Abstract
Two wheat–Thinopyrum substitution lines X479 and X482 selected from the progenies of wheat “Mianyang26 (MY26)” × wheat–Thinopyrum intermedium ssp. trichophorum partial amphiploid were characterized by seed storage protein electrophoresis, genomic in situ hybridization (GISH), fluorescence in situ hybridization (FISH), and PCR-based molecular markers. Seed storage protein analysis showed that X479 expressed some of Th. intermedium ssp. trichophorum-specific gliadin and glutenin bands. Chromosome counting and GISH probed by Pseudoroegneria spicata genomic DNA indicated that two pairs of Thinopyrum-derived chromosomes (St genome and St–JS translocated chromosomes) substituted for two pairs of wheat chromosomes in both X479 and X482. FISH using pAs1 and pHvG38 as probes showed that chromosomes 1B and 4B, and 4D and 6D were absent in X479 and X482, respectively. Using the newly isolated JS chromosome-specific repetitive sequence pDb12H as a probe, the FISH signals revealed that the translocation of St–JS chromosomes in X479 and X482 occurred in repetitive sequence regions of the short arm. The molecular markers based on wheat–rice colinearity confirmed that the chromosome constitutions of X479 and X482 were 1St (1B) + 4St–4JS (4B) and 4St–JS (4D) + 6St (6D), respectively. The substitution lines were both fully fertile which suggests that the Th. intermedium chromosomes in X479 and X482 substitute well for the corresponding wheat chromosomes. The rust resistance and novel agronomic traits revealed that the substitution lines will be potentially useful for genetic improvement of wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a segmental autoallohexaploid wheatgrass, Thinopyrum intermedium consists of two closely related, partially homeologous, genomes and one distinctly diverse genome (Dewey 1984). Efforts to reveal the genomic composition of Th. intermedium have been underway for decades and various hypotheses have been proposed (Chen et al. 1998; Mahelka et al. 2011, 2013). The studies indicated that three distinguishable chromosome sets of Th. intermedium were recently designated J, JS, and St genomes. The J genome was related to both Th. elongatum and Th. bessarabicum; however, the JS genome referred to a modified Th. elongatum/Th. bessarabicum genome (Chen et al. 1998; Chen 2005). Since Th. intermedium has wide range of adaptation to soil and climate, it is endemic to Central and Southeastern Europe through to Turkey and can also now be found growing wild throughout the western half of the North American continent. This results in the vast genetic diversity in the species of Th. intermedium (Wagoner and Schauer 1990). Th. intermedium subspecies has been hybridized extensively with wheat and has proved to be a valuable resource for improving wheat disease resistance and yield potential (Li and Wang 2009). Numerous wheat–Thinopyrum chromosome addition and substitution lines, as well as partial amphiploids, have been developed, which offer solid genetic resources for determining genetic control of novel genes in the Th. intermedium genomes (Chen 2005; Li and Wang 2009). However, extensive genetic diversity exists in the different Th. intermedium subspecies, and chromosomal rearrangements have occurred frequently in Th. intermedium genomes (Friebe et al. 1992; Xu and Conner 1994; Mahelka et al. 2011). Therefore, it is worthwhile continuously introducing Th. intermedium individual chromosomes representing the genetic variation and the novel traits to different wheat background.
Thinopyrum intermedium ssp. trichophorum, a pubescent subspecies of wheatgrass (Dewey 1984), displayed more chromosomal heterochromatic bands than chromosomes of Th. intermedium ssp. intermedium (Xu and Conner 1994) and also appeared to carry novel resistances to several foliar diseases and unique seed storage proteins which may be valuable for wheat improvement (Wills et al. 1998). With the ultimate aim to introduce novel, agronomically important genes from Th. intermedium ssp. trichophorum to wheat, we developed a wheat–Th. intermedium ssp. trichophorum partial amphiploid (Yang et al. 2006). The second step for the introgression alien chromosome segments into wheat is to produce addition and/or substitution lines. This was performed by crossing the wheat–Thinopyrum partial amphiploid to wheat and reported earlier (Hu et al. 2011; Li et al. 2013a, b; Song et al. 2013).
