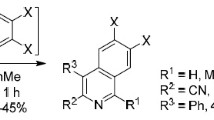
Abstract
A convenient protocol for the synthesis of 5,6-dihydropyrrolo[2,1-a]isoquinolines with various electron-withdrawing substituents at C-2 atom is described. This approach is based on the two-component domino reaction of 1-aroyl-3,4-dihydroisoquinolines with α,β-unsaturated ketones, nitroalkenes and acrylonitrile. Depending on the selected substrates, the reaction was performed in TFE under reflux or under microwave irradiation. Only for the two examples, a transition metal catalyst was used.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyrrolo[2,1-a]isoquinoline ring represents a key structural fragment of different alkaloids such as Erythrina-type alkaloids or compounds isolated from Carduus crispus L. The alkaloids of the Erythrina L. genus (Fabaceae) belong to the one of the most important series of pyrroloisoquinolines exhibiting various pharmacological properties including hypotensive, sedative, anticonvulsive, curare-like and anti-HIV-1 properties [1,2,3,4,5]. Also, the Erythrina extracts and isolated alkaloids demonstrate anxiolytic properties [6,7,8,9,10,11,12,13]. Moreover, Erythrina alkaloids activate GABAA receptors [14] and selectively inhibit nicotinic acetylcholine receptors, especially the receptors of α4β2 subtype [6, 15, 16]. The alkaloids Crispine A ( +) and Crispine B isolated from Carduus crispus L. have been tested for cytotoxic activity on the human cancer cell line. Crispine B showed significant cytotoxicity at micromolar levels [17]. In 2005, Jia et al. have discovered the antitumor activity of Crispine B on cancer cell lines HO-8901 (human ovarian neoplasm) (Fig. 1) [18].
The synthetic methods for the preparation of pyrrolo[2,1-a]isoquinolines are mainly based on two strategies: annulation of the pyrrole ring to the isoquinoline moiety or annulation of the isoquinoline core to the pyrrole or lactam cycle. When the formation of the pyrrolo[2,1-a]isoquinoline moiety proceeds via annulation on the pyrrole core, it is necessary to use palladium-catalyzed reactions, in particular the Mizoroki–Heck coupling reaction [19, 20]. For N-alkylated pyrroles, the intramolecular Heck reaction using Pd(PPh3)4 as a catalyst with sodium acetate as a base or Pd(PPh3)2Cl2 and Ph3P as a catalyst with potassium carbonate as a base allows to obtain pyrroloisoquinolines with good yields [21, 22]. There are also several effective approaches to the synthesis of the pyrrolo[2,1-a]isoquinolines’ frameworks based on the derivatives of butyrolactams [23,24,25,26].
The assembly of the pyrrolo[2,1-a]isoquinoline moieties based on the annulation of the pyrrole fragment to the isoquinoline core is a more common approach. One of the convenient methods includes the use of Morita–Baylis–Hillman carbonates with formal [3 + 2] cycloaddition reaction [27,28,29] that allows obtaining the biologically active ( ±)-Crispine A [27]. Another common method is the intramolecular cyclization of 1-propargyl and 1-allenyl-substituted tetrahydroisoquinolines. The formation of the pyrrole ring proceeds in the presence of silver acetate, and this approach was also used in the synthesis of the alkaloid Crispine A [30]. Intramolecular reactions of 1- and 1,2-functionalized tetrahydro-, 1,2- and 3,4-dihydroisoquinolines are often used for annulation of the pyrrole core [31,32,33,34,35,36]. Recently, our group has demonstrated the preparation of functionally substituted on the pyrrole ring pyrrolo[2,1-a]isoquinolines based on domino reactions of 3,4-dihydro-1-aroylisoquinolines with electron-deficient alkynes, α,β-unsaturated aldehydes and cross-conjugated vinyl ethynyl ketones [37,38,39,40].
Thus, continuing our research of the domino reactions with 3,4-dihydro-1-aroylisoquinolines, we tested conjugated ketones, nitroalkenes and nitriles as substrates. To the best of knowledge, the construction of annulated pyrrole cycle based on the reaction of iminoketones with the conjugated nitroalkenes or nitriles has not been previously described in the scientific literature.
