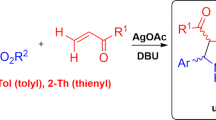
Abstract
The azomethine ylides are generally used in 1,3-dipolar cycloadditions with various dipolarophiles. In this work, a new and diverse route has been developed for the azomethine ylides, for synthesis of novel pyrrole derivatives. The azomethine ylide, produced via C–H activation of unreactive C(sp3)–H bond of 2-methylquinoline, by molecular iodine, in the presence of pyridine. Herein, we represent novel pyrrole derivatives, synthesized from the reaction of pyridinium ylide with olefins, which formed via a reaction of isatin, dialkyl acetylenedicarboxylate derivatives and pyridine as a base in moderate to excellent yields. Various features of this cyclization, discussed.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyrrole is one of the most important nitrogen-containing, five-membered heterocycles in organic chemistry [1]. This compound is the key structural fragment of numerous natural products such as heme, chlorophyll, bile pigments, vitamin B12 and various cytochrome enzymes [2]. Pyrrole and its derivatives demonstrate numeral biological activities such as antibacterial, antiviral, anti-inflammatory, antitumour and antioxidant [3]. These privileged structural motifs are not only wide speared in a vast variety of natural compounds, but also utilized as building blocks in a number of pharmaceuticals, including the blockbuster drug, atorvastatin, used as a cholesterol lowering agent, being the top selling medicine worldwide, the non-steroidal anti-inflammatory compound, Tolmetin and the anticancer drug candidate, Tallimustine [4].
Moreover, many researchers have been concentrated on the biological and pharmaceutical activities of fluorazone (9H-pyrrolo[1,2-a]indole-9-one) and its derivatives [5]. They show a wide range of biological activities, including anti-tubulin [6], psychostimulant [7] and antidiabetic [8]. In addition, novel chromophores exhibit the promising application of dyes having a fluorazone linker as a photosensitizer for solar cells [9]. Also, considerable interest pertaining to fluorazone, arises from the fact that, this fused tricyclic unit, represents the chemical precursor of 9H-pyrrolo[1,2-a]indole (fluorazene), the basic skeleton of cytostatic mitomycine, which serves as cancer chemotherapeutic agents [10] (Fig. 1).
A wealth of synthetic approaches, have been applied for the preparation of pyrrole ring, including the Hantzsch procedure [11], a Paal–Knorr synthesis [12] and the Clauson–Kaus reaction [13]. Despite the existence of these synthetic methods, synthesis of functionalized pyrroles remains intriguing [14, 15] because the above-mentioned routes have some drawbacks such as multi-step synthetic operation, inaccessible starting materials and limited scope [16]. In an attempt to provide access to a broader range of pyrroles, the application of multi-component reactions, specially 1,3-dipolar cycloadditions, has been recently reviewed [17].
C–H bond activations are of practical importance in organic synthesis due to their energy efficient and step-economic process, albeit their low acidities [18]. In the past, C–H bond activation was restricted to the metal-catalyzed methods, involving the scission of a C–H in favor of a metal to build complex structures [19]. Metal-catalyzed C–H bond functionalization is more prevalent and used to transformations where cleavage of a C–H bond is accompanied by a new bond formation at the carbon center [20]. By rising environmental concerns, particularly in the area of pharmaceuticals, recognition of routes that do not need transition-metal catalysts, have been highlighted to solve the problem of metallic impurities elimination from pharmaceutical moieties [21, 22]. In 2011, Kumar and co-workers reported the first molecular iodine-promoted metal-free C–H bond activation of alkylazaarene pyridinium zwitterion [21]. Recently the key role of sp3 C–H bond activation in 1,3-dipolar cycloaddition reactions of azomethine ylides with various alkynes and olefins has been addressed [18, 23, 24]. In 2016, indolizine derivatives were synthesized through 1,3-dipolar cycloaddition reaction of chalcone with an azomethine ylide, generated in-situ by the C–H bond activation of 2-methylquinoline in the presence of iodine and pyridine by Yavari et al. [18]. Also, recently the above-mentioned group, applied the same methodology to alkyne and arylidene pyrazolone derivatives [25].
These reactions lead to the formation of indolizine and spirocyclopropane-linked pyrazolone derivatives, respectively [18, 25]. Herein, we report an innovative pyrrole derivatives synthetic protocol in which, an azomethine ylide undergoes a diverse cyclization reaction with olefin.
