Abstract
Oxidative stress that damages cellular components affects various organs including the brain. It is thus believed to play an active role in neurodegenerative diseases, wherein the intrinsic antioxidant enzymes metabolize toxic intermediates. For therapeutic purpose, instead of antioxidant enzymes, small organic compounds as antioxidants may be more effective. Here, reducing power and electrochemical behavior of some flavanols, flavanonols, flavones, flavonols and O–methylated flavonols have been estimated and confirmed by the calculated bond dissociation energy. Compared to other classes, flavonols exhibited increased reducing power that decreased with methylation of the oxygen atom in the B-ring. Gossypetin emerged as the most effective of these flavonols. Generally, compounds with two hydroxyl groups in two consecutive positions of the phenyl ring and an enolic group in the C-ring with more preference for the hydroxyl group in the ortho position with respect to each other in the catechol moiety showed major activity. 5 position of the A-ring showed the least effect on the activity. The present understanding therefore may be applied for identifying compounds to be used as scaffold for designing potent antioxidants.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidative stress is caused by the imbalance in the redox status of the cells resulting from either excessive generation of reactive oxygen species (ROS) or dysfunction of the antioxidant system [1,2,3]. It is lethal for the normal functioning of the physiological processes for the fact that it is associated with cell membrane damage owing to lipid peroxidation, changes in protein structure and function due to protein oxidation, and finally structural damage to DNA [4]. Unless addressed, it causes severe damage to different organs, the most vulnerable being the brain. This is so because brain has a high oxygen demand which is 20% of the total oxygen consumption of the body [5,6,7,8], also redox active metals such as iron or copper that catalyze ROS formation are abundant there and finally, the presence of high level of polyunsaturated fatty acids in the lipid of brain cells makes it a peroxidation susceptible area [9,10,11,12,13,14].
Thus, oxidative stress is believed to play a central role in the common pathophysiology of neurodegenerative diseases [6, 8, 15] such as amyotrophic lateral sclerosis, Alzheimer's disease [12, 14, 16,17,18,19] and Parkinson's disease [20,21,22]. Antioxidant enzymes [23,24,25] and low molecular weight compounds [24, 26, 27] act as key players of the antioxidant defense machinery. However, small molecules are of much importance due to their ability to penetrate the cellular membrane and thus function both intra- and extracellularly [28, 29].
Flavonoids are the polyphenolic compounds of plant secondary metabolites that are already known to act as non-steroidal anti-inflammatory drugs [30,31,32]. Their polyhydroxyphenolic moiety attached with the electron withdrawing group might exhibit antioxidant activities and therefore may be a better choice for such purposes. Different research groups found that the antioxidant activities of these types of compounds are directly related to their structure [33,34,35,36]. Here, the aim is to study the reducing property and the electrochemical behavior of 16 compounds from various groups and relate their activity to structural features, using theoretical studies.
Reducing agents generally behave as antioxidants; hence, the reducing power of these organic molecules can be an indicator of their antioxidant activity. We have therefore evaluated the reducing power of these compounds estimating their ability to react with potassium ferricyanide to form potassium ferrocyanide, which then reacts with ferric chloride to form ferric–ferrous complex. In conjunction, we have used the same set of compounds for the estimation of electrochemical oxidation.
Electrochemistry is a significant tool to examine the reactions involving transfer of electrons (either reduction or oxidation); hence, redox behaviors of these small organic molecules might add important insight. Various research groups have used a particular type of potentiodynamic electrochemical measurement that is cyclic voltammetry as a valuable tool to assess the redox property of various types of substances [27,28,29, 36, 37]. Lower oxidation potential of certain compounds, that is compounds with low positive potential measured by cyclic voltammetry, reflects greater reducing power and hence is used as an evaluating index of antioxidant activity. The same principle has been used to evaluate the redox behavior of the 16 compounds studied here.
Reducing power and redox activity of certain compounds may be dependent on their atomic hydrogen producing ability because atomic hydrogen serves as a good reducing agent. Alcoholic and phenolic compounds can produce atomic hydrogen by the cleavage of their O–H bond. It may be expected that lower the O–H bond dissociation energy, higher the reducing ability as well as redox activity. So O–H bond dissociation energies have been calculated in addition to the experimental studies to infer the role of the hydroxyl groups at various positions of a flavonoid or a polyhydroxybenzophenone.
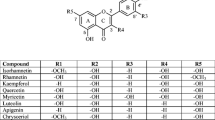
Phenolic hydroxyl group pushes the electron cloud to the benzene ring by positive resonance (+ R effect) increasing the electron density on the phenyl ring. This electron-rich phenyl ring further can transfer electrons to other substances. This phenomenon helps phenolic hydroxyl group containing organic compounds to become good reducing agents. Hence, it is expected that the oxidation potential of these types of organic compounds containing phenolic hydroxyl group will be lower. It is also expected that the reducing power of polyphenolic compounds having more than one phenolic hydroxyl group would increase when the number of hydroxyl groups increases although the positions of these phenolic hydroxyl groups might play a significant role. We have therefore tested various kinds of flavonoids and some polyhydroxybenzophenones. The flavonoids tested are flavanols (epicatechin and epigallocatechin), flavanonols (taxifolin and ampelopsin), flavones (chrysin and luteolin), flavonols (galangin, quercetin, myricetin and gossypetin) and O-methylated flavonols (rhamnetin, isorhamnetin and tamarixetin). The flavonols that we tested initially exhibited reducing activity that did not always increase with increasing number of hydroxyl group but increased with increasing number of catechol like centers. To verify the importance of such centers, we tested three polyhydroxybenzophenones that differed either in the number of hydroxyl groups or in the position of the same number of hydroxyl groups. The compounds chosen were 2,4-dihydroxybenzophenone, 2,2′,4,4′-tetrahydroxybenzophenone and 3,4-dihydroxybenzophenone. Structures of all the compounds studied here are illustrated in Fig. 1
Materials and method
Materials
Potassium ferricyanide was purchased from SRL India, and potassium ferrocyanide was purchased from HiMedia. Disodium hydrogen phosphate, sodium dihydrogen phosphate and ethanol were obtained from Merck Specialities Private Limited. The test compounds, namely chrysin, luteolin, galangin, quercetin, myricetin, taxifolin, epicatechin, epigallocatechin and all polyhydroxybenzophenones, i.e. 2,2′,4,4′-tetrahydroxybenzophenone, 2,4-dihydroxybenzophenone and 3,4-dihydroxybenzophenone, were bought from Sigma-Aldrich. We purchased gossypetin and ampelopsin from Indofine Chemical Company, Inc. Rhamnetin, isorhamnetin and tamarixetin were supplied by Extrasynthese Genay, France. All the compounds obtained from respective companies were used as they were without any further purification.