Results and discussion
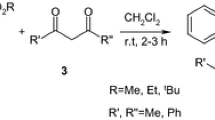
Starting benzoyl-, 4-chloro-, 4-fluoro-, 3,4-diethoxybenzoyl- isoquinolines 1a-d substituted at the C-1 atom were synthesized according to the published procedures [41, 42]. α,β-Unsaturated ketones (methyl vinyl ketone 2a, benzalacetone 2b, furfural acetone 2c, chalcones 2d,e, dibenzalacetones 2f,g), nitroalkenes 2h,i, acrylonitrile 2j, crotononitrile 2k and cinnamoyl nitrile 2l were used in the synthesis of pyrrolo[2,1-a]isoquinolines 3a-y (Scheme 1). Following the protocol developed by us for α,β-unsaturated aldehydes [39], initially, we screened the reaction of isoquinolines 1a-d with α,β-unsaturated ketones in trifluoroethanol under microwave activation. The interaction of 1-aroyl-3,4-dihydroisoquinolines 1a-d with methyl vinyl ketone 2a for 15 min at 150 °C gave the desired pyrrolo[2,1-a]isoquinolines 3a-d in moderate to good isolated yields (40–84% after the recrystallization from EtOAc–hexane mixture). To establish the effect of substituents on the course of the domino process, the isoquinolines 1a-d were reacted with benzal- (2b) and furfural-acetones (2c). The presence of the electron-donating aromatic substituent in the substrate 2b complicates the progress of the reaction in comparison with a methyl vinyl ketone. Wherefore the reaction was carried out under microwave activation at 160 °C for 120 min. Unfortunately, compounds 3e,f were isolated in the mixture with dehydrogenated 1b,d in a 2:1 ratio (3e,f; 47–50% yield). Interestingly, despite the harsh reaction conditions (160 °C), the desired pyrrolo[2,1-a]isoquinolines 3g,h, which contain a sensitive furane ring, were obtained in moderate yields (42% and 56%). It was also found that isoquinolines 1a,c do not react under reflux or under microwave activation with 2b,c yielding only the products of 1a,c aromatization.
Next, the reactions of 1-aroylisoquinolines with chalcones 2d,e and diarylideneacetones 2f,g were studied. We established that 1-aroylisoquinolines react differently with 2d-g. The reaction of 1-aroylisoquinolines 1b,d with chalcones 2d,e under microwave activation at 160 °C for a longer time (40–150 min) yields the expected pyrrolo[2,1-a]isoquinolines 3i-l in low to excellent yields (17–75%). The decreased yields of the desired product for chalcone 2e (3k,l were isolated in 17 and 20% yields, respectively) can be explained by the effect of the electron-donating methoxy group in the phenyl substituent, simultaneously increasing the time of reaction. To increase the yields of 3k,l, the reaction conditions were further optimized (see Schemes S1, S2 and Table S1 in the Supporting Information). As a result, we carried out reactions with 10 mol% AgOAc under microwave activation at 180 °C to obtain pyrrolo[2,1-a]isoquinolines 3k,l in 47% and 60%, respectively. In contrast, diarylideneacetones 2f,g react smoothly with 1-aroylisoquinolines 1b,d and with good yields. Apparently, the carbonyl group in these reagents responds to a much lesser extent to the presence of the aryl substituent.
The reactivity of nitroalkenes 2h,i with 1-aroylisoquinolines was studied on the example of drotaveraldine 1d and phenyl nitroalkene 2h in various alcohols. The use of microwave radiation led to low yields of the corresponding products. For EtOH or MeOH as solvents at reflux for 7–10 days, the yield of 3s was slightly lower than in TFE (2–3 days). Therefore, all reactions of 1-aroyl-3,4-dihydroisoquinolines 1b-d with nitroalkenes 2h,i were subsequently carried out only in trifluoroethanol. As a result, pyrrolo[2,1-a]isoquinolines 3q-v were obtained in 42–66% yields. Contrary to our expectations, the presence of methoxy donor group at the meta position in phenyl ring of nitroalkene did not have any effect on the yield as for the compounds 3k,l.