Results and discussion
We commenced our studies by exploring a pilot experiment. Initially, 2-methylquinoline (1) and pyridine (2) treated to afford azomethine ylide (4) through an iodine-promoted, metal-free C-H activation of 2-methylazaarene. Then, azomethine ylide (4) underwent cyclization reaction with olefin [26] (3a), which formed in-situ, via a reaction of isatin [27, 28] and DMAD in the presence of pyridine as a base, which led to the construction of pyrrole (5a), through a one-pot procedure.
To optimize conditions, the reaction was carried out in various solvents and bases at different temperatures (Table 1). Remarkably, control experiments disclosed that no reaction occurred at ambient temperature (Table 1, entries 3–5). We screened a number of solvents such as H2O, MeOH, DMF and MeCN and found the latter to be the most effective solvent (Table 1, entries 6–10). Base screening studies exploited that surprisingly, the use of DIPEA, instead of DBU, duplicated the yield of product (5a), (Table 1, entry 8). This dramatic increase, promoted us to further optimize the reaction conditions by raising DIPEA loading. Using 2 equivalents of DIPEA, reached the yield to 50% (Table 1, entry 9). To further extend the reaction scope, we gradually elevated the temperature to 70 °C under reflux condition. Thereupon, the yield of product 5a has slightly improved (Table 1, entry 10).

With the optimized condition in hand, we then investigated the reactions scopes, not only with unsubstituted substrates, but also with a wide range of substrates bearing electron-withdrawing and electron-donating functional groups. The reactions proceeded smoothly, affording functionalized pyrrole derivatives (5) in moderate to excellent yields (Table 2).

The structure of product (5a) was elucidated unambiguously on the basis of its spectroscopic data such as 1H NMR, 13C NMR, IR as well as mass spectrometry. The mass of pyrrole (5a), depicted molecular ion peak at m/z = 413. The 1H NMR spectrum of (5a) exhibited two singlet signals at about 3.09 and 3.13 ppm which could be ascribed to the protons of methyl groups attached to the carbonyl groups in the aliphatic region, beside 10 aromatic protons. The observation of 24 distinct signals in the 1H decoupled 13C NMR spectrum of product (5a) is in agreement with the proposed structure.
Also, in the IR spectrum, the absorption band at 1751 cm−1 (C=O), was the most significant stretching frequency, proved the presence of ketone carbonyl group in the structure. As a result, the conversion proceeded in 55% yield to afford pyrrole (5a) using MeCN as a solvent and 2 equivalents of DIPEA as a base, under reflux condition at 70 °C for 3 h (Table 2, entry 1). Therefore, we aimed to propose operationally benign conditions that can be applied to present a wide variety of pyrrole scaffolds (Table1). After reaction completion (monitored by TLC), the reaction solvent evaporated gradually, and then the precipitate was recrystallized from n-hexane–EtOAc (3:1), washed with distilled water and dried at ambient temperature. The structures of pyrrole derivatives (5a–5i) were confirmed on the basis of their spectroscopic data such as 1H NMR, 13C NMR, IR and Mass as shown in supporting information. Regarding the scopes of pyrrole derivatives, the yields of substrates bearing electron-withdrawing groups and unsubstituted substrates were in the same range, and they gave higher yields, as compared to substrates bearing electron-donating groups (Table 2, entries 1–9). We were surprised to find that product (5f) was obtained in 94% yield, the highest yield among other pyrrole derivatives (Table 2, entry 6). Pleasurably, the above-mentioned result, outlined that the reaction proceeded almost complete in this case (Table 2, entry 6). To our delight, the reaction exploited compatibility toward various functional groups, leading to obtain the corresponding substituted pyrrole derivatives (5a–i) in moderate to excellent yields (Table 2, entries 1–9).
Intrigued to take a closer inception of the reaction procedure, our attention was turned to envision a reasonable mechanism for the formation of product (5). Accordingly, a mechanistic rationalization was proposed (Scheme 1). To gain insight into the reaction mechanism, firstly, the C–H activation of 2-methylquinoline (1) by coordination to iodine leads to the formation of intermediate (6). This intermediate is attacked by pyridine to obtain 1-(quinolin-2-ylmethyl) pyridinium iodide (7), which is converted to azomethine ylide (4) by DIPEA. Then, azomethine ylide (4) undergoes a cyclization reaction with olefin [26] (3) to afford the intermediate (8). Then, DIPEA, attacks to intermediate (8) and takes the proton which is attached to the adjacent carbon of 2-methylquinoline. The driving force of this attack causes the elimination of pyridine and affords the intermediate (9). Further attack of DIPEA to the intermediate (9), elimination of the proton and charge transferring, leads to the formation of intermediate (10). Finally, intermediate (10) undergoes an intramolecular cycloaddition in order to gain the intermediate (11), and this intermediate converts to the target pyrrole (5) by DIPEA, through an elimination of water. Significantly, the reaction proceeds via the elimination of the pyridine and protonated DIPEA. It is worth mentioning that olefin [26] (3) was prepared in-situ and has two E and Z isomers. Notably, we did not isolate the isomers, but TLC monitoring elucidated that the Z isomer reacts with the azomethine ylide, and the E isomer remains almost unreacted. Remarkably, driving forces of this cyclization are reactivity of isatin carbonyl group and also aromatic stability of pyrrole product (5) (Scheme 1).