Method
Reducing power assay
Reducing power of various test compounds was measured by the method described by Gow-Chin Yen 1995 [38]. Ferricyanide ion is reduced in the presence of a suitable reducing agent to form the ferrocyanide ion, and this ferrocyanide ion further reacts with ferric ion to form a dark blue (Prussian blue) colored complex which is known as the ferricferrocyanide complex. This complex absorbs visible light with maximum intensity at 700 nm [39, 40].
Using this principle, the reducing power of the flavonoids and some polyhydroxybenzophenones was tested. Higher intensity of the blue colored complex would reflect higher reducing capacity and may therefore be inferred to be effective as an antioxidant.
The method adopted in our study is a modification of the method, followed by Canabady-Rochelle et. al [41]. We have taken 400 µM of potassium ferricyanide and various concentrations of the test sample varying from 0 to 100 µM, co-incubated in the presence of phosphate buffer at 50 °C for 30 min. For control, instead of potassium ferricyanide, 400 µM of potassium ferrocyanide was added and no test sample was added. 250 µL of each solution was mixed with 240 µL of water and 10 µL of 10 mM ferric chloride which was further incubated at room temperature for 20 min. Thereafter, absorbance was measured at 700 nm by using Agilent Technologies Cary 60 UV–Vis spectrophotometer.
whereA0 = absorbance of control.
A = absorbance of the test sample.
Each experiment was carried out in triplicate, and the average was used for subsequent calculation. EC50values were calculated using Origin 8.0.
Electrochemical measurements
The electrochemical measurements of all these compounds were performed using a Basi-C3 Cell instrument at a scan rate of 100 mV s−1 within the potential range of − 0.5 to + 1.50 V with respect to Ag/AgCl. Cyclic voltammograms were recorded using 0.1 M TBAP (tetrabutylammonium perchlorate) as supporting electrolyte and 500 µM of the test compounds in ethanol solution. The working electrode was a glassy carbon disk (0.32 cm2) which was polished with alumina solution, washed with absolute ethanol, and air dried before each electrochemical run. The reference electrode was Ag/AgCl, with platinum as counter electrode. All measurements were carried out in standard electrochemical cells at 25 °C.
Calculation of bond dissociation energy
Initial coordinates of the flavonoids and the polyhydroxybenzophenone molecules were prepared using the molecule builder interface of HyperChem. Thereafter, initial structural optimization was made using the molecular mechanics (MM +) [42] force field option with Polak–Ribiere conjugate gradient algorithm and RMS gradient of 0.001 kcal Å−1 mol−1, followed by ab initio method by using HyperChem release 8.0 software package [43]. In the next phase, quantum chemical calculations were performed by using Gaussian 09 package [44]. Density functional theory [45] method with B3LYP functional [46,47,48,49] and 6-31G(d,p) basis set [50, 51] was deployed. In this phase, at first, geometry of intact molecule obtained from HyperChem (input file prepared by using Gabedit 2.4.8 [52]) was optimized and the corresponding energy was calculated. Then, by deleting the coordinate of the hydrogen atom at a desired position, the structure of the radical was created, its geometry was optimized and energy was calculated. O–H bond dissociation energy was calculated by the following equation [53].
where EBD = bond dissociation energy.
ERAD = energy of corresponding radical.
EH = energy of free hydrogen radical.
EMOL = energy of the intact molecule.
Results and discussion
Here we report reducing power assay and cyclic voltammetry of various kinds of flavonoids and some polyhydroxybenzophenones. The flavonoids include flavones (chrysin and luteolin), flavonols (galangin, quercetin, myricetin and gossypetin), methylated flavonols (rhamnetin, isorhamnetin and tamarixetin), flavanonols (taxifolin and ampelopsin) and flavanols (epicatechin and epigallocatechin). Three polyhydroxybenzophenones, namely 2,4-dihydroxybenzophenone, 2,2′,4,4′-tetrahydroxybenzophenone and 3,4-dihydroxybenzophenone, were also tested.
Reducing power assay
We have measured the reducing power of the compounds at various concentrations. Figure 2 shows the curves for percentage of reduction against different concentration of the chosen compounds.