Here, we report the production and precise characterization of two new, double-disomic Th. intermedium chromosome substitution lines for the purpose of (1) analyzing the ability of the Thinopyrum-genome chromosomes for compensating the loss of homeologous B- and D-genome chromosomes, (2) mapping of agronomically important genes to a Th. intermedium chromosome transferred to wheat, and (3) development of PCR-based markers for identifying the introduced Th. intermedium chromosomes.
Materials and methods
Plant materials
Thinopyrum intermedium ssp. trichophorum accession PI440125 and Pseudoroegneria spicata (St genome, 2n = 2x = 14) accession PI 232131 were obtained from the USDA National Small Grains Collection at Aberdeen, Idaho. The wheat–Th. intermedium ssp. trichophorum partial amphiploid, TE-3 (Yang et al. 2006), and wheat line Mianyang 26 (MY26) are maintained at the Triticeae Research Institute, Sichuan Agricultural University, China. Lines X479 and X482 were developed from the progenies of the cross between TE-3 and MY26, and they are deposited at Xindu Experimental Station of University of Electronic Science and Technology of China.
Sequential C-banding and GISH
Seedling root tips were collected, pretreated in water at 0 °C for 24 h, and fixed in ethanol–acetic acid (3:1) for 1 week. Root-tip squashes were stained using the conventional Feulgen method for chromosome counting according to Gill et al. (1991). For GISH analysis, total genomic DNA from Ps. spicata was labeled with digoxigenin-11-dUTP by nick translation following the manufacturer’s instructions (Roche Diagnostics, Indianapolis, IN). Sheared genomic DNA of Chinese Spring wheat (CS, genomes ABD, 2n = 42) was used as blocking DNA. The hybridization mixture was prepared as described by (Mukai et al. 1993). The GISH signal was detected with fluorescein-conjugated antidigoxigenin antibody (Roche Diagnostics, Indianapolis, IN), and the slide was mounted in propidium iodide dissolved in Vectashield® antifade solution (Vector Laboratories, Burlingame, CA). Microphotographs of C-banded and GISH chromosomes were taken with an Olympus BX-51 microscope using a DP-70 CCD camera.
Fluorescence in situ hybridization (FISH)
The probes pAs1, containing a 1-kb DNA fragment isolated from Aegilops tauschii in the plasmid pUC8 (Rayburn and Gill 1986), and pHvG38 (Pedersen and Langridge 1997), with GAA repeats were used to identify the B-genome of wheat, were generously provided by Dr. B. Friebe, Wheat Genetic and Genomic Resources Centre, Department of Plant Pathology, Kansas State University, USA. An LTR probe pDbH12 was used to distinguish the JS genome of Th. intermedium as reported by Liu et al. (2009). The probe labeling, hybridization, and detection for FISH were the same as in the GISH protocol.
Seed storage protein electrophoresis
Acid polyacrylamide gel electrophoresis (APAGE) and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) were used to separate endosperm gliadin proteins and glutenin subunits, respectively. The procedures were described by Yang et al. (2001).
Disease resistance screening
Wheat- and Thinopyrum-derived lines X479, X482, and their parents were evaluated in the field during 2010–2013 at Xindu Experimental Station, Sichuan Academy for Agricultural Sciences. The adult-plant resistance to P. striiformis f. sp. tritici strains CYR31, CYR32, and CYR33 were provided by the Plant Protection Institute, Sichuan Academy of Agricultural Sciences. A subset of 20 plants was selected for these assessments, and their represented parents were also tested. Infection types were evaluated 2–3 weeks after inoculation when uredinia were fully developed. Stripe rust responses were recorded following Ma et al. (1995).