Conclusion
Totally, azomethine ylides react differently with various dipolarophiles. N-metalled azomethine ylides underwent 1,3-dipolar cycloaddition reactions with quinolyl α,β-unsaturated esters and led to the synthesis of polysubstituted 3-pyrrolidinylquinolinyl derivatives [29]. Also, the reactions of azomethine ylides with chalcones and arylidene pyrazolones have been recently reviewed and led to synthesis of indolizines through 1,3-dipolar cycloaddition reactions and spirocyclopropane derivatives via elimination of pyridine [18],25. In the present work, we have successfully accomplished an elegant approach in order to synthesize novel poly substituted pyrrole derivatives with aromatic stabilization, comprising the diverse cyclization reaction of azomethine ylides generated in-situ through the iodine-mediated reaction of 2-methylquinoline and pyridine with in-situ prepared olefins. Apart from the moderate to excellent yields of the desired products and short reaction time, the present work is bestowed with several advantages over previous key protocols for synthesis of pyrrole-based scaffolds [16, 17, 30], such as, one pot procedure, toleration of an array of functional groups, simple work-up procedure, besides straight-forward isolation and purification of target pyrroles, since all derivatives were purified by recrystallization without using silica gel column chromatography. It is worth mentioning that the reaction is catalyst-free and proceeds well without the formation of any side products, irradiation, metals or ligands. We hope our novel protocol provides a new access to pyrrole-based scaffolds of pharmaceutical applications.
Experimental section
General remarks
All reagents were purchased from Merck and Fluka companies and used without further purifications. The melting points were recorded on an Electro thermal type 9100 melting point apparatus. The IR spectra were obtained on Avatar 370 FT-IR Thermo Nicolet, and only noteworthy absorptions are mentioned. The 1H NMR (300 MHz) and the 13C NMR (75 MHz) spectra were recorded on a Brucker DRX-300 Avance, using CDCl3 as applied solvent and TMS as internal standard at 300 and 75 MHz, respectively; δ in ppm, J in Hz. The mass spectra were scanned on a Varian Mat CH-7 at 70 eV and AB SCIEX 3200 QTRAP apparatuses.
General procedure for the synthesis of pyrrole 5a–i
-
1.
A mixture of quinaldine (1) (0.143 g, 1 mmol), pyridine (2) (0.158 g, 2 mmol) and I2 (0.253 g, 1 mmol) in the presence of DIPEA (0.258 g, 2 mmol) in MeCN (3 mL) was warmed to 60 °C to afford azomethine ylide (4). Then, the formed azomethine ylide (4) was added dropwise to the mixture of olefin [26] (3) (1 mmol) which is prepared in-situ, through a one pot procedure. The reaction mixture was settled under reflux conditions at 70 °C for 3 h. The reaction solvent was evaporated gradually, and the precipitate was recrystallized from n-hexane–EtOAc (3:1). Finally, the precipitate was filtered, washed with distilled water repeatedly and dried at ambient temperature, to give the pyrroles 5a–c. It is noteworthy to mention that in the cases of products 5d, 5e, 5h and 5i, a gum-shaped product was gained after recrystallization. So, the formed gum was dissolved in the minimum amount of EtOAc in order to remove the impurities. In the case of product 5g, in order to more purification after recrystallization, the precipitate was washed with boiling n-hexane. Moreover, in the case of product 5f in which the reaction proceeded almost completely, the precipitate was purified only by washing with EtOAc in 50 °C.
Complete spectroscopic and analytical data for products 5a–i are given below.