Flavones
As already mentioned, the hydroxyl group of phenolic compounds donates electron to the benzene ring through + R effect, which increases the electron density in the ring, thereby increasing the tendency of electron donation thus giving rise to compounds with increased reduction power over compounds containing only benzene ring. Reducing power of two flavones, chrysin and luteolin has been studied. Chrysin contains two hydroxyl groups at 5- and 7-position of the A-ring, while luteolin contains two more additional hydroxyl groups in the B-ring at 3′- and 4′-positions. Figure S1 may be referred to follow the ring nomenclature and atom numbering of the compounds. Our findings indicate that chrysin has no reducing capacity, but luteolin is a better reducing agent with EC50 value of 72.90 µM (Fig. 2a). This refers to the importance of two consecutive hydroxyl groups in the phenyl ring and hence the importance of the catechol moiety for exhibiting the reducing property.
Flavonols
In comparison with chrysin, galangin a flavonol contains only one extra hydroxyl group at 3-position of the C-ring. This hydroxyl group is also unable to show any effect on the reducing capacity to the flavonol (Fig. 2b). However, another flavonol, quercetin seems to be a good reducing agent having EC50 of 32.42 µM once again indicating the importance of the catechol moiety. It is also interesting to note that the reducing power of quercetin is greater than luteolin (flavone) which implies that though the hydroxyl group at 3-position itself appears to have no importance on reducing power, when connected to the catechol moiety it plays a role in increasing the reducing power.
Myricetin which has further addition of a hydroxyl group in its B-ring shows quite similar reducing power as quercetin, with EC50 of 35.62 µM. Thus, increase in the number of hydroxyl group above two, in the B-ring, does not hold a linear relationship with reducing power. This implies that, in case of flavonol, the pyrogallol moiety has very little effect on the reducing property over catechol moiety. Like quercetin, gossypetin also contains a catechol moiety as B-ring. But, in the A-ring, gossypetin contains three hydroxyl groups at 5-, 7- and 8-positions instead of two. Here the two consecutive hydroxyl groups at 7- and 8-positions serve as another catechol like center in gossypetin. It showed an EC50 value of 24.25 µM which is the best not only with respect to flavonols but also among all the compounds examined in the study. It is to be noted that both myricetin and gossypetin contain the same number of hydroxyl groups.
O-Methylated flavonols
Methylation on various oxygen atoms of quercetin may hamper the reduction ability as the methoxy group is unable to donate atomic hydrogen unlike phenolic hydroxyl group that can easily do so by dissociating the covalent bond between hydrogen and oxygen. We have already seen that hydroxyl group at 7-position of A-ring has no effect on reducing power so we may expect methylation of this oxygen not to show any increment on the reducing power. Rhamnetin which is 7-O-methylquercetin shows a reducing power with EC50 value of 30.93 µM which is quite close to that of quercetin (Fig. 2c). On the other hand, B- ring as catechol moiety has shown significance and absence of this moiety makes flavonols inert. We therefore studied the reducing power of isorhamnetin and tamarixetin in order to see the effect of methylation in the B-ring at 3′- and 4′-oxygen atom of quercetin, respectively. Methylation at 3′-position decreases the reduction ability of isorhamnetin (3′-O-methylquercetin) as reflected from large EC50 (50.88 µM). Effect of O-methylation at 4′-position resulted in an even larger EC50 of tamarixetin (98.19 µM).
Flavanonols
Like flavonols, flavanonols contain double bonded oxygen atom at position 4 of C-ring although double bond between C2 and C3 is absent. Ampelopsin, the pyrogallol moiety containing flavanonol, has quite lower value of EC50 (38.82 µM) than other flavanols but is still greater than the flavonols studied here (Fig. 2d). The catechol moiety containing taxifolin has EC50 as 44.36 µM. It may be due to the fact that absence of double bond between C2 and C3 atoms in flavanonols as well as in flavanols gives rise to non-planar sp3 hybridized carbon containing compounds. These compounds are unable to drag unpaired electron of the free radical intermediates from B- ring. Hence, the formation of such unpaired electron containing free radical intermediates is not facilitated.
Flavanols
Unlike flavonol, flavanol lacks the double bond between 2- and 3-position of C-ring and also the double bonded oxygen atom at 4-position of C-ring. We realized the importance of the double bond between C2 and C3 and also the double bonded oxygen atom of 4-position of flavonol during the study of the reducing power of flavanols. Epicatechin is a flavanol which contains a catechol moiety. Reducing power assay of epicatechin, a catechol moiety containing flavanol, confirmed the EC50 value as 47.03 µM (Fig. 2e) which is quite greater than that of quercetin. Such kind of increment in EC50 was also obtained for epigallocatechin which too is a flavanol, but like myricetin it contains a pyrogallol moiety as B-ring. Epigallocatechin showed EC50 of 44.31 µM which is comparable to epicatechin but greater than that of its corresponding flavonol myricetin.
Polyhydroxybenzophenone
Importance of the catechol moiety could be further verified through estimation of reducing power of polyhydroxybenzophenones. Here, the keto group has electron withdrawing capability. So addition of polyhydroxyphenyl group joined with keto group might exhibit considerable reducing power. In this series, first we have studied 2,4-dihydroxybenzophenone which contains one resorcinol moiety. In the given condition, this compound showed no reducing capability (Fig. 2f). Even upon addition of two resorcinol moiety as in 2,2′,4,4′-tetrahydroxybenzophenone, no reducing activity was observed. Thus, it becomes evident that the number of resorcinol moiety plays no role, on the contrary resorcinol moiety containing compounds turned out to be in capable of showing any reducing power. We also found 3,4-dihydroxybenzophenone has a reducing power with EC50 value 71.19 µM. Though 3,4-dihydroxybenzophenone is not so good as a reducing agent in comparison with flavonoids, but it is much better than the other two polyhydroxybenzophenones. So it is clear that reducing capability does not depend on the number of polyhydroxyphenyl group but the arrangement of the hydroxyl groups on phenyl ring matters.