Molecular marker analysis
DNA was extracted from fresh leaves of X479, X482, TE-3, and CS. PCR-based Landmark Unique Gene (PLUG) primers were according to Ishikawa et al. (2009). Polymerase chain reaction (PCR) was performed in an Icycler thermal cycler (Bio-RAD Laboratories, Emeryville, CA) in reaction volumes 25 μl, containing 10 mmol Tris–HCl (pH 8.3), 2.5 mmol MgCl2, 200 μmol of each dNTP, 100 ng template DNA, 0.2 U Taq polymerase (Takara, Japan) and 400 nmol primer. The cycling parameters were 94 °C for 3 min for denaturing; followed by 35 cycles at 94 °C for 1 min, 55 °C for 1 min, 72 °C for 2 min, and then, a final extension at 72 °C for 10 min. The amplified products were cloned and sequenced.
Results
Spike and seeds morphology of X479 and X482
Among BC1F5 progenies from the cross between CS-Th. intermedium ssp. trichophorum partial amphiploid TE-3 and wheat line MY26, each four plants of lines X479 and X482 were selected. As shown in Fig. 1, the spikes morphology of X479 and X482 displayed similar agronomic traits and high seed set to the wheat parent MY26. The lines X479 and X482 had 16–20 spikelets per spike, with shorter spikes than either MY26 or TE-3. The seeds of X479 were relatively thinner and longer than MY26, while the grains of X482 were larger and had a higher 1,000-kernel weight than MY26 (Supplementary Table 1). X479 carried a trait of pubescence on the glumes of spikes, which originated from Th. intermedium ssp. trichophorum (Yang et al. 2006).
Seed storage protein analysis
The endosperm storage proteins have been considered as useful genetic markers and also utilized on cultivar identification. APAGE produced distinctive bands in the ω, γ, β, and α zones of seed gliadin storage proteins from seeds of CS, TE-3, X479, and X482 plants (Fig. 2a). X479 and X482 displayed clearly different gliadin bands patterns. Within X479 and X482 lines, different seeds produced identical band patterns, indicating their genetic homogeneity at the gliadin loci. The line X479 produced two strong bands in the ω-gliadin zones (arrowed), which are identical to those in the partial amphiploid TE-3. Based on our previous study, the ω-gliadin bands represented the Th. intermedium ssp. trichophorum-specific bands (Yang et al. 2006).
The high molecular weight glutenin subunit (HMW-GS) composition of X479 and X482 and their parents TE-3 and MY26 were analyzed by SDS–PAGE (Fig. 2b, c). The HMW-GS in TE-3 included Glu-A1 null, Glu-B1 subunits 7 + 8, and Glu-D1 subunits 2 + 12. In MY26, Glu-A1 subunit 1, Glu-B1 subunits 7 + 9, and Glu-D1 subunits 2 + 12 were present. Line X482 showed the HMW-GS of Glu-B1 subunits 7 + 9 and Glu-D1 subunits 2 + 12. Line X479 had the Glu-D1 2 + 12 subunits, but there were no bands for the Glu-B1 locus, which is on the long arm of chromosome 1B. It is clear that the Th. intermedium group 1 chromosome in X479 contained the Thinopyrum-specific HMW-GS and ω-gliadin genes.
GISH and FISH
Root-tip chromosome counts and meiotic observation of PMCs were conducted on 20 plants of lines X479 and X482. We found that all plants had 42 chromosomes and 21 bivalents at meiotic MI, indicating cytological stability of X479 and X482 (Supplementary Fig. 1).
