DNA was extracted from ground seeds of the parents and F6:7 RILs using a modified cetyl trimethylammonium bromide (CTAB) protocol (Kidwell and Osborn 1992). The extracted DNA was quantified using an ND 1000 Spectrophotometer (NanoDrop Technologies, Delaware, USA). The population was genotyped using SNP markers obtained from two approaches: (1) the 9000 Infinium iSelect SNP assay (9K; Cavanagh et al. 2013) and (2) genotyping by sequencing (GBS; Elshire et al. 2011).
For genotyping using the Infinium iSelect assay, DNA suspended in ddH2O at approximately 80 ng/µl was submitted to the USDA-ARS Small Grain Genotyping Center, Fargo, ND, USA. The data generated were manually called using Illumina’s GenomeStudio 2011.1 (Illumina Inc, Hayward, CA, USA). Briefly, monomorphic markers (markers with the same calls for the entire population), markers with more than 10 % missing data, and markers that deviated from a 1:1 segregation ratio were discarded. Markers with 5 % or less heterozygous calls were retained to avoid false purging of heterozygous loci. This resulted in 1050 high-quality markers that were retained for linkage mapping.
To increase mapping resolution, and partly to investigate the feasibility of genome mapping using markers obtained from next-generation sequencing, the population was also genotyped using the GBS method (Elshire et al. 2011). In the GBS approach, a double-digested library was created using the restriction enzymes PstI and MspI on 200 ng of DNA per sample, following Poland et al. (2012) with modifications. Each library was 76-plexed, with the parents repeated six times each, and the libraries were sequenced in two lanes of Illumina HiSeq 2000, generating 100-bp paired-end sequences. The sequences were processed using the UNEAK pipeline (Lu et al. 2013) using the parameters -c 10 −e 0.025 to obtain GBS SNPs. Reads containing SNPs were used as query sequences and BLASTN-searched against the wheat chromosome survey sequences (CSS) to assign SNPs to unique chromosomes. The wheat CSS were obtained by assembling reads obtained from sequencing flow-sorted wheat chromosomes from the ‘Chinese Spring’ variety (International Wheat Genome Sequencing Consortium, http://wheaturgi.versailles.inra.fr/Seq-Repository/). To ensure that correct SNPs were obtained, only the full-length alignment of a query sequence with the survey sequences allowing either one base mismatch or one gap was permitted. To circumvent retaining of redundant SNPs on paralog sequences and duplicated regions among the A, B, and D subgenomes, SNPs thus obtained were filtered to remove those that mapped more than once to multiple chromosomes. SNPs that were monomorphic, had no allele calls for >10 individuals (>7 % missing data), and were heterozygous in >10 individuals (7 % heterozygosity) were also discarded. This process resulted in 932 high-quality SNP markers that were retained for linkage mapping. Sequences and allele types of these GBS SNPs are available in Supplementary File 1.
Linkage map construction and QTL mapping
SNPs obtained from both genotyping approaches (9K, GBS) were combined to assign markers to linkage groups. Linkage groups were constructed using Mapdisto V1.7.7.0.1 (Lorieux 2012) using a minimum logarithm of odds (LOD) value of 3.0. Genetic distances between the markers were calculated based on the Kosambi mapping function (Kosambi 1943). The program Windows QTL Cartographer 2.5_011, which implements composite interval mapping (CIM) to identify QTL, was used to analyze marker–trait associations (Wang et al. 2012). The LOD threshold for declaring a significant QTL was calculated by 1000 permutations at α = 0.05 and set at 2.5. A walk speed of 1 cM was used for QTL detection. QTL effects were estimated as the proportion of phenotypic variance (R 2) explained by the QTL. If multiple QTL were detected in an environment, digenic additive x additive epistatic interactions were tested among the detected QTL using the multiple interval mapping (MIM) algorithm available in the same program. Using multiple marker intervals simultaneously, the MIM procedure fits multiple putative QTL directly in the QTL mapping model and estimates several genetic architecture parameters including the effects of and interactions among significant QTL. Epistatic interaction among all SNP markers, irrespective of their association with the detected QTL, was also carried out using the MIM algorithm. A QTL × QTL interaction was declared significant if the LOD threshold was ≥1.0.
Results
Disease evaluation
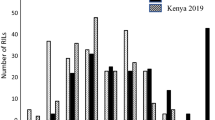
Both parents conferred medium to high levels of resistance in the field in Kenya (Table 1), but were seedling susceptible to TTKSK, TTKST, TTTSK races (IT 3+ for both lines to all three races). The disease pressure observed in each environment was adequate for good discrimination among stem rust phenotypes of the RILs and the mapping of loci associated with quantitative resistance. The disease severity distributions skewed toward the lower percent severity responses overall (Fig. 1). The highest disease pressure was observed in Ethiopia in 2013 with lines showing up to 70 % severity. Disease scores recorded for the RIL population along with the parents in all four environments are presented in Table 1. There was no significant difference between MN06113-8 and RB07 for their average stem rust responses across all environments (t test P value of 0.25 at α = 0.05). This is not completely unexpected, as both parent lines exhibit similar levels of APR in the field (Table 1; Fig. 1). When differences were observed, such as in the three African environments, MN06113-8 displayed a lower severity compared to RB07. Disease severity distributions for the RILs across all environments were continuous, suggestive of quantitative and polygenic resistance. Disease reactions of RILs between Kenya and Ethiopia nurseries were strongly correlated, whereas correlations with the St. Paul reactions were lower, yet still significant (Table 2). The lower correlation coefficients between St. Paul and the Kenyan environments suggest the presence of genotype by environment (G×E) interaction, corroborated by the significant F test value of 3.1 (significant at P < 0.001) in the combined ANOVA. Some of the factors that could have contributed toward G×E could be the differences between Pgt races, inoculum load, and differences in environmental conditions.