Electrochemical behavior
Cyclic voltammetry (CV) is a powerful electrochemical technique popularly applied to study the reduction and oxidation processes of molecular species. More importantly, CV is employed to study the electron transfer-initiated chemical reactions. We have therefore recorded the response in current when a voltage is being applied across individual solution of the compounds in ethanol and the reference electrode. Figure 3 shows cyclic voltammograms (current versus potential plot) for all the compounds.
Flavones
For flavone type of molecules, cyclic voltammogram of chrysin shows no prominent peak on forward or reverse scan, but for luteolin one peak is observed at E1 = + 0.71 V on forward scan and another at E1 = + 0.50 V on reverse scan due to the almost reversible oxidation process (Fig. 3a). The absence of any peak in cyclic voltammogram of chrysin indicates the redox inactive nature of this compound. Hence, it is clear that hydroxyl group of 5- and 7-positions of A-ring is unable to exert much redox activity to these compounds. A single peak in cyclic voltammogram of luteolin signifies that the compound is oxidized in one step. Luteolin becomes a redox active system when the normal B-ring of chrysin transforms to catechol moiety and the peak in cyclic voltammogram corresponds to the catechol moiety. The B-ring as catechol moiety is thus important for redox activity.
Flavonols
Addition of one hydroxyl group at 3-position of redox inactive flavone chrysin makes it the flavonol galangin. In the cyclic voltammogram of galangin, one peak is observed at E1 = + 0.82 V on forward scan and another at E1 = + 0.70 V on reverse scan implying one step oxidation/reduction process at the electrode surface (Fig. 3b). So it is clear that this hydroxyl group at position 3 has an impact on redox activity. Likewise, in case of quercetin that has an extra hydroxyl group at 3- position in comparison with another flavone luteolin, an extra peak is observed on forward anodic scan. Compound quercetin in ethanol (Fig. 3b) shows two waves on forward anodic scan (− 0.5 to + 1.50 V) for the two step oxidation process at the electrode surface. The peaks are observed at E1 = + 0.65 V and E2 = + 1.0 V, and for myricetin, they are observed at E1 = + 0.54 V and E2 = + 1.02 V. These clearly indicate that oxidation of the catechol moiety in quercetin and of the pyrogallol moiety in myricetin has a similar nature. However, gossypetin shows three peaks at E1 = + 0.49 V, E2 = + 0.70 V and E3 = + 1.15 V with respect to Ag/AgCl electrode. The presence of this third redox active center in gossypetin may be due to the presence of two consecutive hydroxyl groups at the 7- and 8-position of A-ring. On reverse scan, oxidized products of these three compounds are reduced through two steps at the electrode surface and the corresponding peaks are observed at E1 = + 0.05 V and E2 = + 0.51 V for quercetin; at E1 = + 0.42 V and E2 = + 0.86 V for myricetin and at E1 = + 0.30 V and E2 = + 0.57 V for gossypetin. The three compounds (quercetin, myricetin and gossypetin) show similar type of CVs with two peaks for two quasi-reversible oxidation processes. Among these flavonols, the oxidation potential (E1) of gossypetin is the lowest.
O-Methylated flavonols
The cyclic voltammograms of O-methylated flavonols also show two waves on forward anodic scan (Fig. 3c), and the peaks are observed at E1 = + 0.69 V and E2 = + 1.04 V for rhamnetin; at E1 = + 0.71 V and E2 = + 1.14 V for isorhamnetin and at E1 = + 0.74 V and E2 = + 0.99 V for tamarixetin. Here also two very small peaks are observed at E1 = + 0.51 V and E2 = + 0.91 V for rhamnetin; at E1 = + 0.52 V and E2 = + 0.94 V for isorhamnetin and at E1 = + 0.63 V and E2 = + 0.87 V for tamarixetin. Beside this, rhamnetin shows another peak at E3 = + 0.04 V for irreversible reduction process. In these molecules, the two peaks on forward scan are due to the two quasi-reversible oxidation processes.
Flavanonols
In case of flavanonols, the cyclic voltammograms of each of the compounds show two peaks. For ampelopsin, the peaks are at E1 = + 0.64 V and E2 = + 1.04 V (Fig. 3d), but for taxifolin, the two peaks are merged to one broad peak at E1 = + 0.86 V. Ampelopsin also shows a small peak at E1 = + 0.90 V, whereas taxifolin shows two peaks at E1 = + 0.21 V and E2 = + 0.73 V on reverse scan. The two peaks on forward anodic scan indicate the two step oxidation of the compounds. Therefore, the oxidation process of taxifolin is quasi-reversible in nature, but for ampelopsin, it is almost irreversible in nature.
Flavanol
For flavanol, the cyclic voltammograms of each of the compounds also show two peaks (Fig. 3e): at E1 = + 0.76 V and E2 = + 1.02 V for epicatechin; at E1 = + 0.77 V and E2 = + 0.96 V for epigallocatechin and on reverse scan, epicatechin shows one prominent peak at E1 = + 0.04 V and a very small peak at E2 = + 0.65 V, whereas epigallocatechin shows only a small peak at E1 = + 0.12 V. Thus, oxidation of epicatechin is quasi-reversible and is almost irreversible for epigallocatechin.