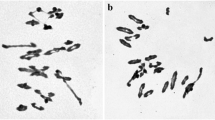
GISH, using total genomic DNA from Ps. spicata (St genome) as a probe on mitotic metaphase chromosomes of X479 and X482, was used to determine the introgression of segments of the Th. intermedium chromosomes. The St genome-based GISH procedure can distinguish the individual J, JS, and St chromosomes in Th. intermedium (Chen et al. 1998). As indicated in Fig. 3a, e, GISH results show that both X479 and X482 contained two pairs of strongly hybridized chromosomes, of which one pair of chromosomes had signals uniformly along the entire chromosome lengths, indicating that they belonged to Th. intermedium St chromosomes. Another pair of chromosomes showed strong GISH signals around the centromeric regions and weaker signals at the telomeric regions, indicating that this pair of chromosomes belonged to the JS genome, with half St genome fragment translocated in the short arms. Therefore, it can be concluded that both lines X479 and X482 contained two St chromosomes and two St–JS chromosomes.
In order to determine the constitution of wheat chromosomes in X479 and X482, a total of twenty mitotic metaphase chromosomes in X479 and X482 were hybridized by with probes pAs1 and pHvG38 (Fig. 3), respectively. The presence of wheat D-genome chromosomes was demonstrated using probe pAs1 (Rayburn and Gill 1986), while the pHvG38 can be easily detected for the presence of B-genome chromosomes (Pedersen and Langridge 1997). The FISH signals by pAs1 revealed that X479 contained the complete D-genome (Fig. 3b), while X482 had ten D-genome chromosomes and was missing chromosomes 4D and 6D (Fig. 3f). The FISH signals by pHvG38 indicated that chromosomes 1B and 4B were absent in X479 (Fig. 3c), while X482 contained the complete B-genome (Fig. 3g).
FISH using pDb12H as a probe assisted in the identification of the St–JS chromosomes in X479 and X482 (Fig. 3d, h). A pair of chromosomes in both X479 and X482 displayed identical hybridization signals. The signals covered the most regions of long arm except the centromeric and telomeric regions, and the limited regions in the middle of short arm. Based on the chromosomes distributions of sequential St genome GISH and pDb12H FISH signals outlined by Liu et al. (2009), we considered that the translocation of St–JS chromosomes in X479 and X482 appeared in the intercalary breakpoint at the short arm of JS chromosome (Fig. 4).
Molecular markers
The PLUG primers were designed based on rice syntenic region, and presumably amplify fragments corresponding to the similar linkage group(s) of wheat genomes (Ishikawa et al. 2009). Our previous studies showed that the PLUG markers were useful for producing Thinopyrum chromosome-specific markers (Hu et al. 2012). In total, 145 PLUG markers were tested, including X479, X482, and their parents MY26 and TE-3. Six PLUG markers from homeologous group 1, 6 markers from group 4 and 3 markers from group 6 clearly produced Th. intermedium-specific bands in X479 and X482 (Table 1). By using the Th. intermedium and Ps. spicata (St genome) as control, the results of PLUG primers amplification showed that the Th. intermedium chromosomes in line X479 belong to groups 1 and 4, while those of line X482 belong to groups 4 and 6 (Fig. 5).
PCR amplification using PLUG primers TNAC1026 (a), TNAC1412 (b, c), and TNAC1743 (d). The arrows indicate the X479- or X482-specific bands identical to that of Th. intermedium-derived bands, star indicates that the wheat bands absent in X479 or X482. Thi and St refer Th. intermedium and Ps. spicita, respectively
Chinese Spring nulli-tetrasomic lines were also used to identify the target wheat bands. The amplification of primer TNAC1026 showed the absence of the 1B bands in X479 (Fig. 5a). Similarly, for TNAC1412 (Fig. 5b, c), 4B-specific bands were absent in X479, and 4D bands absent in X482. The amplification of TNAC1743 showed that X482 was missing 6D-specific bands (Fig. 5d). Therefore, we concluded that X479 was a double-disomic 1St (1B), 4St–Js (4B) substitution line, while X482 was a double-disomic 4St–Js (4D), 6St (6D) substitution line.