Dimethyl 9-oxo-1-(quinolin-2-yl)-9H-pyrrolo[1,2-a]indole-2,3-dicarboxylate (5a)

Yellow powder; mp 178–180 °C; yield: 0.22 g (55%). FT-IR (KBr, υ (cm−1)): 1258, 1445, 1596, 1697, 1729, 1751, 2953, 3047. 1H NMR (300 MHz, CDCl3) δ ppm: 3.99 (s, 3H), 4.06 (s,3H), 7.30 (d, J = 6.0 Hz,1H), 7.49–7.64 (m, 2H), 7.65–7.78 (m, 2H), 7.83 (d, J = 9.0 Hz, 1H), 7.98 (d, J = 12.0 Hz, 1H), 8.26 (d, J = 9.0 Hz, 1H), 8.34 (d, J = 9.0 Hz, 1H), 8.82 (d, J = 9.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ ppm = 52.4 (CH3), 52.9 (CH3), 116.6 (CH), 121.6 (CH), 123.5 (CH), 124.8 (C), 126.2 (C), 126.8 (C), 126.9 (C), 127.5 (CH), 127.9 (CH), 128.8 (CH), 129.0 (CH), 129.6 (C), 129.7 (CH), 132.0 (CH), 132.7 (C), 135.9 (CH), 136.8 (C), 147.7 (C), 148.5 (C), 159.2 (C = O), 163.9 (C = O), 178.7 (C = O). MS m/z (%) = 412 [M] +, Chemical formula, C24H16N2O5, Exact Mass = 412.
Diethyl 9-oxo-1-(quinolin-2-yl)-9H-pyrrolo[1,2-a]indole-2,3-dicarboxylate (5b)

Yellow powder; mp 180–182 °C; Yield: 0.39 g (90%); FT-IR (KBr, υ (cm−1)): 1249, 1468, 1597,1695, 1724, 2896, 2978, 3051; 1H NMR (300 MHz, CDCl3) δ ppm: 1.41 (t, J = 6 Hz, 3H), 1.43 (t, J = 6 Hz, 3H), 4.43 (q, J = 6.0 Hz, 2H), 4.54 (q, J = 6.0 Hz, 2H), 7.16–7.25 (m, 1H), 7.46–7.52 (m, 2H), 7.63–7.69 (m, 2H), 7.78 (d, J = 6.0 Hz, 1H), 7.96 (d, J = 9.0 Hz, 1H), 8.22 (d, J = 9.0 Hz, 1H), 8.27 (d, J = 9.0 Hz, 1H), 8.76 (d, J = 6.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ ppm: 14.0 (CH3), 14.2 (CH3), 61.4 (CH2), 62.1 (CH2), 116.6 (CH), 121.7 (CH), 123.7 (CH), 124.6 (C), 126.6 (C), 126.7 (C), 127.4 (C), 127.5 (CH), 127.7 (CH), 128.8 (CH), 129.3 (CH), 129.5 (C), 129.5 (CH), 130.6 (CH), 135.7 (C), 136.7 (CH), 143.7 (C), 147.5 (C), 149.2 (C), 158.9 (C=O), 165.2 (C=O), 179.8 (C=O). Synthesis of compound (5b) with chemical formula (C26H20N2O5) was confirmed with LC/MS/MS [M + H]+ ion at m/z = 441.145.
Diisopropyl 9-oxo-1-(quinolin-2-yl)-9H-pyrrolo[1,2-a]indole-2,3-dicarboxylate (5c)

Yellow powder; mp 182–184 °C; Yield: 0.22 g (48%); FT-IR (KBr, υ (cm−1)): 1258, 1468, 1695, 1613, 1726, 2851, 2920, 2953; 1H NMR (300 MHz, CDCl3) δ ppm: 1.22 (d, J = 6.0 Hz, 6H), 1.37 (d, J = 6.0 Hz, 6H), 5.23–5.31 (m, 2H), 7.44–7.51 (m, 3H), 7.63 (t, J = 9.0 Hz, 2H), 7.77(d, J = 6.0 Hz, 1H), 7.98 (d, J = 6.0 Hz, 1H), 8.20 (d, J = 6.0 Hz, 1H), 8.22 (d, J = 6.0 Hz, 1H), 8.60 (d, J = 6.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ ppm: 21.7 (2 CH3), 22.0 (2 CH3), 69.6 (CH), 70.8 (CH), 117.9 (CH), 121.7 (CH), 124.5 (CH), 124.8 (C), 126.9 (CH), 127.6 (C), 127.7 (C), 128.3 (CH), 129.2 (CH), 129.5 (CH), 129.6 (CH), 130.4 (C), 130.5 (CH), 132.6 (CH), 135.0 (C), 136.7 (CH), 142.1 (C), 147.7 (C), 149.2 (C), 158.7 (C=O), 164.1 (C=O), 178.5 (C=O). Synthesis of compound (5c) with chemical formula (C28H24N2O5) was confirmed with LC/MS/MS [M + H]+ ion at m/z = 469.178 and LC/MS/MS [M + Na]+ adduct ion at m/z = 491.169.