Frequency distribution of stem rust severity (%) for the RB07/MN06113-8 population comprised of 141 recombinant inbred lines evaluated in four environments: two at Njoro, Kenya, one at Debre Zeit, Ethiopia, and one at St. Paul, MN, USA. Square and circle symbols (with the same color scheme as the bars) represent the average disease severity of the parents MN06113-8 and RB07, respectively, in each environment. Ken12 Kenya 2012, Ken13 Kenya 2013, Eth13 Ethiopia 2013, StP13 St. Paul 2013
Construction of linkage maps of the RIL population
SNPs obtained from both genotyping methods—the 9K SNP chip and de novo GBS SNPs—were combined to develop linkage maps to represent wheat chromosomes. Of the 1982 markers used for linkage mapping, 1899 (972 9K, 927 GBS) were assigned to 29 linkage groups. All 21 wheat chromosomes were represented by the linkage groups, with 8 chromosomes represented by two linkage groups. These markers covered 1950 cM of the genome, with an average interval of 1.03 cM. Most SNPs were assigned to the A and B genomes, with 855 and 909 SNPs, respectively, while 135 SNPs were assigned to the D genome (Fig. 2).
Quantitative mapping of resistance to stem rust
The CIM method of QTL mapping detected six significant QTL involved in APR in the African environments and four QTL involved in APR in St. Paul (Table 3) with one QTL significant in all four environments. The detected QTL ranged in their LOD scores from 2.6 to 16, with four QTL contributed by MN06113-8 and five contributed by RB07. The QTL QSr.umn-2B.2 was detected in all environments (Fig. 3) and explained the observed phenotypic variation (R 2) as follows: 31.4 % in Ken12, 27.1 % in Ken13, 46.9 % in Eth13, and 8.7 % in StP13. This QTL, derived from MN06113-8, had the largest effect in all environments except in StP13 where the QTL QSr.umn-4B.2 was the largest in terms of both R 2 and allelic effect (Table 3). Other QTL explained 5–13 % of the resistance variation, but were significant only in one of the four environments. Multiple QTL on a single chromosome were detected in two environments: Ken12 with two QTL on chromosome 2B and StP13 with two QTL on chromosome 4B.
Quantitative trait loci (QTL) interval map for QSr.umn-2B.2 detected on wheat chromosome 2B. The QTL is associated with stem rust resistance in the RB07/MN06113-8 population of recombinant inbred lines and provided resistance in all environments. The Y-axis indicates the logarithm of odds (LOD) values with the dotted line representing the threshold LOD score of 2.5. The X-axis is labeled with SNP markers and the genetic distances in centimorgan (cM) between the markers. Markers beginning with ‘TP’ are SNPs discovered from GBS approach. The arrowed line shows the orientation of the linkage map, with the circle representing the centromere. Ken12 Kenya 2012, Ken13 Kenya 2013, Eth13 Ethiopia 2013, StP13 St. Paul 2013
Test for additive × additive epistasis among the detected QTL revealed no significant interactions. Test for epistasis among all loci (significant or not) also detected no significant interactions. All two QTL models were generated to estimate the average reduction in severity (Table 4). The results indicate that combinations of at least two QTL in a gene-pyramiding scheme reduced disease severity values from 9.2 to 52.2 % in the environments where the QTL were detected.
Discussion
In this study, we report several genomic regions associated with APR to African Pgt races in the Ug99 race group and North American Pgt races in lines derived from the University of Minnesota wheat breeding program. Analysis of the segregating population in four environments led to identification of nine QTL on chromosomes 1A, 2B, 2D, 4A, 4B, 6D, and 7A, explaining from 4.5 to 46.9 % of resistance variation in the field.
The QTL QSr.umn-2B.2 was significant in all four environments and was contributed by the resistant parent MN06113-8 (Table 3). A small-effect QTL, QSr.umn-2B.1, was also detected on chromosome 2B in the Ken12 environment. Based on mapping of significant SNP sequences to the wheat CSS and also the published 9K map (Cavanagh et al. 2013), the chromosomal locations of QSr.umn-2B.1 and QSr.umn-2B.2 were determined to be on the long arm and short arm of 2B, respectively. QTL located on 2B that provide resistance to African stem rust races have been reported previously as summarized by the Ug99 resistance loci consensus map including QTL mapped to 2B from unpublished the International Center for the Improvement of Maize and Wheat (CIMMYT) populations (Yu et al. 2014). Singh et al. (2013a) reported QSr.cim-2BS on 2BS between the Diversity Arrays Technology (DArT) markers wPt-9230 and wPt-744022 that explained 3.2–6.2 % of the resistance expressed in the CIMMYT population PBW343/Muu. Bhavani et al. (2011) also detected a QTL on chromosome 2B between the DArT markers wPt-7829 and wPt-2266 that provided moderate to low levels of resistance to Ug99 races in the CIMMYT population PBW343/Juchi. Additionally, in an association analysis of CIMMYT spring wheat germplasm, Yu et al. (2011) reported that the markers wPt-7750, wPt-8460, and wPt-7200 on chromosome 2B were significantly associated with resistance to races of the Ug99 lineage. Crossa et al. (2007) detected a non-Ug99 stem rust QTL associated with the 2BS markers wPt-0100 and wPt-4916, in the same region as QSr.umn-2B.2, in CIMMYT’s elite spring wheat germplasm. We used the integrated genetic map consisting of different marker types generated by Maccaferri et al. (2015) to compare the QTL positions in our study with significant markers reported in previous studies and found that the marker wPt-7750 to be the closest to the QSr.umn-2B.2 peak, at a distance of 34 cM. Thus, neither of these reported markers nor the mapped locations of the seedling genes on 2B (Sr9h, Sr28, Sr36, Sr39, Sr40, and Sr47) that provide resistance to the Ug99 race group (Hiebert et al. 2010; Klindworth et al. 2012; Wu et al. 2009; Tsilo et al. 2008; Niu et al. 2011; Rouse et al. 2012; 2014a) overlap or closely flank the QTL region on 2BS reported in this study.