Then, conjugated nitriles have been screened in the reactions with 1-aroyl-3,4-dihydroisoquinolines. The reactions of isoquinolines 1b-d with acrylonitrile 2j were carried out in trifluoroethanol for 2–3 days at reflux, whereas the reactions under microwave activation led to a decreased yield of target compounds. Pyrrolo[2,1-a]isoquinolines 3w-y were formed in moderate to good yields (40–70%). But in comparison with nitro derivatives, the reaction with unsaturated nitriles has significant limitations. The reaction of 1a-d with crotononitrile 2k or with cinnamoyl nitrile 2l was tested not only under the above-described conditions, but also at reflux. Unfortunately, the desired products have not been detected.
The structures of the obtained pyrrolo[2,1-a]isoquinolines 3a-y were confirmed by 1H, 13C NMR and mass spectrometry.
The possible pathway for the reactions with conjugated ketones, nitroalkenes and acrylonitrile is proposed as illustrated in Scheme 2. Initially, the Michael-type zwitterion A is formed, whereupon the carbanion attacks the carbonyl group with subsequent five-membered ring closure (intermediate B). Next, the proton transfer occurs to give C, further dehydration of which leads to the ylide D, giving ultimately the products 3a-y.
Conclusion
Various 5,6-dihydropyrrolo[2,1-a]isoquinolines containing keto-, enone-, nitro-, and nitrile electron-withdrawing groups at C2-position can be obtained in moderate to good yields by a convenient protocol via a domino reaction of 1-aroyl-3,4-dihydroisoquinolines with conjugated alkenes. As far as we are aware, the synthesis of the annulated pyrrole ring via the interaction of the iminoketone fragment with nitroalkenes or acrylonitrile has never previously been reported. Selected α,β-unsaturated ketones contain more challenging groups than in our previous works. However, only in the case of reaction with methoxy chalcone derivative catalyst was used.
References
Boekelheide V (1960) Chapter 11 The Erythrina alkaloids. Alkaloids Chem Physiol 7:201–227. https://doi.org/10.1016/S1876-0813(08)60005-6
Hill RK (1967) Chapter 12 The Erythrina alkaloids. Alkaloids Chem Physiol 9:483–515. https://doi.org/10.1016/S1876-0813(08)60208-0
Dyke SF, Quessy SN (1981) Chapter 1 Erythrina and related alkaloids. Alkaloids Chem Physiol 18:1–98. https://doi.org/10.1016/S1876-0813(08)60236-5
Chawala AS, Jackson AH (1984) Erthrina and related alkaloids. Nat Prod Rep 1:371–373. https://doi.org/10.1016/S1876-0813(08)60208-0
Mohammed MMD, Ibrahim NA, Awad NE, Matloub AA, Mohamed-Ali AG, Barakat EE, Mohamed AE, Colla PL (2012) Anti-HIV-1 and cytotoxicity of the alkaloids of Erythrina abyssinica Lam. growing in Sudan. Nat Prod Res 26:1565–1575. https://doi.org/10.1080/14786419.2011.573791
Decker MW, Anderson DJ, Brioni JD, Donnelly-Roberts DL, Kang CH, O'Neil AB, Piattoni-Kaplan M, Swanson S, Sullivan JP (1995) Erysodine, a competitive antagonist at neuronal nicotinic acetylcholine receptors. Eur J Pharmacol 280:79–89. https://doi.org/10.1016/0014-2999(95)00191-M
Garín-Aguilar ME, Luna JE, Soto-Hernández M, Valencia del Toro G, Vázquez MM (2000) Effect of crude extracts of Erythrina americana Mill. on aggressive behavior in rats. J Ethnopharmacol 69:189–196. https://doi.org/10.1016/S0378-8741(99)00121-X