Dimethyl 7-chloro-9-oxo-1-(quinolin-2-yl)-9H-pyrrolo[1,2-a]indole-2,3-dicarboxylate (5d)

Yellow powder; mp 184–186 °C; Yield: 0.25 g (57%); FT-IR(KBr, υ (cm−1)): 837, 1292, 1468, 1595, 1696, 1726, 2923, 3080; 1H NMR (300 MHz, CDCl3) δ ppm: 3.99 (s, 3H), 4.06 (s, 3H) 7.50–7.57 (m, 2H), 7.67–7.73 (m, 2H), 7.82 (d, J = 9.0 Hz, 1H), 7.97 (d, J = 9.0 Hz, 1H), 8.27 (d, J = 9.0 Hz, 1H), 8.32 (d, J = 9.0 Hz, 1H), 8.76 (d, J = 9.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ ppm: 61.5 (CH3), 62.3 (CH3), 117.9 (CH), 121.6 (CH), 123.9 (CH), 124.8 (C), 126.9 (C), 127.5 (C), 127.8 (C), 128.1 (CH), 129.5 (CH), 129.5 (CH), 129.6 (C), 130.3 (CH), 130.5 (C), 132.6 (CH), 135.1 (C), 136.8 (CH), 142.0 (C), 147.6 (C), 148.9 (C), 158.9 (C=O), 165.0 (C=O), 178.5 (C=O). Synthesis of compound (5d) with chemical formula (C24H15ClN2O5) was confirmed with LC/MS/MS [M + H]+ ion at m/z = 447.060 and LC/MS/MS [M + Na]+ adduct ion at m/z = 469.062.
Diethyl 7-chloro-9-oxo-1-(quinolin-2-yl)-9H-pyrrolo[1,2-a]indole-2,3-dicarboxylate (5e)

Yellow powder; mp 183–185 °C; Yield:0.28 g (60%); FT-IR (KBr, υ (cm−1)): 839, 1469, 1597, 1699, 1723, 1740, 2974, 3072; 1H NMR (300 MHz, CDCl3) δ ppm: 1.40 (t, J = 9.0 Hz, 3H), 1.43 (t, J = 9.0 Hz, 3H), 4.44 (q, J = 6.0 Hz, 2H), 4.53 (q, J = 6.0 Hz, 2H), 7.56–7.48 (m, 2H), 7.65–7.72 m, 2H), 7.82 (d, J = 12.0 Hz, 1H), 7.96 (d, J = 9.0 Hz, 1H), 8.25 (d, J = 9.0 Hz, 1H), 8.31 (d, J = 9.0 Hz, 1H), 8.73 (d, J = 9.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ ppm: 13.9 (CH3), 14.2 (CH3), 61.5 (CH2), 62.3 (CH2), 117.9 (CH), 121.6 (CH), 123.9 (CH), 124.8 (C), 126.9 (C), 127.5 (C), 127.8 (C), 128.1 (CH), 129.5 (CH), 129.6 (CH), 129.67 (C), 130.3 (CH), 130.5 (C), 132.6 (CH), 135.1 (C), 136.8 (CH), 142.0 (C), 147.6 (C), 148.9 (C), 158.9 (C=O), 165.0 (C=O), 178.5 (C=O). Synthesis of compound (5e) with chemical formula (C26H19ClN2O5) was confirmed with LC/MS/MS [M + H]+ ion at m/z = 475.088.
Diisopropyl 7-chloro-9-oxo-1-(quinolin-2-yl)-9H-pyrrolo[1,2-a]indole-2,3-dicarboxylate (5f)

Yellow powder; mp 184–186 °C; Yield: 0.47 g (94%); FT-IR(KBr, υ (cm−1)): 835, 1470, 1598, 1697, 1718, 1734, 2982, 3076; 1H NMR (300 MHz, CDCl3) δ ppm: 1.29 (d, J = 6.0 Hz,6H), 1.43 (d, J = 6.0 Hz, 6H), 5.28–5.40 (m, 2H), 7.47–7.56 (m, 2H), 7.65–7.71 (m, 2H), 7.82 (d, J = 9.0 Hz, 1H), 8.03 (d, J = 9.0 Hz, 1H), 8.25 (d, J = 6.0 Hz, 1H), 8.28 (d, J = 6.0 Hz, 1H), 8.62 (d, J = 9.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ ppm: 21.8 (2CH3), 22.1 (2CH3), 69.7(CH), 70.9 (CH), 118.0 (CH), 121.8 (CH), 124.6 (CH), 124.8 (C), 127.0 (C), 127.7 (C), 127.8 (C), 128.0 (CH), 129.3 (CH), 129.5 (CH), 129.7 (C), 130.4 (CH), 130.5 (C), 132.7 (CH), 135.1 (CH), 136.8 (C), 142.2 (C), 147.8 (C), 149.3 (C), 159.2 (C=O), 164.6 (C=O), 178.2 (C=O). Synthesis of compound (5f) with chemical formula (C28H23ClN2O5) was confirmed with LC/MS/MS [M + H] + ion at m/z = 503.133 and LC/MS/MS [M + Na]+ adduct ion at m/z = 525.129.