Polyhydroxybenzophenone
In another type of hydroxybenzophenone molecules, cyclic voltammogram of 2,2′,4,4′-tetrahydroxybenzophenone does not show any peak on forward or reverse scan (Fig. 3f), whereas compound 2,4-dihydroxybenzophenone shows only one small peak at E1 = + 0.94 V on forward scan but no peak on reverse scan due to the irreversible oxidation process. The cyclic voltammogram of compound 3,4-dihydroxybenzophenone shows one peak at E1 = + 0.95 V on forward scan and at E1 = + 0.37 V on reverse scan for the nearly reversible oxidation process. Here also the absence of any significant peak in cyclic voltammogram of 2,2′,4,4′-tetrahydroxybenzophenone and a very small peak in cyclic voltammogram of 2,4-dihydroxybenzophenone indicate that both compounds have similar type of redox inactive nature. Among these three compounds, only 3,4-dihydroxybenzophenone reversibly oxidizes, whereas the other two compounds are almost redox inactive. These results once again show the importance of the catechol moiety in redox activity
Bond dissociation energy calculation
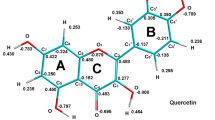
Compounds that can readily dissociate atomic hydrogen can easily reduce other substances. For polyphenolic compounds we can say that the bond dissociation energies of O–H bonds are very important to justify the mechanism of the exhibited redox property. Hydroxyl group having weak O–H bond gives away the atomic hydrogen easily; hence, it takes part in the reduction reaction more responsibly. Figure 4 shows the bond dissociation energies of the hydroxyl groups present in the chosen compounds.
Flavones
Chrysin has two hydroxyl groups at 5- and 7- position of the A-ring. In our study we have found that O–H bond dissociation energy of 5-position is greater than that of 7-position indicating removal of hydrogen atom from hydroxyl group at 5-position by homolytic bond cleavage is quite difficult than removal of atomic hydrogen from the hydroxyl group at 7-position. The radical formed from hydroxyl group of 5- position destabilizes due to field effect of double bonded oxygen atom of C- ring (Fig. S2), whereas this kind of repulsive, destabilizing field effect is absent in case of the radical formed from 7-OH. We also can see that O–H bond dissociation energy of hydroxyl group of 3′-position and 4′-position of B-ring is lower than bond dissociation energy of the other two hydroxyl groups of the A-ring. Hence, we can say that rather than A-ring, removal of hydrogen atom by homolytic bond cleavage of O–H bond from B-ring is favorable. After removal of hydrogen from the hydroxyl group at the 4′-position of B-ring, the resulting radical is stabilized by hydrogen bond formation with the hydroxyl group of the next carbon (Fig. S3) and also by resonance. It may be noted that in this case, the unpaired electron is delocalized through the B- ring and up to the ketonic oxygen of C-ring via double bond between C2 and C3 (Fig. S4). On the other hand, presence of similar radical at the 3′-position is also stabilized by the formation of hydrogen bond with neighboring hydroxyl group, but in case of resonance the unpaired electron is delocalized only within the B-ring (Fig. S5). Hence, the removal of hydrogen from the 4′-position is quite easier than that of the 3′-position. This is also reflected by the bond dissociation energy of these two O–H bonds, where we can see that bond dissociation energy of 4′-hydroxyl group is quite lower than that of 3′-hydroxyl group. Here also we can see that presence of the catechol moiety in two consecutive position of benzene ring plays a significant role to release atomic hydrogen and thus become a good reducing agent.
Flavonols
Since the arrangement of hydroxyl groups present in the A-ring of the flavonol galangin is similar with the flavone chrysin, we got quite similar pattern of O–H bond dissociation energy of hydroxyl groups present in the A-ring of galangin. We also found that enolic hydroxyl group in galangin (which was absent in case of flavones) has lower value of O–H bond dissociation energy than other hydroxyl groups present in the same molecule. It may be due to the delocalization of the unpaired electron through the double bond between C2 and C3. Quercetin also has quite similar pattern to its flavone homolog, luteolin; in addition, like galangin, it has lower value of O–H bond dissociation energy at 3-position of the C-ring. 3-, 5- and 7- hydroxyl groups of myricetin have quite similar O–H bond dissociation energy value with quercetin. Unlike quercetin, myricetin contains three hydroxyl group in the B-ring. Since 3′- and 5′-both positions of B-ring are meta- position with respect to the chromone moiety, hydroxyl radicals of these two positions have similar O–H bond dissociation energy. It is observed that 4′ hydroxyl group of myricetin has lower O–H bond dissociation energy than quercetin. It is because the radical formed by the release of atomic hydrogen from 4′-hydroxyl group was stabilized by hydrogen bonding with the hydroxyl groups of 3′- and 5′-positions, but for quercetin there was only one hydrogen bond forming hydroxyl group (Fig. S3). For gossypetin we found hydroxyl groups connected with the 3-, 3′- and 4′-positions that have quite larger values of O–H bond dissociation energy, but it is also notable that hydroxyl groups of 5-, 7- and 8-positions have quite lesser value of O–H bond dissociation energy. From this observation, it is evident that unlike other flavonols tested here, A-ring of gossypetin takes active part in reducing activity.
O-Methylated flavonols
Methylation of the oxygen atom present in the 7-position of quercetin has almost zero impact on O–H bond dissociation energy of all other hydroxyl groups present in the same molecule. So we may expect very faint effect of this methylation on the reducing behavior. On the other hand, methylation of the oxygen atom which is connected to 3′-position of B-ring is showing an impact on the O–H bond dissociation of 4′-hydroxyl group. Because in that situation radical produced by the removal of atomic hydrogen cannot be stabilized by hydrogen bonding with neighboring atoms. For similar reason O–H bond dissociation energy of 4′-hydroxyl group of isorhamnetin is quite larger than that of quercetin, whereas other hydroxyl groups have quite similar values with quercetin. Our observation for tamarixetin was quite similar to isorhamnetin, but here instead of 4′-hydroxyl group the change was observed for 3′ hydroxyl group.