Garcia-Mateos R, Garín Aguilar ME, Soto-Hernández M, Martínez-Vázquez M (2000) Effect of beta-erythroidine and beta-dihydroerythroidine from Erythrina americana on rats aggressive behaviour. Pharm Pharmacol Lett 10:34–37 https://www.researchgate.net/publication/293212601_Effect_of_b-erythroidine_and_b-dihydroerythroidine_from_Erythrina_americana_on_rats_aggressive_behaviour. Accessed 3 June 2020
Onusic GM, Nogueira RL, Pereira AM, Flausino OA Jr, Viana MB (2003) Effects of chronic treatment with a water–alcohol extract from Erythrina mulungu on anxiety-related responses in rats. Biol Pharm Bull 26:1538–1542. https://doi.org/10.1248/bpb.26.1538
Flausino OA Jr, Pereira AM, Bolzani VS, Nunes-de-Souza RL (2007) Effects of Erythrinian alkaloids isolated from Erythrina mulungu (Papilionaceae) in mice submitted to animal models of anxiety. Biol Pharm Bull 30:375–378. https://doi.org/10.1248/bpb.30.375
Santos Rosa D, Faggion SA, Gavin AS, Anderson de Souza M, Fachim HA, Ferreira dos Santos W, Soares Pereira AM, Siqueira Cunha AO, Beleboni RO (2012) Erysothrine, an alkaloid extracted from flowers of Erythrina mulungu Mart. ex Benth: evaluating its anticonvulsant and anxiolytic potential. Epilepsy Behav 23:205–212. https://doi.org/10.1016/j.yebeh.2012.01.003
Dias SA, Neves AEO, Ferraz ABF, Picada JN, Pereira P (2013) Neuropharmacological and genotoxic evaluation of ethanol extract from Erythrina falcata leaves, a plant used in Brazilian folk medicine. Rev Bras Farmacogn 23:335–341. https://doi.org/10.1590/S0102-695X2013005000015
Bonilla JA, Santa Maria AM, Toloza G, Espinoza MP, Avalos JN, Nuñez MJ, Moreno M (2014) Sedative, anxiolytic and toxicological effect of an aqueous extract from Erythrina berteroana (pito) flowers in mice. Rev Cuba Plantas Med 19:383–398. https://www.revplantasmedicinales.sld.cu/index.php/pla/article/view/91/106. Accessed 3 June 2020
Carvalho ACCS, Almeida DS, Melo MG, Cavalcanti SC, Marçal RM (2009) Evidence of the mechanism of action of Erythrina velutina Willd (Fabaceae) leaves aqueous extract. J Ethnopharmacol 122:374–378. https://doi.org/10.1016/j.jep.2008.12.019
Iturriaga-Vásquez P, Carbone A, García-Beltrán O, Livingstone PD, Biggin PC, Cassels BK, Wonnacott S, Zapata-Torres G, Bermudez I (2010) Molecular determinants for competitive inhibition of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol 78:366–375. https://doi.org/10.1124/mol.110.065490
Setti-Perdigao P, Serrano MA, Flausino OA Jr, Bolzani VS, Guimarães MZ, Castro NG (2013) Erythrina mulungu alkaloids are potent inhibitors of neuronal nicotinic receptor currents in mammalian cells. PLoS ONE 8:e82726. https://doi.org/10.1371/journal.pone.0082726
Zhang Q, Tu G, Zhao Y, Cheng T (2002) Novel bioactive isoquinoline alkaloids from Carduus crispus. Tetrahedron 58:6795–6798. https://doi.org/10.1016/S0040-4020(02)00792-5
Xie WD, Li PL, Jia ZJ (2005) A new flavone glycoside and other constituents from Carduus crispus. Pharmazie 60:233–236. https://www.ingentaconnect.com/contentone/govi/pharmaz/2005/00000060/00000003/art00016. Accessed 3 June 2020
Coya E, Sotomayor N, Lete E (2014) Intramolecular direct arylation and Heck reactions in the formation of medium-sized rings: selective synthesis of fused indolizine, pyrroloazepine and pyrroloazocine systems. Adv Synth Catal 356:1853–1865. https://doi.org/10.1002/adsc.201400075