No known major genes effective to Ug99 races are postulated in the population as the parents are susceptible at the seedling stage to the races TTKSK, TTKST, and TTTSK with IT 3+ to these races. Therefore, the QTL QSr.umn-2B.2 on 2BS could be an important discovery with a relatively large effect on disease reduction. More importantly, this QTL was detected in all environments we used to screen the population, suggesting that it is effective to all of the races used in these environments. Although, it is possible that the QTL may not be effective to one or more races that is present at a low frequency in the disease nursery, such as in the St. Paul nursery. Screening of the population in single-race nurseries would elucidate the efficacy of this QTL to the races. Regardless, the use of QSr.umn-2B.2 in breeding for APR to stem rust globally could be a major advantage in the fight against wheat stem rust. In addition, some wheat APR genes are known to be effective against more than one pathogen, either due to pleiotropy or due to colocalization of genes resistant to multiple pathogens (Risk et al. 2013; Suenaga et al. 2003; William et al. 2003). The short arm of chromosome 2B reportedly contains APR QTL conferring resistance to stripe rust (Carter et al. 2009; Prins et al. 2011) and leaf rust (Tsilo et al. 2014). Therefore, it may be worth investigating whether the QTL QSr.umn-2B.2 also provides resistance to other pathogens such as leaf rust and stripe rust.
The minor effect QTL QSr.umn-1A and QSr.umn-2D were both detected in the Ken13 environment. Other loci on chromosome 1A that are significantly associated with resistance to African stem rust races have been reported by Rouse et al. (2014b) in ‘Thatcher’ wheat (QTL QSr.cdl-1AL), Bhavani et al. (2011) in the CIMMYT biparental population PBW343/Kingbird (QTL between the markers wPt-0128 and wPt-734078), Yu et al. (2012) in CIMMYT’s winter wheat breeding germplasm, Pozniak et al. (2008) in a durum wheat (Triticum durum Desf.) association mapping panel, and Singh et al. (2013b) in the durum wheat population Sachem/Strongfield. The only QTL located on chromosome 2D providing APR to the African stem rust races was reported by Bhavani et al. (2011) in the CIMMYT population PBW343/Kiritati, which is located 14 cM from the position of QSr.umn-2D.
Another minor effect QTL observed in our study was QSr.umn-4A, located on the short arm of chromosome 4A. Previous reports of QTL located on 4A include the mapping studies by Bhavani et al. (2011) and Yu et al. (2011). The QTL QSr.umn-4B.1 and QSr.umn-4B.2 were both detected in the StP13 environment, yet the former was contributed by RB07 and the latter by MN06113-8. Previous reports of QTL on 4B include a minor effect QTL detected by Bhavani et al. (2011) in the PBW343/Kingbird population; QTL contributed by the Canadian cultivar ‘Carberry’ (Singh et al. 2013a); and an APR QTL contributed by the Indian cultivar ‘WL711’ in the RIL population HD2009/WL711 (Kaur et al. 2009).
The QTL QSr.umn-6D was detected only in the Ken12 environment, yet could be novel as no QTL effective to the Ug99 races have been reported in this region. This QTL originates from RB07 and is located on the short arm of chromosome 6D. The all-stage resistance gene Sr42 is also located on 6DS and provides resistance to the African races TTKSK, TTKST, and TTTSK (Ghazvini et al. 2012). Since both parents showed susceptible infection types to these three races during seedling screening and were negative for the presence of Sr42 during marker screening, Sr42 may not be present in either parent. Another all-stage resistance gene present on 6DS, Sr5, is not effective against races in the Ug99 lineage (Singh et al. 2011) and does not provide APR to stem rust when deployed singly, but it may be involved in APR when used with other genes (Nazareno and Roelfs 1981; Knott 2001). We do not have sufficient information to investigate the relationship between QSr.umn-6D and Sr5.
The QTL QSr.umn-7A, also contributed by RB07, mapped on the distal end of chromosome 7AS (0.8 cM) and explained 4.5 % of the phenotypic variance. Singh et al. (unpublished; see Yu et al. 2014) reported a QTL located between 2.9 and 5.6 cM on chromosome 7AS in the PBW343/Kenya Nyangumi population, which could be the same as the QTL detected in our study. Further studies including fine mapping of the region are essential to establish the novelty of this resistance locus. Also, given that we implemented SNP markers and other studies have used either DArT or SSR markers, a satisfactory direct comparison with published QTL cannot be made with existing information. Characterizing the QTL detected in our study in relation to QTL identified in other studies can be helpful in terms of sustainable gene deployment strategies. However, the use of different marker systems in different studies makes it difficult to do so. Regardless, description of these QTL within breeding germplasm can aid significantly in the fight against the disease.