Rust resistances
Lines X479 and X482 and the parental lines TE-3 and MY26 were inoculated with P. striiformis f. sp. tritici races CYR31, CYR32, and CYR33 at the adult plant stage. TE-3 was immune to these isolates, whereas wheat parent MY26 was highly susceptible. X479 was highly resistant to stripe rust, while X482 was susceptible (Fig. 1c). These results indicated that the stripe rust resistance in X479 was from the chromosome 1St to Th. intermedium ssp. trichophorum, while the 4St–JS and 6St may not contain the stripe rust resistance gene(s), or the resistance is not expressed.
Discussion
Thinopyrum intermedium consists of three distinguishable chromosome sets which have been designated as the J, JS, and St genomes (Chen et al. 1998; Chen 2005). Molecular and cytogenetic evidence has also revealed that remarkable genetic diversity and genomic structural modifications exist across inter-population and intra-population germplasm accessions of Th. intermedium (Mahelka et al. 2011, 2013). The study of the variation of agronomical traits is an essential starting point for further utilization of the Th. intermedium novel gene(s) for wheat improvement (Li and Wang 2009). In the present study, the Th. intermedium ssp. trichophorum accession, which displayed a distinctly different chromosomal heterochromatin constitution from the related Th. intermedium ssp. Intermedium, was used to as donor to produce wheat–Th. intermedium substitution lines (Xu and Conner 1994; Yang et al. 2006). The incorporation of Th. intermedium chromosomes to wheat enables the effect of individual Thinopyrum chromosomes to be determined and clarifies inter-chromosomal homeologies (Hu et al. 2011; Li et al. 2013a, b).
In the present study, we obtained fertile progeny from wheat–Th. intermedium ssp. trichophorum double-disomic substitution lines X479 and X482. Based on GISH patterns and C-banding analysis, the Th. intermedium chromosomes 1St and 4JS–St substituted wheat chromosomes 1B and 4B in X479, while chromosomes 4JS–St and 6St substituted wheat chromosomes 4D and 6D in X482. We previous identified a 1St (1D) substitution line (Hu et al. 2011). The results suggested that the groups 1, 4, and 6 Th. intermedium chromosomes can be easy to compensate for the loss of corresponding homeologous chromosomes of wheat B- and D-genomes. Wheat-alien chromosome substitution lines are not only of great theoretical interest for elucidating evolutionary relationships, but also of immense practical interest for introducing the rich genetic diversity of donor species for crop improvement (Jiang et al. 1994). In the present study, the C-banding and GISH pattern of chromosome 1St in X479 is identical to our previously identified chromosome 1St#2 in wheat–Th. intermedium ssp. trichophorum substitution line AS1677 (Hu et al. 2011). The chromosome 1St#2 carried a novel stripe rust resistance gene(s), which when transferred to line X479, conferred resistance to that serious cereal disease. Li et al. (2013a, b) located a Th. intermedium-specific HMW-GS, encoded by gene Glu-1St#2x, on chromosome 1St#2, which is identical to the additional HMW-GS band scored in X479. However, we also found the Thinopyrum-specific ω-gliadin in X479, indicating that chromosome 1St in X479 contained the novel Th. intermedium-specific Gli-1 loci. Meanwhile, the trait for Th. intermedium ssp. Trichophorum-specific pubescence on the glumes was also observed in X479, suggesting that the gene(s) were located on chromosome 1St of X479.
Recently, Han et al. (2014) reported that four different types of chromosome 6P of Agropyron cristatum possessed different desirable genes in wheat–Ag. cristatum disomic addition lines. Similar diversity of the 1St chromosomes from different wheat–Th. intermedium ssp. trichophorum substitution lines may exist across germplasm stocks Friebe et al. (1992) identified a wheat–Th. intermedium disomic addition lines L7 (6Ai) originated from TAF 46 (Cauderon et al. 1973), and Chen et al. (1999) further assigned the Th. intermedium chromosome in L7 as 6St. The C-banding pattern of the chromosome 6St in X482 appeared clearly different from that the chromosome 6St in L7. We thus have named the group 6 T. intermedium-derived chromosome in X482 as “6St#2.” We found X482 showed higher grain weight than X479 and wheat parent MY26. It is likely that the chromosome 6St#2 in X482 may confer the important trait of high grain weight useful for wheat breeding.