Azcargorta AR, Coya E, Barbolla I, Lete E, Sotomayor N (2016) Generation of tertiary and quaternary stereocentres through palladium-catalysed intramolecular Heck-type reactions for the stereocontrolled synthesis of pyrrolo[1,2-b]isoquinolines. Eur J Org Chem 2016:2054–2063. https://doi.org/10.1002/ejoc.201600082
Olsen CA, Parera N, Albericio F, Álvareza M (2005) 5,6-Dihydropyrrolo[2,1-b]isoquinolines as scaffolds for synthesis of lamellarin analogues. Tetrahedron Lett 46:2041–2044. https://doi.org/10.1016/j.tetlet.2005.01.145
Chávez-Santos RM, Reyes-Gutiérrez PE, Torres-Ochoa RO, Ramírez-Apan MT (2017) Martínez R (2017) 5,6-Dihydropyrrolo[2,1-a]isoquinolines as alternative of new drugs with cytotoxic activity. Chem Pharm Bull 65:973–981. https://doi.org/10.1248/cpb.c17-00409
Jebalia K, Planchata A, Amri H, Mathé-Allainmat M, Lebreton J (2016) A short and efficient approach to pyrrolo[2,1-a]isoquinoline and pyrrolo[2,1-a]benzazepine derivatives. Synthesis 48:1502–1517. https://doi.org/10.1055/s-0035-1561398
Selvakumar J, Mangalaraj S, Achari KMM, Mukund K, Ramanathan CR (2017) Triflic acid mediated cyclization of unsymmetrical N-phenethyl- and N-(3-indolylethyl)succinimides: regio- and diastereoselective synthesis of substituted pyrroloisoquinolinones and indolizinoindolones. Synthesis 49:1053–1064. https://doi.org/10.1055/s-0036-1588639
Stepakov AV, Ledovskaya MS, Boitsov VM, Molchanov AP, Kostikov RR, Gurzhiy VV, Starova GL (2012) Synthesis of isoxazolopyrroloisoquinolines by intramolecular cyclizations of 5-(2-arylethyl)-6-hydroxytetrahydro-4H-pyrrolo[3,4-d]isoxazol-4-ones. Tetrahedron Lett 53:5414–5417. https://doi.org/10.1016/j.tetlet.2012.07.114
Lenshmidt LV, Ledovskaya MS, Larina AG, Filatov AS, Molchanov AP, Kostikov RR, Stepakov AV (2018) Synthesis of isoxazolopyrrolo[2,1-a]isoquinoline, isoxazolo[5',4': 1,2]indolizino[8,7-b]indole, and isoxazolo[5,4-a]thieno[2,3-g]indolizine derivatives by intramolecular cyclization of hydroxylactams constituting a fragment of the pyrroloisoxazole system. Russ J Org Chem 54:112–125. https://doi.org/10.1134/S1070428018010116
Basavaiah D, Lingaiah B, Reddy GC, Sahu BH (2016) Baylis-Hillman acetates in synthesis: copper(I)/tert -butyl hydroperoxide promoted one-pot oxidative intramolecular cyclization protocol for the preparation of pyrrole-fused compounds and the formal synthesis of (±)-Crispine A. Eur J Org Chem 2016:2398–2403. https://doi.org/10.1002/ejoc.201600384
Tang X, Yang MC, Ye C, Liu L, Zhou HL, Jiang XJ, You XL, Han B, Cui HL (2017) Catalyst-free [3+2] cyclization of imines and Morita–Baylis–Hillman carbonates: a general route to tetrahydropyrrolo[2,1-a]isoquinolines and dihydropyrrolo[2,1-a]isoquinolines. Org Chem Front 4:2128–2133. https://doi.org/10.1039/C7QO00492C
Cui HL, Jiang L, Liu S (2019) Direct synthesis of dihydropyrrolo[2,1-a]isoquinolines through FeCl3 promoted oxidative aromatization. Adv Synth Cat 361:4772–4780. https://doi.org/10.1002/adsc.201900756
Agarwal S, Kataeva O, Schmidt U, Knölker HJ (2013) Silver(i)-promoted oxidative cyclisation to pyrrolo[2,1-a]isoquinolines and application to the synthesis of (±)-crispine A. RSC Adv 3:1089–1096. https://doi.org/10.1039/C2RA22823H
Punirun T, Soorukram D, Kuhakarn C, Reutrakul V, Pohmakotr M (2018) Oxidative difluoromethylation of tetrahydroisoquinolines using TMSCF2SPh: synthesis of fluorinated pyrrolo[2,1-a]isoquinolines and benzo[a]quinolizidines. J Org Chem 83:765–782. https://doi.org/10.1021/acs.joc.7b02783