Dimethyl 7-methyl-9-oxo-1-(quinolin-2-yl)-9H-pyrrolo[1,2-a]indole-2,3-dicarboxylate (5g)

Yellow powder; mp 181–183 °C; Yield: 0.19 g (45%); FT-IR (KBr, υ ( cm−1)): 1458, 1593, 1706, 1727, 1744, 2853, 2924, 2956; 1H NMR (300 MHz, CDCl3) δ ppm: 2.40 (s, 3H), 3.98 (s, 3H), 4.02 (s, 3H), 7.36 (d, J = 9.0 Hz, 1H), 7.53 (s, 1H), 7.67 (t, J = 9.0 Hz, 1H), 7.83 (t, J = 9.0 Hz, 1H), 7.91 (d, J = 9.0 Hz, 1H), 7.94 (d, J = 9.0 Hz, 1H), 8.50 (m, 3H). 13C NMR (75 MHz, CDCl3) δ ppm: 21.0 (CH3), 69.4 (CH3), 70.5 (CH3), 116.3 (CH), 121.9 (CH), 124.1 (CH), 125.3 (C), 126.8 (C),127.4 (C),127.6 (C),127.6 (CH), 128.6 (CH), 129.1 (CH), 129.4 (C), 129.5 (CH), 130.6 (CH), 136.1 (C), 136.7 (C),136.9 (CH), 141.7 (C), 147.8 (C), 149.8 (C), 159.0 (C),164.5 (C), 180.0 (C). Synthesis of compound (5 g) with chemical formula (C25H18N2O5) was confirmed with LC/MS/MS [M + H]+ ion at m/z = 427.126.
Diethyl 7-methyl-9-oxo-1-(quinolin-2-yl)-9H-pyrrolo[1,2-a]indole-2,3-dicarboxylate (5h)

Yellow powder; mp 183–185 °C; Yield: 0.21 g (48%); IR( KBr, υ (cm−1): 1255, 1489, 1599, 1695, 1726, 2847, 2958, 3056; 1H NMR (300 MHz, CDCl3) δ ppm: 1.40 (t, J = 6.0 Hz, 3H), 1.43 (t, J = 6.0 Hz, 3H), 2.33 (s, 3H), 4.43 (q, J = 6.0 Hz, 2H), 4.53 (q, J = 6.0 Hz, 2H),7.26–7.31 (m,1H), 7.46 – 7.53 (m, 2H), 7.64– 7.70 (m, 1H), 7.80 (d, J = 9.0 Hz, 1H), 7.96 (d, J = 9.0 Hz, 1H), 8.15 (d, J = 9.0 Hz, 1H), 8.23 (d, J = 9.0 Hz, 1H), 8.76 (d, J = 9.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ ppm: 14.0 (CH3), 14.2 (CH3), 20.9 (CH3), 61.4 (CH2), 62.0 (CH2), 116.4 (CH), 121.7 (CH), 123.5 (C), 125.2 (C), 126.7 (C), 127.2 (C), 127.5 (C), 127.7 (CH), 128.9 (CH), 128.9 (CH), 129.5 (C), 130.7 (CH), 136.0 (CH), 136.7 (C), 136.8 (C), 141.7 (CH), 147.6 (C), 149.4 (C), 159.0 (C), 161.0 (C=O), 165.3 (C=O), 180.1 (C=O). Synthesis of compound (5h) with chemical formula (C27H22N2O5) was confirmed with LC/MS/MS [M + H]+ ion at m/z = 455.160.