One interesting discovery made in this study is the lack of detection of QTL on chromosomes with genes exhibiting pleiotropic APR to stem rust and other diseases, as reported in published studies. No QTL were detected on chromosomes 1B (location of Sr58), 3B (location of Sr2), 4D (location of Sr55), 5B (location of Sr56), and 7D (location of Sr57). The lack of QTL detection on these chromosomes suggests that a previously undetected APR gene could be present in the RB07/MN06113-8 population. However, the marker density on chromosomes 4D and 7D (11 and 4 markers, respectively) in our study limited our ability to potentially detect Sr55 or Sr57. Screening for the presence of Sr57 (colocalized with Lr34/Yr18/Pm38) using the STS marker csLV34 developed by Lagudah et al. (2006) showed that neither parent carried Sr57. Screening of other APR genes Sr55, Sr56, and Sr58 was not possible due to unavailability of diagnostic markers. Our discovery of QSr.umn-2B.2 in all four environments, as well as other QTL in previously unreported chromosomal locations, provides a strong case for the existence of new QTL in the RB07/MN06113-8 population that confer APR against African and North American stem rust races. Identification of new APR genes is significant because it would provide breeders with new and possibly durable tools to develop varieties resistant to highly virulent Pgt races such as the Ug99 race group. The discovery of new APR genes and their deployment can also alleviate the selection pressure put on the pathogen by newly deployed race-specific resistance genes (Evanega et al. 2014; Singh et al. 2008) and therefore prolong their durability. Further field screening and tests are required to confirm the novelty of these discovered regions. Also, identifying markers linked to these QTL would aid in marker-assisted selection to enhance resistance.
The emergence of widely virulent African stem rust races is often credited to the pathogen’s ability to defeat singly deployed resistance genes in areas conducive to the pathogen’s growth, development, and evolution. To slow down the pathogen from developing virulence by single-step mutations for deployed resistance genes, the scheme of resistance breeding by pyramiding multiple genes has been proposed (Knott 1989). As pyramiding of multiple genes in a single line can be resource intensive given the large number of progeny needed for screening (Bonnett et al. 2005), combining fewer genes may be desirable to obtain immediate resistance to the disease. The combination of only two QTL detected in our study showed clear reduction in disease severity (Table 4) and can be implemented to lower the disease pressure. Combining multiple QTL as the long-term breeding goal could be of greater interest, especially in disease hotspots, as pyramiding APR genes with major genes may also increase the durability of major genes (Burdon et al. 2014; Mundt 2014). Further work to fine map the regions and to identify diagnostic markers is required to accomplish successful pyramiding of multiple QTL.
Conclusions
It is generally considered that the widely virulent African stem rust races reaching the breadbaskets of Asia and the Americas is a real possibility (Hodson et al. 2011). Understanding the threat of their possible arrival, several mapping studies in different types of mapping populations have been conducted to discover resistance loci effective against the Ug99 race group. Here, we report a large-effect APR QTL, QSr.umn-2B.2, and other sources of resistance that are effective against African and North American Pgt races. The RIL mapping population was developed using advanced breeding lines from the University of Minnesota wheat breeding program. An advantage of mapping resistant QTL in a population obtained from crossing two elite parent lines is that the resistant line can be used as a donor parent in a recurrent breeding scheme without linkage drag. The discovery of these QTL in our elite parent lines should offer value to their utilization for APR to the Ug99 race group in other breeding programs globally.