Flavanonols
We mentioned earlier that radical formed from hydroxyl group of 3-position of flavonol is stabilized by the delocalization of unpaired electron through the double bond between C2 and C3 atom of the C-ring. Due to the absence of double bond between the C2 and C3 atom of C-ring, the delocalization of unpaired electron is not possible. Hence, the radical formed from hydroxyl group of the 3- position cannot be stabilized. As a result, we can see that, in case of the flavanonol taxifolin, the O–H bond dissociation energy of hydroxyl group of 3-position is quite higher than that of its flavonol homolog, quercetin. Also, we have seen that the 3′- and 4′ hydroxyl group of both luteolin and quercetin have different O–H bond dissociation value due to their different position and hence different delocalization behavior of their corresponding radical. But for taxifolin, due to the absence of the double bond between C2 and C3, delocalization of free electron of the radical corresponding to 4′-position up to the ketonic oxygen of the C-ring does not occur. Hence, we got similar O–H bond dissociation values of 3′ and 4′ hydroxyl groups of B-ring. The environment of hydroxyl groups present in 3-, 5- and 7-positions of ampelopsin are totally similar with that of taxifolin; these hydroxyl groups have quite similar O–H bond dissociation energy. Moreover, 3′- and 5′-positions are quite similar; hydroxyl groups of these positions therefore have similar values of O–H bond dissociation energy. Like B-ring of myricetin, radical formed from 4′-hydroxyl group of ampelopsin gets stability by the hydrogen bonding with hydroxyl groups of 3′- and 5′-positions.
Flavanol
Flavanols also do not contain any double bond between C2 and C3 atoms like flavanonols; hence, O–H bond dissociation energy of hydroxyl group present in flavanols are quite similar with the flavanonols. It is interesting to note that unlike all other flavonoids the O–H bond dissociation energy of hydroxyl group present at the 5-position of A-ring of flavanols is quite low. This is so because this group of compounds does not contain any ketonic oxygen at 4-position. We mentioned earlier that hydroxyl group at 5 position has larger O–H bond dissociation value due to the presence of the ketonic oxygen at 4-position. Except this ketonic oxygen, flavanonol and flavanol are similar in all other respect. Hence, the O–H bond dissociation energy of all hydroxyl groups present in the B-ring of epicatechin have similar pattern with its flavanonol homolog, taxifolin, whereas epigallocatechin shows similarity with ampelopsin.
Polyhydroxybenzophenone
Though 2,4-dihydroxybenzophenone and 2,2′,4,4′-tetrahydroxybenzophenone differ in the number of hydroxyl group but positions of their hydroxyl groups with respect to keto group are quite similar. However, 3,4-dihydroxybenzophenone has quite different arrangement. Here we have seen O–H bond dissociation energy values of hydroxyl radicals present in 3,4-dihydroxybenzophenone are smaller than that of 2,4-dihydroxybenzophenone and 2,2′,4,4′-tetrahydroxybenzophenone. So, it is clear that two consecutive hydroxyl groups show lower bond dissociation energy with respect to any other arrangements.
Conclusions
It is evident from this study, that not only the number of hydroxyl groups, also their position plays a vital role in exhibiting significant reducing property in flavonoids. Despite having the same number of hydroxyl groups, gossypetin is a better reducing agent than myricetin. According to Firuzi et al. a pair of hydroxyl group in the B-ring and the enolic group of C-ring are most important for antioxidant activity [54]. However, we found the hydroxyl group at 3-position is much more effective for exhibiting the property. More precisely, without the catechol moiety (B-ring), the sole presence of the enolic group does not contribute to the reducing power. Likewise, without the enolic group, the catechol moiety containing flavone, luteolin, showed a reducing power similar to that of 3,4-dihydroxybenzophenone, a nonflavonoid class of compound. Additionally, we found that, of the two hydroxyl groups at 3′- and 4′-position, the one at 4′- is more important toward exhibiting reducing activity. We believe that polyhydroxyphenolic compounds reduce other substances by releasing atomic hydrogen from the hydroxyl group. It is expected that during this process unpaired electron containing the radical will be formed as an intermediate. This unpaired electron of the radical can be pulled from para position through –R effect and hence may stabilize the radical intermediate which further favors the reduction process. This is by far not possible for a hydroxyl group in the meta position (3′-position). Another requirement is the hydroxyl group at 3-position of the C-ring. The presence of an enolic group involving the C2 and C3 atom of C- ring creates a positive impact on the reducing behavior of flavonoids. From the electrochemical measurement we found that the chosen compounds are showing peaks during forward scan either corresponding to a pair of hydroxyl group present in the ortho position with respect to each other or hydroxyl group present in 3-position of the flavonoids. From bond dissociation energy calculation we found that hydroxyl groups of two consecutive position of phenyl ring or enolic hydroxyl group have lower O–H bond dissociation energy implying these positions to release atomic hydrogen more easily.