Chen J, Xu Q, Liao W (2014) Metal-free intramolecular carbocyanation of alkenes: catalytic stereoselective construction of pyrrolo[2,1-a]isoquinolines with multiple substituents. Chem Eur J 20:13876–13880. https://doi.org/10.1002/chem.201404217
Qin TY, Cheng L, Ho-Chol J, Zhang SXA, Liao WW (2016) Facile synthesis of multifunctional pyrrolo[2,1-a]isoquinolin-3(2H)-ones via sulfa-Michael-triggered one-pot reactions. Synthesis 48:357–364. https://doi.org/10.1055/s-0035-1560974
Imbri D, Tauber J, Opatz T (2013) A high-yielding modular access to the lamellarins: synthesis of lamellarin G trimethyl ether, lamellarin η and dihydrolamellarin η. Chem Eur J 19:15080–15083. https://doi.org/10.1002/chem.201303563
Mandrekar KS, Kadam HK, Tilve SG (2018) Domino Bischler-Napieralski – Michael reaction and oxidation – new route to coumarin-pyrrole-isoquinoline fused pentacycles. Eur J Org Chem 2018:6665–6670. https://doi.org/10.1002/ejoc.201801244
Vyasaamudri S, Yang DY (2018) Application of differential reactivity towards synthesis of lamellarin and 8-oxoprotoberberine derivatives: Study of photochemical properties of aryl-substituted benzofuran-8-oxoprotoberberines. Tetrahedron 74:1092–1100. https://doi.org/10.1016/j.tet.2018.01.042
Voskressensky LG, Borisova TN, Matveeva MD, Khrustalev VN, Aksenov AV, Vartanova AE, Varlamov AV (2016) A novel multi-component approach to the synthesis of pyrrolo[2,1-a] isoquinoline derivatives. RSC Adv 6:74068–74071. https://doi.org/10.1039/C6RA15810B
Voskressensky LG, Borisova TN, Matveeva MD, Khrustalev VN, Titov AA, Aksenov AV, Dyachenko SV, Varlamov AV (2017) A facile synthesis of 1-oxo-pyrrolo[2,1-a]isoquinolines. Tetrahedron Lett 58:877–879. https://doi.org/10.1016/j.tetlet.2017.01.061
Matveeva MD, Borisova TN, Titov AA, Anikina LV, Dyachenko SV, Astakhov GS, Varlamov AV, Voskressensky LG (2017) Domino reactions of 1-aroyl-3,4-dihydroisoquinolines with α, β-unsaturated aldehydes. Synthesis 49:5251–5257. https://doi.org/10.1055/s-0036-1588486
Matveeva M, Golovanov A, Borisova T, Titov A, Varlamov A, Shaabani A, Obydennik A, Voskressensky L (2018) Domino reactions of vinyl ethynyl ketones with 1-aryl-3,4-dihydroisoquinolines — Search for selectivity. Mol Cat 461:67–72. https://doi.org/10.1016/j.mcat.2018.09.020
Cho SD, Kweon DH, Kang YJ, Lee SGm Lee WS, Yoon YJ, (1999) Synthesis of 6,7-dimethoxy-1-halobenzyl-1,2,3,4-tetrahydroisoquinolines. J Heterocycl Chem 36:1151–1156. https://doi.org/10.1002/jhet.5570360507
Awuah E, Capretta A (2010) Strategies and synthetic methods directed toward the preparation of libraries of substituted isoquinolines. J Org Chem 75:5627–5634. https://doi.org/10.1021/jo100980p
Acknowledgements
This work has been supported by the «RUDN University Program 5-100». The elemental analysis was performed using the equipment of the Center for Molecular Composition Studies at the INEOS RAS under support of the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Astakhov, G.S., Shigaev, R.R., Borisova, T.N. et al. Facile synthesis of pyrrolo[2,1-a]isoquinolines by domino reaction of 1-aroyl-3,4-dihydroisoquinolines with conjugated ketones, nitroalkenes and nitriles. Mol Divers 25, 2441–2446 (2021). https://doi.org/10.1007/s11030-020-10146-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10146-7