References
Anderson JA, Busch RH, Mcvey DV, Kolmer JA, Linkert GL, Wiersma JV, Dill-Macky R, Wiersma JJ, Hareland GA (2005) Registration of ‘Oklee’ wheat. Crop Sci 45:784–785
Anderson JA, Linkert GL, Busch RH, Wiersma JJ, Kolmer JA, Jin Y, Dill-Macky R, Wiersma JV, Hareland GA, McVey DV (2009) Registration of ‘RB07’ wheat. J Plant Regist 3(2):175–180. doi:10.3198/jpr2008.08.0478crc
Anderson JA, Wiersma JJ, Linkert GL, Kolmer JA, Jin Y, Dill-Macky R, Wiersma JV, Hareland GA, Busch RH (2012) Registration of ‘Tom’ wheat. J Plant Regist 6(2):180–185. doi:10.3198/jpr2011.06.0339crc
Ayliffe M, Singh R, Lagudah E (2008) Durable resistance to wheat stem rust needed. Curr Opin Plant Biol 11(2):187–192. doi:10.1016/j.pbi.2008.02.001
Bansal U, Bariana H, Wong D, Randhawa M, Wicker T, Hayden M, Keller B (2014) Molecular mapping of an adult plant stem rust resistance gene Sr56 in winter wheat cultivar Arina. Theor Appl Genet 127(6):1441–1448. doi:10.1007/s00122-014-2311-1
Bhavani S, Singh RP, Argillier O, Huerta-Espino J, Singh S, Njau P, Brun S, Lacam S, Desmouceaux N (2011) Mapping durable adult plant stem rust resistance to the race Ug99 group in six CIMMYT wheats. In: McIntosh RA (ed) Proceedings of the Borlaug Global Rust Initiative 2011 technical workshop, June 13–16, 2011, Saint Paul, Minnesota. Borlaug Global Rust Initiative, Ithaca, NY, pp 43–53
Bonnett DG, Rebetzke GJ, Spielmeyer W (2005) Strategies for efficient implementation of molecular markers in wheat breeding. Mol Breed 15(1):75–85. doi:10.1007/s11032-004-2734-5
Burdon JJ, Barrett LG, Rebetzke G, Thrall PH (2014) Guiding deployment of resistance in cereals using evolutionary principles. Evol Appl 7:609–624
Busch RH, McVey DV, Linkert GL, Wiersma JV, Warnes DO, Wilcoxson RD, Hareland GA, Edwards I, Schmidt H (1996) Registration of ‘Verde’ wheat. Crop Sci 36:1418
Busch RH, McVey DV, Linkert GL, Wiersma JV, Dill-Macky R, Hareland GA, Edwards I, Schmidt HJ (2000) Registration of ‘HJ98’ wheat. Crop Sci 40:296–297
Carter AH, Chen XM, Garland-Campbell K, Kidwell KK (2009) Identifying QTL for high-temperature adult-plant resistance to stripe rust (Puccinia striiformis f. sp. tritici) in the spring wheat (Triticum aestivum L.) cultivar ‘Louise’. Theor Appl Genet 119(6):1119–1128. doi:10.1007/s00122-009-1114-2
Cavanagh CR, Chao S, Wang S, Huang BE, Stephen S, Kiani S, Forrest K, Saintenac C, Brown-Guedira GL, Akhunova A, See D, Bai G, Pumphrey M, Tomar L, Wong D, Kong S, Reynolds M, da Silva ML, Bockelman H, Talbert L, Anderson JA, Dreisigacker S, Baenziger S, Carter A, Korzun V, Morrell PL, Dubcovsky J, Morell MK, Sorrells ME, Hayden MJ, Akhunov E (2013) Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Natl Acad Sci. doi:10.1073/pnas.1217133110
Crossa J, Burgueño J, Dreisigacker S, Vargas M, Herrera-Foessel SA, Lillemo M, Singh RP, Trethowan R, Warburton M, Franco J, Reynolds M, Crouch JH, Ortiz R (2007) Association analysis of historical bread wheat germplasm using additive genetic covariance of relatives and population structure. Genetics 177(3):1889–1913. doi:10.1534/genetics.107.078659
Dyck PL (1992) Transfer of a gene for stem rust resistance from Triticum araraticum to hexaploid wheat. Genome 35(5):788–792. doi:10.1139/g92-120
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6(5):e19379. doi:10.1371/journal.pone.0019379
Evanega SD, Singh RP, Coffman R, Pumphrey MO (2014) The Borlaug Global Rust Initiative: reducing the genetic vulnerability of wheat to rust. In: Tuberosa R, Graner A, Frison E (eds) Genomics of plant genetic resources. Springer, Netherlands, pp 317–331. doi:10.1007/978-94-007-7575-6_13
Ghazvini H, Hiebert C, Zegeye T, Liu S, Dilawari M, Tsilo T, Anderson J, Rouse M, Jin Y, Fetch T (2012) Inheritance of resistance to Ug99 stem rust in wheat cultivar Norin 40 and genetic mapping of Sr42. Theor Appl Genet 125(4):817–824. doi:10.1007/s00122-012-1874-y
Hare RA, McIntosh RA (1979) Genetic and cytogenetic studies of durable adult-plant resistances in Hope and related cultivars to wheat rusts. Zeitschrift für Pflanzenzüchtung 83:350–367
Hayes HK, Ausemus ER, Stakman EC, Bailey CH, Wilson HK, Bamberg RH, Morkley MC, Crim RF, Levine MN (1936) Thatcher wheat. Stn Bull Minn Agric Exp Stn 325:1–36
Herrera-Foessel S, Singh R, Lillemo M, Huerta-Espino J, Bhavani S, Singh S, Lan C, Calvo-Salazar V, Lagudah E (2014) Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor Appl Genet 127(4):781–789. doi:10.1007/s00122-013-2256-9
Hiebert C, Fetch T Jr, Zegeye T (2010) Genetics and mapping of stem rust resistance to Ug99 in the wheat cultivar Webster. Theor Appl Genet 121(1):65–69. doi:10.1007/s00122-010-1291-z
Hodson DP, Nazari K, Park RF, Hansen J, Lassen P, Arista J, Fetch T, Hovmøller M, Jin Y, Pretorius ZA, Sonder K (2011) Putting Ug99 on the map: an update on current and future monitoring. In: McIntosh RA (ed) Borlaug Global Rust Initiative 2011 technical workshop. Borlaug Global Rust Initiative, St Paul, pp 3–13