It is thus confirmed that out of all types of compounds studied here, flavonols are the most efficient reducing agents and the most efficient flavonol is gossypetin with lowest EC50 as confirmed through reducing power assay. It showed three peaks during the study of the electrochemical behavior—one may be associated with hydroxyl groups at 3′-, 4′-positions of the B-ring; another may be for the hydroxyl groups at 7-, 8-positions of the A-ring and the third for the enolic group involving the C2 and C3 atoms of the C-ring. Besides, estimation of the bond dissociation energy revealed greater contribution of the hydroxyl groups at 7-, 8-positions of gossypetin over those at 3′-, 4′-positions toward reducing power. And any modifications if demanded for flavonoids, the 5-position may be a good choice because this position has very little effect toward the reducing behavior due to high value of O–H bond dissociation energy. This indicates that gossypetin has three redox active centers whereas others have one or two that can be oxidized. It is noteworthy that the value of the potential corresponding to the first peak for forward scan is the smallest with respect to the same for all other compounds we have studied. Moreover, the O–H bond dissociation energy of the hydroxyl groups of gossypetin is lower than similar hydroxyl groups present in other compounds. Gossypetin thus emerges out to be the most potent reducing agent among the compounds studied here that may play an imperative role in preventing diseases associated with oxidative stress.
References
Valko M, Leibfritz D, Moncol J et al (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Pizzino G, Irrera N, Cucinotta M et al (2017) Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. https://doi.org/10.1155/2017/8416763
Chelombitko MA (2018) Role of reactive oxygen species in inflammation: a minireview. Moscow Univ Biol Sci Bull 73:199–202. https://doi.org/10.3103/S009639251804003X
Gandhi S, Abramov AY (2012) Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev 2012:1–11. https://doi.org/10.1155/2012/428010
Masamoto K, Vazquez A, Wang P, Kim SG (2009) Brain tissue oxygen consumption and supply induced by neural activation: determined under suppressed hemodynamic response conditions in the anesthetized rat cerebral cortex. Adv Exp Med Biol 645:287–292. https://doi.org/10.1007/978-0-387-85998-9_43
Jain V, Langham MC, Wehrli FW (2010) MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab 30(9):1598–1607. https://doi.org/10.1038/jcbfm.2010.49 (Epub 2010 Apr 21)
Chakrabarti S, Munshi S, Banerjee K, Thakurta IG, Sinha M, Bagh MB (2011) Mitochondrial dysfunction during brain aging: role of oxidative stress and modulation by antioxidant supplementation. Aging Dis 2(3):242–256
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360(1):201–205. https://doi.org/10.1124/jpet.116.237503
Yasuda M (1937) Lipids analysis of the human brain. J Biochem 26(2):203–210. https://doi.org/10.1093/oxfordjournals.jbchem.a125663
O’Brien JS, Sampson EL (1965) Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res 6(4):537–544
Scandroglio F, Venkata JK, Loberto N, Prioni S, Schuchman EH, Chigorno V, Prinetti A, Sonnino S (2008) Lipid content of brain, brain membrane lipid domains, and neurons from acid sphingomyelinase deficient mice. J Neurochem 107(2):329–338. https://doi.org/10.1111/j.1471-4159.2008.05591.x
Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA et al (2012) Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem 287(4):2678–2688. https://doi.org/10.1074/jbc.M111.274142
Dawson G (2015) Measuring brain lipids. Biochim Biophys Acta Mol Cell Biol Lipids 1851(8):1026–1039. https://doi.org/10.1016/j.bbalip.2015.02.007
Torres M, Busquets X, Escribá PV (2016) Brain lipids in the pathophysiology and treatment of Alzheimer’s disease. Updat Dement. https://doi.org/10.5772/64757
Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2(12):1–13. https://doi.org/10.3389/fnagi.2010.00012
Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225(4667):1168–1170. https://doi.org/10.1126/science.6474172
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259. https://doi.org/10.1007/bf00308809
Cutler RG, Kelly J, Storie K et al (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci 101(7):2070–2075. https://doi.org/10.1073/pnas.0305799101
Cruz-Sánchez FF, Gironès X, Ortega A, Alameda F, Lafuente JV (2010) Oxidative stress in Alzheimer’s disease hippocampus: a topographical study. J Neurol Sci 299(1–2):163–167. https://doi.org/10.1016/j.jns.2010.08.029
Hirsch E, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 334(6180):345–348. https://doi.org/10.1038/334345a0
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122(8):1437–1448. https://doi.org/10.1093/brain/122.8.1437
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39(6):889–909. https://doi.org/10.1016/s0896-6273(03)00568-3
Mate JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153(1–3):83–104. https://doi.org/10.1016/s0300-483x(00)00306-1
Tokarz P, Kaarniranta K, Blasiak J (2013) Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related molcular degeneration (AMD). Biogerontology 14(5):461–482. https://doi.org/10.1007/s10522-013-9463-2
Lei XG, Zhu JH, Cheng WH, Bao Y, Ho YS, Reddi AR (2016) Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol Rev 96(1):307–364. https://doi.org/10.1152/physrev.00010.2014
Amari F, Fettouche A, Samra MA, Kefalas P, Kampranis SC, Makris AM (2008) Antioxidant small molecules confer variable protection against oxidative damage in yeast mutants. J Agric Food Chem 56(24):11740–11751. https://doi.org/10.1021/jf802829r
Lincoln KM, Gonzalez P, Richardson TE, Julovich DA, Saunders R, Simpkins JW, Green KN (2013) A potent antioxidant small molecule aimed at targeting metal-based oxidative stress in neurodegenerative disorders. Chem Commun (Camb) 49(4):2712–2714. https://doi.org/10.1039/c2cc36808k