Diisopropyl 7-methyl-9-oxo-1-(quinolin-2-yl)-9H-pyrrolo[1,2-a]indole-2,3-dicarboxylate (5i)

Yellow powder; mp 185–187 °C; Yield: 0.24 g (50%); IR (KBr, υ (cm−1)): 1218, 1477, 1600, 1697, 1723, 1744, 2924, 3080; 1H NMR (300 MHz, CDCl3) δ ppm: 1.22 (d, J = 6.0 Hz, 6H). 1.35 (d, J = 9.0 Hz, 6H), 2.28 (s, 3H), 5.20–5.33 (m,2H), 7.18–7.24 (m, 1H), 7.39–7.46 (m, 2H), 7.56–7.62 (m, 1H), 7.73 (d, J = 9.0 Hz, 1H), 7.95 (d, J = 9.0 Hz, 1H), 8.04 (d, J = 9.0 Hz, 1H), 8.16 (d, J = 9.0 Hz, 1H), 8.57 (d, J = 9.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ ppm: 20.9 (CH3), 21.7(2CH3), 22.0 (2CH3), 69.4 (CH), 70.4 (CH), 116.3 (CH), 121.8 (CH), 124.1 (CH), 125.1 (C), 126.7 (C), 127.3 (C), 127.5 (C), 127.6 (CH), 128.6 (CH), 129.1 (CH), 129.4 (C), 129.4 (CH), 130.55 (CH), 135.9 (C), 136.5 (CH), 136.8 (C), 141.8 (C), 147.6 (C), 149.6 (C), 158.8 (C=O), 164.4 (C=O), 180.11 (C=O). Synthesis of compound (5i) with chemical formula (C29H26N2O5) was confirmed with LC/MS/MS [M + H]+ ion at m/z = 483.198.
References
Estévez V, Villacampa M, Menéndez JC (2014) Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem Soc Rev 43:4633–4657
Alizadeh A, Rezvanian A, Bijanzadeh HR (2008) Synthesis of highly functionalized pyrrole derivatives via a four-component reaction of two primary amines and diketene in the presence of nitrostyrene. Synthesis (Stuttg) 2008:725–728
Sobenina LN, Stepanova ZV, Petrova OV et al (2013) Synthesis of 3-[5-(biphenyl-4-yl)pyrrol-2-yl]-1-phenylprop-2-yn-1-ones by palladium-free cross-coupling between pyrroles and haloalkynes on aluminum oxide. Russ Chem Bull 62:88–92. https://doi.org/10.1007/s11172-013-0011-7
Oldfield E (2010) Targeting isoprenoid biosynthesis for drug discovery: bench to bedside. Acc Chem Res 43:1216–1226. https://doi.org/10.1021/ar100026v
Aiello F, Garofalo A, Grande F (2010) Efficient synthesis of 9H-pyrrolo[1,2-a]indol-9-one derivatives based on active manganese dioxide promoted intramolecular cyclization. Tetrahedron 66:274–277. https://doi.org/10.1016/j.tet.2009.10.111
Lisowski V, Léonce S, Kraus-Berthier L et al (2004) Design, synthesis, and evaluation of novel thienopyrrolizinones as antitubulin agents. J Med Chem 47:1448–1464. https://doi.org/10.1021/jm030961z
Quermonne MA, Dallemagne P, Louchahi-Raoul J et al (1992) Effective cerebral antihypoxic activity of new aminocyclopentanones. Eur J Med Chem 27:961–965. https://doi.org/10.1016/0223-5234(92)90029-Z
Cartoon MEK, Cheeseman GWH (1981) Lithiation reactions of 1-(2′-bromophenyl)pyrrole and related compounds. J Organomet Chem 212:1–9. https://doi.org/10.1016/S0022-328X(00)85520-5
Mátravölgyi B, Hergert T, Thurner A et al (2017) Synthesis and investigation of solar-cell photosensitizers having a fluorazone backbone. Eur J Org Chem 2017:1843–1854. https://doi.org/10.1002/ejoc.201601622
Mátravölgyi B, Hergert T, Bálint E et al (2018) Access to fluorazones by intramolecular dehydrative cyclization of aromatic tertiary amides: a synthetic and mechanistic study. J Org Chem 83:2282–2292. https://doi.org/10.1021/acs.joc.7b03176
Leonardi M, Estévez V, Villacampa M, Menéndez JC (2019) The Hantzsch pyrrole synthesis: non-conventional variations and applications of a neglected classical reaction. Synthesis (Stuttg) 51:816–828
Vladimirova S (2021) Synthesis of new pyrrole compounds with potential antihtyperlipidemic effect. J Chem Technol Metall 56:720–724
Alvi S, Ali R (2021) Design, synthesis and photophysical properties of novel star-shaped truxene-based heterocycles utilizing ring-closing metathesis, Clauson-Kaas, Van Leusen and Ullmann-type reactions as key tools. Beilstein J Org Chem 17:1374–1384
Zuo L, Yang Y, Guo W (2021) Modular domino process toward highly functionalized pyrroles via Pd-catalyzed [4+ 1] annulation under mild conditions. Org Lett 23:2013–2018