Jin Y, Singh RP, Ward RW, Wanyera R, Kinyua M, Njau P, Fetch T, Pretorius ZA, Yahyaoui A (2007) Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f. sp. tritici. Plant Dis 91(9):1096–1099. doi:10.1094/PDIS-91-9-1096
Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, Fetch T (2008) Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis 92(6):923–926. doi:10.1094/PDIS-92-6-0923
Jin Y, Szabo LJ, Rouse MN, Fetch T, Pretorius ZA, Wanyera R, Njau P (2009) Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f. sp. tritici. Plant Dis 93:367–370
Kaur J, Bansal U, Khanna R, Saini R, Bariana H (2009) Molecular mapping of stem rust resistance in HD2009/WL711 recombinant inbred line population. Int J Plant Breed 3:28–33
Kerber ER, Dyck PL (1990) Transfer to hexaploid wheat of linked genes for adult-plant leaf rust and seedling stem rust resistance from an amphiploid of Aegilops speltoides × Triticum monococcum. Genome 33(4):530–537. doi:10.1139/g90-079
Kidwell K, Osborn T (1992) Simple plant DNA isolation procedures. In: Beckmann JS, Osborn TC (eds) Plant genomes: methods for genetic and physical mapping. Springer, Netherlands, pp 1–13. doi:10.1007/978-94-011-2442-3_1
Kislev ME (1982) Stem rust of wheat 3300 years old found in Israel. Science 216(4549):993–994. doi:10.1126/science.216.4549.993
Klindworth DL, Niu Z, Chao S, Friesen TL, Jin Y, Faris JD, Cai X, Xu SS (2012) Introgression and characterization of a goatgrass gene for a high level of resistance to Ug99 stem rust in tetraploid wheat. G3: Genes|Genomes|Genetics 2(6):665–673. doi:10.1534/g3.112.002386
Knott DR (1968) The inheritance of resistance to stem rust races 56 and 15B-IL (Can.) in the wheat varieties Hope and H-44. Can J Genet Cytol 10(2):311–320. doi:10.1139/g68-043
Knott DR (1982) Multigenic inheritance of stem rust resistance in wheat. Crop Sci 22(2):393–399. doi:10.2135/cropsci1982.0011183X002200020045x
Knott DR (1989) The wheat rusts—breeding for resistance. Theor Appl Genet 12:1–201. doi:10.1007/978-3-642-83641-1
Knott DR (2001) The relationship between seedling and field resistance to two races of stem rust in Thatcher wheat. Can J Plant Sci 81(3):415–418. doi:10.4141/P00-027
Kolmer JA, Dyck PL, Roelfs AP (1991) An appraisal of stem and leaf rust resistance in North American hard red spring wheats and the probability of multiple mutations to virulence in populations of cereal rust fungi. Phytopathology 81:237–239
Kosambi DD (1943) The estimation of map distance from recombination values. Ann Eugenics 12(1):172–175. doi:10.1111/j.1469-1809.1943.tb02321.x
Lagudah ES, McFadden H, Singh RP, Huerta-Espino J, Bariana HS, Spielmeyer W (2006) Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. Theor Appl Genet 114(1):21–30. doi:10.1007/s00122-006-0406-z
Liu W, Rouse M, Friebe B, Jin Y, Gill B, Pumphrey M (2011) Discovery and molecular mapping of a new gene conferring resistance to stem rust, Sr53, derived from Aegilops geniculata and characterization of spontaneous translocation stocks with reduced alien chromatin. Chromosome Res 19(5):669–682. doi:10.1007/s10577-011-9226-3
Lorieux M (2012) MapDisto: fast and efficient computation of genetic linkage maps. Mol Breed 30(2):1231–1235. doi:10.1007/s11032-012-9706-y
Lu F, Lipka AE, Glaubitz J, Elshire R, Cherney JH, Casler MD, Buckler ES, Costich DE (2013) Switchgrass genomic diversity, ploidy, and evolution: novel insights from a network-based SNP discovery protocol. PLoS Genet 9(1):e1003215. doi:10.1371/journal.pgen.1003215
Maccaferri M, Zhang J, Bulli P, Abate Z, Chao S, Cantu D, Bossolini E, Chen X, Pumphrey M, Dubcovsky J (2015) A genome-wide association study of resistance to stripe rust (Puccinia striiformis f. sp. tritici) in a worldwide collection of hexaploid spring wheat (Triticum aestivum L.). G3 Genes|Genomes|Genetics 5(3):449–465. doi:10.1534/g3.114.014563
Mago R, Lawrence G, Ellis J (2011) The application of DNA marker and doubled-haploid technology for stacking multiple stem rust resistance genes in wheat. Mol Breed 27(3):329–335. doi:10.1007/s11032-010-9434-0
McIntosh RA, Gyarfas J (1971) Triticum timopheevi as a source of resistance to wheat stem rust. Zeitschrift für Pflanzenzüchtung 66:240–248
McIntosh RA, Wellings CR, Park RF (1995) Wheat rusts: an atlas of resistance genes. CSIRO Publications, Victoria
McIntosh RA, Yamazaki Y, Devos KM, Dubcovsky J, Rogers WJ, Appels R (2003) Catalogue of gene symbols for wheat. In: Pogna NE, Romano M, Pogna EA, Galterio (eds) Proceedings of the 10th international wheat genetics symposium, 1–6 September, 2003, Paestum, Italy. Istituto Sperimentale per la Cerealicoltura, Rome, pp 1–34
Mundt CC (2014) Durable resistance: a key to sustainable management of pathogens and pests. Infect Genet Evol 27:446–455. doi:10.1016/j.meegid.2014.01.011