Kohen R (1999) Skin antioxidants: their role in aging and in oxidative stress-new approaches for their evaluation. Biomed Pharmacother 53(4):181–192. https://doi.org/10.1016/S0753-3322(99)80087-0
Kohen R, Vellaichamy E, Hrbac J, Gati IO, Tirosh O (2000) Quantification of the overall reactive oxygen species scavenging capacity of biological fluids and tissues. Free Radic Biol Med 28(6):871–879. https://doi.org/10.1016/s0891-5849(00)00191-x
Tunon MJ, Garcia-Mediavilla MV, Sanchez-Campos S, Gonzalez-Gallego J (2009) Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr Drug Metab 10(3):256–271. https://doi.org/10.2174/138920009787846369
Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K (2009) Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets 8(3):229–235. https://doi.org/10.2174/187152809788681029
Serafini M, Peluso I, Raguzzini A (2010) Flavonoids as anti-inflammatory agents. Proc Nutr Soc 69(3):273–278. https://doi.org/10.1017/S002966511000162X
Arora A, Nair MG, Strasburg GM (1998) Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic Biol Med 24(9):1355–1363. https://doi.org/10.1016/S0891-5849(97)00458-9
Dugas AJ, Castañeda-Acosta J, Bonin GC et al (2000) Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: structure-activity relationships. J Nat Prod 63:327–331. https://doi.org/10.1021/np990352n
Firuzi O, Mladênka P, Petrucci R, Mladênka P, Marrosu G, Saso L (2004) Hypochlorite scavenging activity of flavonoids. J Pharm Pharmacol 56(6):801–807. https://doi.org/10.1211/0022357023556
Frontana CE, González I (2007) Structural factors affecting the reactivity of the natural a-Hydroxy Benzoquinones. An electrochemical and ESR Study 3(29):13–23. https://doi.org/10.1149/1.2753287
Zhang D, Chu L, Liu Y, Wang A, Ji B, Wu W et al (2011) Analysis of the antioxidant capacities of flavonoids under different spectrophotometric assays using cyclic voltammetry and density functional theory. J Agric Food Chem 59(18):10277–10285. https://doi.org/10.1021/jf201773q
Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem 43(1):27–32. https://doi.org/10.1021/jf00049a007
Graham HD (1992) Stabilization of the Prussian blue color in the determination of polyphenols. J Agric Food Chem 40:801–805. https://doi.org/10.1021/jf00017a018x
Nóbrega JA, Lopes GS (1996) Flow-injection spectrophotometric determination of ascorbic acid in pharmaceutical products with the Prussian blue reaction. Talanta 43:971–976. https://doi.org/10.1016/0039-9140(95)01830-1
Canabady-Rochelle LLS, Harscoat-Schiavo C, Kessler V, Aymes A, Fournier F, Girardet JM (2015) Determination of reducing power and metal chelating ability of antioxidant peptides: revisited methods. Food Chem 183:129–135. https://doi.org/10.1016/j.foodchem.2015.02.147
Hocquet A, Langgård M (1998) An evaluation of the MM+ force field. J Mol Model 4(3):94–112. https://doi.org/10.1007/s008940050128
HyperChem(TM) Professional 8.0, Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. (2009) Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford CT. Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136(3B):B864–B871. https://doi.org/10.1103/PhysRev.136.B864
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis Can. J Phys 58:1200–1211
Lee C, Hill C, Carolina N (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789. https://doi.org/10.1103/PhysRevB.37.785
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98(45):11623–11627. https://doi.org/10.1021/j100096a001
Binkley JS, Pople JA, Hehre WJ (1980) Self-consistent molecular 21. orbital methods small split valence basis sets for first-row elements. J Am Chem Soc 102(3):939–947. https://doi.org/10.1021/ja00523a008
Curtiss LA, Redfern PC, Rassolov V, Kedziora G, Pople JA (2001) Extension of Gaussian-3 theory to molecules containing third-row atoms K, Ca. Ga-Kr J Chem Phys 114(21):9287–9295. https://doi.org/10.1063/1.1366337
Allouche AR (2011) Gabedit—A graphical user interface for computational chemistry softwares. J Comput Chem 32:174–182. https://doi.org/10.1002/jcc.216
Parkinson CJ, Mayer PM, Radom L (1999) An assessment of theoretical procedures for the calculation of reliable radical stabilization energies. J Chem Soc Perkin Trans 2(11):2305–2313. https://doi.org/10.1039/A905476F
Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L (2005) Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta 1721(1–3):174–184. https://doi.org/10.1016/j.bbagen.2004.11.001
Acknowledgements
MSD acknowledges Swami Vivekananda Merit-cum-means Scholarship scheme of Government of West Bengal for his fellowship. The authors gratefully acknowledge Dr. Subinit Roy of Saha Institute of Nuclear Physics for allowing MSD to use ORIGIN 8.0 and Prof. Ansuman Lahiri of Department of Biophysics, Molecular Biology & Bioinformatics of University of Calcutta for providing the computational facility of his laboratory and use the Gaussian 09 software.
Funding
This work was funded by Department of Science and Technology, Government of West Bengal, West Bengal, India. [Grant No. 159(Sanc.)/ST/P/S&T/2G-6/2008].
Author information
Authors and Affiliations
Contributions
MSD has determined the reducing power and calculated the bond dissociation energy of the compounds under the supervision of SB, while PM has carried out the electrochemical study with cyclic voltammetry under the supervision of AG. MSD and SB has written the article and given the final form.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dutta, M.S., Mahapatra, P., Ghosh, A. et al. Estimation of the reducing power and electrochemical behavior of few flavonoids and polyhydroxybenzophenones substantiated by bond dissociation energy: a comparative analysis. Mol Divers 26, 1101–1113 (2022). https://doi.org/10.1007/s11030-021-10232-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10232-4