Zhao K, Du R, Wang B et al (2019) RhCl3· 3H2O-catalyzed regioselective C (sp2)–H alkoxycarbonylation: efficient synthesis of indole-and pyrrole-2-carboxylic acid esters. ACS Catal 9:5545–5551
He X-L, Zhao H-R, Song X et al (2019) Asymmetric Barton–Zard reaction to access 3-pyrrole-containing axially chiral skeletons. ACS Catal 9:4374–4381. https://doi.org/10.1021/acscatal.9b00767
Lin Z, Li C, Zhou Z et al (2019) Copper (II)-promoted oxidation/[3+ 2] cycloaddition/aromatization cascade: efficient synthesis of tetrasubstituted NH-pyrrole from chalcones and iminodiacetates. Synlett 30:1442–1446
Yavari I, Naeimabadi M, Halvagar MR (2016) FeCl3-catalyzed formation of indolizine derivatives via the 1, 3-dipolar cycloaddition reaction between azomethine ylides and chalcones or dibenzylideneacetones. Tetrahedron Lett 57:3718–3721
Labinger JA, Bercaw JE (2002) Understanding and exploiting C–H bond activation. Nature 417:507–514. https://doi.org/10.1038/417507a
Ritleng V, Sirlin C, Pfeffer M (2002) Ru-, Rh-, and Pd-catalyzed C–C bond formation involving C–H activation and addition on unsaturated substrates: reactions and mechanistic aspects. Chem Rev 102:1731–1770. https://doi.org/10.1021/cr0104330
Kumar A, Gupta G, Srivastava S (2011) Synthesis of new class of alkyl azarene pyridinium zwitterions via iodine mediated sp3 C–H bond activation. Org Lett 13:6366–6369. https://doi.org/10.1021/ol202654j
Breugst M, Reissig H-U (2020) The Huisgen reaction: Milestones of the 1,3-dipolar cycloaddition. Angew Chemie Int Ed 59:12293–12307. https://doi.org/10.1002/anie.202003115
Yavari I, Naeimabadi M (2018) Synthesis of 3-(quinolin-2-yl)indolizines through iodine-mediated sp3 C–H functionalization of azaarenes. Synth Commun 48:632–637. https://doi.org/10.1080/00397911.2017.1362436
Yavari I, Naeimabadi M, Halvagar MR (2018) A diastereoselective synthesis of functionalized bis-spirorhodanine-linked cyclopentanes via C (sp3)–H activation. Tetrahedron 74:4145–4150. https://doi.org/10.1016/j.tet.2018.06.029
Yavari I, Naeimabadi M, Sheykhahmadi J et al (2017) Diastereoselective synthesis of spirocyclopropane-linked pyrazolones from azomethine ylides via C(sp3)-H activation. ChemistrySelect 2:11370–11375. https://doi.org/10.1002/slct.201701906
Shahraki A, Hassanabadi A (2014) Pyridine-mediated N-vinylation of heterocyclic compounds: a mild, stereoselective and general synthesis. J Chem Res 38:586–588
Ribeiro NM, Da SBV, de Almeida VF et al (2005) 5-Chloro- and 5,7-dichloroisatin by chlorination of isatin with trichloroisocyanuric acid. Org Prep Proced Int 37:265–267. https://doi.org/10.1080/00304940509354956
Singh A, Raghuwanshi K, Patel VK et al (2017) Assessment of 5-substituted isatin as surface recognition group: design, synthesis, and antiproliferative evaluation of hydroxamates as novel histone deacetylase inhibitors. Pharm Chem J 51:366–374
Bouraiou A, Debache A, Rhouati S et al (2008) 1,3-Dipolar cycloaddition of stabilized azomethine ylides to alkenyl quinolines: an efficient route to polyfunctionalized 3-pyrrolidinylquinoline derivatives. J Heterocycl Chem 45:329–333. https://doi.org/10.1002/jhet.5570450206
Wang S-F, Guo C-L, Cui K et al (2015) Lactic acid as an invaluable green solvent for ultrasound-assisted scalable synthesis of pyrrole derivatives. Ultrason Sonochem 26:81–86
Acknowledgements
We gratefully appreciate Ferdowsi university of the Mashhad Research council for their financial support of this work (Grant: 3/52224).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sayebani, S., Eshghi, H. & Naeimabadi, M. Synthesis of functionalized pyrrole derivatives via diverse cyclization of azomethine ylide and olefins. Mol Divers 26, 2221–2230 (2022). https://doi.org/10.1007/s11030-021-10328-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10328-x