Due to the highly polyploid nature of the Th. intermedium species, the inter- and intra-genomic chromosomal rearrangements also occur commonly in the wheat–Th. intermedium partial amphiploids (Chen et al. 1999; Yang et al. 2006; Zeng et al. 2013). The Th. intermedium genomic compositions involved different St–JS genome chromosomes as either Robertsonian, intercalary, or terminal interchanges were found in several partial amphiploids and addition lines (Chen 2005). Tang et al. (2000) identified Th. intermedium St–JS translocated chromosomes in addition lines Z1, Z2, and Z6, which belong to homeologous group 2. In the present study, the GISH–FISH patterns and molecular markers revealed that St–JS chromosomes in X479 and X482 belonged to homeologous group 4, which differs from the previously reported St–JS chromosomes in wheat–Th. intermedium addition or substitutions (Chen 2005; Li and Wang 2009). Li et al. (2005) reported that Th. intermedium JS chromosomes of homeologous group 4 conferring novel genes for resistance to wheat streak mosaic, wheat curl mite, and eyespot in wheat background. Further disease screening of the 4St–JS chromosomes in X479 and X482 may discover additional resistance genes. Production of a complete set of Th. intermedium substitution and addition lines in a common wheat background will facilitate the study of genomic structures of the introgression of Thinopyrum chromosomes. It is possible to reduce the size of alien translocations by inducing recombination between the Thinopyrum chromosomes and the corresponding related wheat chromosome(s) after crossing the substitution lines X479 and X482 to wheat using the approaches of marker-assisted chromosome engineering (Niu et al. 2014).
The molecular markers based on comparative genome analysis provided a simple and precise method to target the alien species in a wheat background. The PLUG markers can be useful for alien chromatin identification and assignment of their corresponding linkage groups (Hu et al. 2011; Song et al. 2013; Li et al. 2013a, b). In the present study, the group 1 and group 4 PLUG markers detected polymorphic fragments specific to the Th. intermedium chromosome in X479, and the group 4 and group 6 PLUG markers detected for X482 (Fig. 5). The PCR amplification of Th. intermedium-specific bands in group 4 of X479 and X482 was identified that the part of short arm was derived from the St genome and the long arm related to JS genome. These St–JS chromosomes were not inherited from their parents TE-3 (Yang et al. 2006). Therefore, St–JS translocation occurred during the processes of cross between wheat and TE-3. Meanwhile, the St–JS chromosomes can be clearly discriminated by C-banding and FISH by multiple probes such as pDb12H, pHvG38, and pAs1, as well as GISH with St genomic DNA (Fig. 4). From observations of chromosomes after FISH using pDb12H as the probe, it is apparent that the translocation between the JS and St chromosomes occurred at the short arm. The phenomenon was also commonly observed in the Aegilops chromosomes translocation among the species, reported by Molnár et al. (2011). It is suggested that the inter-genomic translocation breakpoints are frequently mapped to repetitive sequences rich chromosomal regions in the allopolyploid species.