Nazareno NRX, Roelfs AP (1981) Adult plant resistance of Thatcher wheat to stem rust. Phytopathology 71:181–185
Niu Z, Klindworth DL, Friesen TL, Chao S, Jin Y, Cai X, Xu SS (2011) Targeted introgression of a wheat stem rust resistance gene by DNA marker-assisted chromosome engineering. Genetics 187:1011–1021. doi:10.1534/genetics.110.123588
Njau PN, Jin Y, Huerta-Espino J, Keller B, Singh RP (2010) Identification and evaluation of sources of resistance to stem rust race Ug99 in wheat. Plant Dis 94(4):413–419. doi:10.1094/PDIS-94-4-0413
Peterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity of leaves and stem of cereals. Can J Res 26c(5):496–500. doi:10.1139/cjr48c-033
Poland JA, Brown PJ, Sorrells ME, Jannink J-L (2012) Development of high-density genetic maps for barley and wheat using a aovel two-enzyme genotyping-by-sequencing approach. PLoS ONE 7(2):e32253. doi:10.1371/journal.pone.0032253
Pozniak CJ, Reimer S, Fetch T, Clarke JM, Clarke FR, Somers DJ, Knox RE, Singh AK (2008) Association mapping of Ug99 resistance in diverse durum wheat population. In: Rudi A, Russell E, Peter L, Michael M, Lynne M, Peter S (eds) Proceedings of the 11th international wheat genetics symposium, 24–29 August, 2008, Brisbane, Australia. Sydney University Press, Sydney, pp 809–811
Pretorius ZA, Singh RP, Wagoire WW, Payne TS (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis 84:203
Pretorius ZA, Szabo LJ, Boshoff WHP, Herselman L, Visser B (2012) First report of a new TTKSF race of wheat stem rust (Puccinia graminis f. sp. tritici) in South Africa and Zimbabwe. Plant Dis 96(4):590. doi:10.1094/PDIS-12-11-1027-PDN
Prins R, Pretorius ZA, Bender CM, Lehmensiek A (2011) QTL mapping of stripe, leaf and stem rust resistance genes in a Kariega × Avocet S doubled haploid wheat population. Mol Breed 27(2):259–270. doi:10.1007/s11032-010-9428-y
Risk JM, Selter LL, Chauhan H, Krattinger SG, Kumlehn J, Hensel G, Viccars LA, Richardson TM, Buesing G, Troller A, Lagudah ES, Keller B (2013) The wheat Lr34 gene provides resistance against multiple fungal pathogens in barley. Plant Biotechnol J 11(7):847–854. doi:10.1111/pbi.12077
Roelfs AP (1985) Wheat and rye stem rust. In: Roelfs AP, Bushnell WR (eds) The cereal rusts, vol 2. Academic Press, Orlando, pp 3–37
Roelfs AP, Singh RP, Saari EE (1992) Rust diseases of wheat: concepts and methods of disease management. CIMMYT, Mexico
Rouse MN, Jin Y (2011) Stem rust resistance in A-genome diploid relatives of wheat. Plant Dis 95:941–944
Rouse M, Nava I, Chao S, Anderson J, Jin Y (2012) Identification of markers linked to the race Ug99 effective stem rust resistance gene Sr28 in wheat (Triticum aestivum L.). Theor Appl Genet 125(5):877–885. doi:10.1007/s00122-012-1879-6
Rouse MN, Nirmala J, Jin Y, Chao S, Fetch TG Jr, Pretorius ZA, Hiebert CW (2014a) Characterization of Sr9h, a wheat stem rust resistance allele effective to Ug99. Theor Appl Genet 127(8):1681–1688. doi:10.1007/s00122-014-2330-y
Rouse MN, Talbert LE, Singh D, Sherman JD (2014b) Complementary epistasis involving Sr12 explains adult plant resistance to stem rust in Thatcher wheat (Triticum aestivum L.). Theor Appl Genet 127(7):1549–1559. doi:10.1007/s00122-014-2319-6
Singh RP (2012) Pros and cons of utilizing major, race-specific resistance genes versus partial resistance in breeding rust resistant wheat. In: McIntosh RA (ed) Borlaug Global Rust Initiative 2012 technical workshop. International Maize and Wheat Improvement Center (CIMMYT), Beijing, pp 57–65
Singh RP, Huerta EJ, Manilal HM (2005) Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. Turk J Agric For 29:121–127
Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Njau P, Wanyera R, Herrera-Foessel SA, Ward RW (2008) Will stem rust destroy the world’s wheat crop? In: Donald LS (ed) Advances in agronomy, vol 98. Academic Press, pp 271–309. doi:10.1016/S0065-2113(08)00205-8
Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Bhavani S, Njau P, Herrera-Foessel S, Singh PK, Singh S, Govindan V (2011) The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu Rev Phytopathol 49(1):465–481. doi:10.1146/annurev-phyto-072910-095423
Singh A, Knox RE, DePauw RM, Singh AK, Cuthbert RD, Campbell HL, Singh D, Bhavani S, Fetch T, Clarke F (2013a) Identification and mapping in spring wheat of genetic factors controlling stem rust resistance and the study of their epistatic interactions across multiple environments. Theor Appl Genet 126(8):1951–1964. doi:10.1007/s00122-013-2109-6
Singh A, Pandey MP, Singh AK, Knox RE, Ammar K, Clarke JM, Clarke FR, Singh RP, Pozniak CJ, DePauw RM, McCallum BD, Cuthbert RD, Randhawa HS, Fetch TG Jr (2013b) Identification and mapping of leaf, stem and stripe rust resistance quantitative trait loci and their interactions in durum wheat. Mol Breed 31(2):405–418. doi:10.1007/s11032-012-9798-4