References
Cauderon Y, Saigne B, Dauge M (1973) The resistance to wheat rusts of Agropyron intermedium and its use in wheat improvement. In: Sears ER, Sears LMS (eds) Proc 4th Int Wheat Genet Symp, University of Missouri, Columbia, pp 401–407
Chen Q (2005) Detection of alien chromatin introgression from Thinopyrum into wheat using S genomic DNA as a probe: a landmark approach for Thinopyrum genome research. Cytogenet Genome Res 109:350–359
Chen Q, Conner RL, Laroche A, Thomas JB (1998) Genome analysis of Thinopyrum intermedium and Th. ponticum using genomic in situ hybridization. Genome 141:580–586
Chen Q, Conner RL, Laroche A, Ji W, Armstrong KC, Fedak G (1999) Genomic in situ hybridization analysis of Thinopyrum chromatin in a wheat–Th. intermedium partial amphiploid and six derived chromosome addition lines. Genome 42:1217–1223
Dewey DR (1984) The genomic system of classification as a guide to intergeneric hybridization with the perennial Triticeae. In: Gustafson JP (ed) Gene manipulation in plant improvement, vol 16. Plenum Press, New York, pp 209–279
Friebe B, Mukai Y, Gill BS, Cauderon Y (1992) C-banding and in situ hybridization analyses of Agropyron intermedium, a partial wheat -Ag. intermedium amphiploid, and six derived chromosome addition lines. Theor Appl Genet 84:899–905
Gill BS, Friebe B, Endo TR (1991) Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome 34:830–839
Han H, Bai L, Su J, Zhang J, Song L, Gao A, Yang X, Li X, Liu W, Li L (2014) Genetic rearrangements of six wheat–Agropyron cristatum 6P addition lines revealed by molecular markers. PLoS ONE 9:e91066
Hu LJ, Li GR, Zeng ZX, Chang ZJ, Liu C, Zhou JP, Yang ZJ (2011) Molecular cytogenetic identification of a new wheat–Thinopyrum substitution line with stripe rust resistance. Euphytica 177:169–177
Hu LJ, Li GR, Zhan HX, Liu C, Yang ZJ (2012) New St-chromosome specific molecular markers for identifying wheat–Thinopyrum intermedium derivative lines. J Genet 91:e69–e74
Ishikawa G, Nakamura T, Ashida T, Saito M, Nasuda S, Endo TR, Wu J, Matsumoto T (2009) Localization of anchor loci representing five hundred annotated rice genes to wheat chromosomes using PLUG markers. Theor Appl Genet 118:499–514
Jiang J, Friebe B, Gill BS (1994) Recent advances in alien gene transfer in wheat. Euphytica 73:199–212
Li H, Wang X (2009) Thinopyrum ponticum and the promising source of resistance to fungal and viral diseases of wheat. J Genet Genomics 36:557–565
Li HJ, Arterburn M, Jones SS, Murray TD (2005) Resistance to eyespot of wheat, caused by Tapesia yallundae, derived from Thinopyrum intermedium homoeologous group 4 chromosomes. Theor Appl Genet 111:932–940
Li GR, Liu C, Li CH, Zhao JM, Zhou L, Dai G, Yang EN, Yang ZJ (2013a) Introgression of a novel Thinopyrum intermedium St-chromosome-specific HMW-GS gene into wheat. Mol Breed 31:843–853
Li J, Endo TR, Saito M, Ishikawa G, Nakamura T, Nasuda S (2013b) Homoeologous relationship of rye chromosome arms as detected with wheat PLUG markers. Chromosoma 122:555–564
Liu C, Yang ZJ, Jia JQ, Li GR, Zhou JP, Ren ZL (2009) Genomic distribution of a long terminal repeat (LTR) Sabrina-like retrotransposon in Triticeae species. Cereal Res Commun 37:363–372
Ma H, Singh RP, Mujeeb-Kazi A (1995) Suppression/expression of resistance to stripe rust in synthetic hexaploid wheat (Triticum turgidum × T. tauschii). Euphytica 83:87–93
Mahelka V, Kopecky D, Pastova L (2011) On the genome constitution and evolution of intermediate wheatgrass (Thinopyrum intermedium: Poaceae, Triticeae). BMC Evol Biol 11:127
Mahelka V, Kopecky D, Baum BR (2013) Contrasting patterns of evolution of 45S and 5S rDNA families uncover new aspects in the genome constitution of the agronomically important grass Thinopyrum intermedium (Triticeae). Mol Biol Evol 30:2065–2086
Molnár I, Cifuentes M, Schneider A, Benavente E, Molnár-Láng M (2011) Association between simple sequence repeat-rich chromosome regions and intergenomic translocation breakpoints in natural populations of allopolyploid wild wheats. Ann Bot 107:65–76
Mukai Y, Friebe B, Hatchett JH, Yamamoto M, Gill BS (1993) Molecular cytogenetic analysis of radiation-induced wheat rye terminal and intercalary chromosomal translocations and the detection of rye chromatin specifying resistance to Hessian fly. Chromosoma 102:88–95
Niu Z, Klindworth DL, Yu G, Friesen TL, Chao S, Jin Y, Cai X, Ohm JB, Rasmussen JB, Xu SS (2014) Development and characterization of wheat lines carrying stem rust resistance gene Sr43 derived from Thinopyrum ponticum. Theor Appl Genet 127:969–980
Pedersen C, Langridge P (1997) Identification of the entire chromosome complement of bread wheat by two-colour FISH. Genome 40:589–593
Rayburn AL, Gill BS (1986) Isolation of a D genome specific repeated DNA sequence from Aegilops squarrosa. Plant Mol Biol Rep 4:102–109
Song XJ, Li GR, Zhan HX, Liu C, Yang ZJ (2013) Molecular identification of a new wheat–Thinopyrum intermedium ssp. trichophorum addition line for resistance to stripe rust. Cereal Res Commun 41:211–220
Tang S, Li Z, Jia X, Larkin PJ (2000) Genomic in situ hybridization (GISH) analyses of Thinopyrum intermedium, its partial amphiploid Zhong 5, and disease-resistant derivatives in wheat. Theor Appl Genet 100:344–352
Wagoner P, Schauer A (1990) Intermediate wheatgrass as a perennial grain crop. In: Janick J, Simon JE (eds) Advances in new crops. Timber Press, Portland, pp 143–145
Wills BJ, Douglas GB, McKenzie J, Trainor KD, Foote AG (1998) Thinopyrum intermedium (Host) Barkw and Dewey—a review, and evaluation of intermediate and pubescent wheatgrass for dryland agriculture in New Zealand. Procee N Z Grassl Assoc 60:233–241
Xu J, Conner RL (1994) Intravarietal variation in satellites and C-banded chromosomes of Agropyron intermedium ssp. trichophorum cv. Greenleaf. Genome 37:305–310
Yang ZJ, Li GR, Jiang HR, Ren ZL (2001) Expression of nucleolus, endosperm storage proteins and disease resistance in an amphiploid between Aegilops tauschii and Secale silvestre. Euphytica 119:317–321
Yang ZJ, Li GR, Chang ZJ, Zhou JP, Ren ZL (2006) Characterization of a partial amphiploid between Triticum aestivum cv. Chinese Spring and Thinopyrum intermedium ssp. trichophorum. Euphytica 149:11–17
Zeng J, Cao W, Fedak G, Sun S, McCallum B, Fetch T, Xue A, Zhou Y (2013) Molecular cytological characterization of two novel durum-Thinopyrum intermedium partial amphiploids with resistance to leaf rust, stem rust and Fusarium head blight. Hereditas 150:10–16
Acknowledgments
We particularly thank Dr. I. Dundas at University of Adelaide, Australia, for reviewing the manuscript. We thank the National Natural Science Foundation of China (Nos. 31101143, 31171542, 31201203), and Sichuan Wheat Breeding Community for the financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, G., Lang, T., Dai, G. et al. Precise identification of two wheat–Thinopyrum intermedium substitutions reveals the compensation and rearrangement between wheat and Thinopyrum chromosomes. Mol Breeding 35, 1 (2015). https://doi.org/10.1007/s11032-015-0202-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-015-0202-z