Singh RP, Herrera-Foessel SA, Huerta-Espino J, Lan CX, Basnet BR, Bhavani S, Lagudah ES (2013c) Pleiotropic gene Lr46/Yr29/Pm39/Ltn2 confers slow rusting, adult plant resistance to wheat stem rust fungus. In: Borlaug Global Rust Initiative 2013 technical workshop. New Delhi, India, p 17
Stakman EC, Stewart DM, Loegering WQ (1962) Identification of physiologic races of Puccinia graminis var. tritici. US Dep Agric Agric Res Serv E 6/7
Stubbs RW, Prescott JM, Saari EE, Dubin HJ (1986) Cereal disease methodology manual. CIMMYT, Mexico
Stuthman DD, Leonard KJ, Miller‐Garvin J (2007) Breeding crops for durable resistance to disease. In: Donald LS (ed) Advances in agronomy, vol 95. Academic Press, pp 319–367. doi:10.1016/S0065-2113(07)95004-X
Suenaga K, Singh RP, Huerta-Espino J, William HM (2003) Microsatellite markers for genes Lr34/Yr18 and other quantitative trait Loci for leaf rust and stripe rust resistance in bread wheat. Phytopathology 93(7):881–890. doi:10.1094/phyto.2003.93.7.881
Tsilo TJ, Jin Y, Anderson JA (2008) Diagnostic microsatellite markers for the detection of stem rust resistance gene Sr36 in diverse genetic backgrounds of wheat. Crop Sci 48(1):253–261. doi:10.2135/cropsci2007.04.0204
Tsilo TJ, Kolmer JA, Anderson JA (2014) Molecular mapping and improvement of leaf rust resistance in wheat breeding lines. Phytopathology 104(8):865–870. doi:10.1094/PHYTO-10-13-0276-R
Wang S, Basten CJ, Zeng Z-B (2012) Windows QTL cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC
Wanyera R, Kinyua MG, Jin Y, Singh RP (2006) The spread of stem rust caused by Puccinia graminis f. sp. tritici, with virulence on Sr31 in wheat in Eastern Africa. Plant Dis 90(1):113. doi:10.1094/PD-90-0113A
William M, Singh RP, Huerta-Espino J, Islas SO, Hoisington D (2003) Molecular marker mapping of leaf rust resistance gene Lr46 and its association with stripe rust resistance gene Yr29 in wheat. Phytopathology 93(2):153–159. doi:10.1094/phyto.2003.93.2.153
Wu S, Pumphrey M, Bai G (2009) Molecular mapping of stem-rust-resistance gene Sr40 in wheat. Crop Sci 49(5):1681–1686. doi:10.2135/cropsci2008.11.0666
Yu L-X, Lorenz A, Rutkoski J, Singh R, Bhavani S, Huerta-Espino J, Sorrells M (2011) Association mapping and gene–gene interaction for stem rust resistance in CIMMYT spring wheat germplasm. Theor Appl Genet 123(8):1257–1268. doi:10.1007/s00122-011-1664-y
Yu L-X, Morgounov A, Wanyera R, Keser M, Singh S, Sorrells M (2012) Identification of Ug99 stem rust resistance loci in winter wheat germplasm using genome-wide association analysis. Theor Appl Genet 125(4):749–758. doi:10.1007/s00122-012-1867-x
Yu L-X, Barbier H, Rouse M, Singh S, Singh R, Bhavani S, Huerta-Espino J, Sorrells M (2014) A consensus map for Ug99 stem rust resistance loci in wheat. Theor Appl Genet 127(7):1561–1581. doi:10.1007/s00122-014-2326-7
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14(6):415–421. doi:10.1111/j.1365-3180.1974.tb01084.x
Acknowledgments
We thank the University of Minnesota Genomics Center, University of Minnesota Supercomputing Institute, the Microbial & Plant Genomics Institute, the University of Minnesota Graduate School, Kenya Agriculture and Livestock Research Organization, Ethiopia Institute of Agricultural Research, USDA-ARS Cereal Disease Laboratory personnel, and Anderson Wheat Lab for their help and support during various phases of the project. Funding for this work was provided by the United States Department of Agriculture, Agriculture and Food Research Initiative and 2011-68002-30029 (Triticeae Coordinated Agricultural Project, www.triticeaecap.org), the Borlaug Global Rust Initiative Durable Rust Resistance in Wheat Project (administered by Cornell University with a grant from the Bill & Melinda Gates Foundation), and the UK Department for International Development.
Author contribution
P.B. supervised planting of the RIL population in St. Paul, MN; recorded the phenotype of the RIL population in St. Paul, MN; carried out genotyping of the plant materials and data analysis; and drafted the manuscript. M.N.R. recorded the phenotype in East African nurseries and assisted in preparing the manuscript. S.B. supervised planting of and disease inoculation on the population in East African rust nurseries. J.A.A. conceived the study, developed the population, supervised the project, and assisted in preparing the manuscript. All authors contributed to and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Human participants and/or animals statement
Not applicable.
Informed consent
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bajgain, P., Rouse, M.N., Bhavani, S. et al. QTL mapping of adult plant resistance to Ug99 stem rust in the spring wheat population RB07/MN06113-8. Mol Breeding 35, 170 (2015). https://doi.org/10.1007/s11032-015-0362-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0362-x