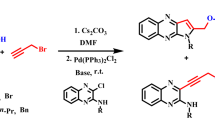
Abstract
The reactions of several 2-chloroquinoline-3-carboxylate esters with propargyl alcohol and a secondary amine in the presence of palladium catalyst leads to the formation of new alkyl 1-amino substituted pyrrolo[1,2-a]quinoline-4-carboxylate derivatives. This one-pot process, carried out in the absence of any copper salt, provides an efficient method for the synthesis of functionalized pyrrolo[1,2-a]quinolines in good-to-high yields.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Intoduction
Among all kinds of transition metal-catalyzed coupling reactions, the Sonogashira cross-coupling reaction [1] provides a powerful route to the C(sp)–C(\(\hbox {sp}^{2}\)) bond formation. It is a useful method for the synthesis of a variety of compounds including arylalkynes and conjugated enynes [2], heterocycles [3, 4], several natural products, pharmaceuticals [5], and oligomers and polymers [6].
In general, the usual catalytic system for a Sonogashira coupling reaction is made up of palladium–phosphine complexes with copper(I) iodide in the presence of an excess or a stoichiometric amount of a base [7, 8]. In the recent years, although a significant modification has been reported for the Sonogashira coupling procedure, efficient copper-free reactions have been developed to prevent the oxidative homocoupling reaction of acetylenes (Glaser-type reaction) [9, 10]. The byproducts of the homocoupling reactions are usually difficult to separate from the desired products, and the copper acetylide formed in the reaction is a potentially explosive reagent [11]. Although copper-free Sonogashira coupling reactions have been widely investigated [12–14], few examples with aryl chlorides [15–17] and heteroaryl chlorides such as pyridyl chlorides [18] and 2-chloroquinolines [19] with terminal alkynes have been reported.
Sonogashira coupling/cyclization reaction of alkyl 2-chloroquinoline-3-carboxylate with propargyl alcohol and a secondary amine. \(^{\mathrm{a}}\)Reaction conditions: 2a–c (1 mmol), 3 (1.25 mmol), secondary amine (3 mmol), \(\hbox {Et}_{3}\hbox {N}\) (3 mmol), Pd(\(\hbox {PPh}_{3})_{2}\hbox {Cl}_{2}\) (0.05 mmol), distilled \(\hbox {CH}_{3}\hbox {CN}\) (5 mL), 80 \({^{\circ }}\)C, 18 h, argon atmosphere
Quinolines are an important class of nitrogen-containing heterocyclic compounds, due to their wide occurrence in natural products [20] and their interesting biological properties [21]. Pyrrolo[1,2-a]quinolines, found extensively in nature in alkaloids such as gephyrotoxin [22, 23], are natural alkaloids that have been the subject of many investigations. They also show a wide range of pharmaceutical activities, such as anti-inflammatory [24], anti-viral [25], analgesic [26], and antitumor [27] activities.
In view of their significant biological importances, many efforts have been dedicated to the development of new synthetic methodologies for the preparation of pyrrolo[1,2-a]quinolines [28–30]. Synthesis of these compounds based on the C–C bond formation synthetic strategies via transition metal-catalyzed coupling reactions has been little explored. In continuation as part of our research [31–34] on the Pd-catalyzed reaction of acetylenes leading to heterocyclic compounds of biological significance, we decided to develop a modified Sonogashira coupling reaction/heteroannulation for the one-pot synthesis of new pyrrolo[1,2-a]quinoline esters. In this paper, a simple, direct, and convenient multicomponent approach to the synthesis of alkyl 1-aminopyrrolo[1,2-a]quinoline-4-carboxylate derivatives from readily available materials is presented (Scheme 1).
To introduce diversity to our pyrrolo[1,2-a]quinoline derivatives, our retrosynthetic strategy chose the use of alkyl 2-chloroquinoline-3-carboxylate esters, propargyl alcohol, and a secondary amine as starting materials (Scheme 2). The Sonogashira cross-coupling reaction is the key step in this synthesis.
The starting materials 2a–c were prepared from commercially available acetanilide in several steps via a Vilsmeier-Haack reaction [35], followed by substituent oxidation/esterification in alcohol in the presence of iodine [36] (Scheme 3; Table 1).
Initially, we chose the reaction of methyl 2-chloroquinoline-3-carboxylate ester (2a) with propargyl alcohol (3) and morpholine as the model reaction to study the copper-free Sonogashira coupling and optimize the reaction conditions. In order to find the optimized conditions for the synthesis of methyl 1-morpholinopyrrolo[1,2-a]quinoline-4-carboxylate (5a), the effects of various reaction parameters were studied (Table 2). We studied two catalytic systems: \(\hbox {Pd}(\hbox {PPh}_{3})_{2}\hbox {Cl}_{2 }\)and Pd/C, in different solvents and in the presence of several bases such as \(\hbox {Et}_{3}\hbox {N}\), \(\hbox {K}_{2}\hbox {CO}_{3}\), and DIPEA. We found that Pd(\(\hbox {PPh}_{3})_{2}\hbox {Cl}_{2}\) was the optimal catalyst based on product yields. Amongst the solvents tested, acetonitrile was found to be the most suitable one, and \(\hbox {Et}_{3}\hbox {N}\) was the optimal base to use.
After optimizing the reaction conditions, in order to explore the scope and generality of this protocol, we applied the reaction on three series of alkyl 2-chloroquinoline-3-carboxylate esters (2a–c) in the presence of propargyl alcohol (3) and a number of secondary amines (4), which afforded the corresponding products 5a–g in good-to-high yields (Table 3).
The structural assignments of compounds 5a–g were based on spectroscopic data and mass analysis. The \(^{1}\hbox {H}\) NMR spectrum for methyl 1-morpholinopyrrolo[1,2-a]quinoline-4-carboxylate (5a) showed a doublet at \(\delta \) 9.47, which is characteristic of an aromatic proton at position 9 of this heterocyclic system; it was deshielded by the diamagnetic pyrrole ring system. A singlet at \(\delta \) 7.78 is characteristic of the proton at position 5, and the other three aromatic protons in the quinoline ring appeared at \(\delta \) 7.32–7.75. The two doublets at \(\delta \) 7.23 and \(\delta \) 6.54 were assigned to the two protons at positions 2 and 3 in the fused pyrrole ring. In the aliphatic region, the 11 protons of the morpholine and methoxy substituents of this heterocyclic system appeared at \(\delta \) 2.96–4.04.
Mechanistically, the key step of the process is the Sonogashira coupling reaction, catalyzed by a Pd(II) complex together with CuI as co-catalyst. Copper(I) iodide reacts with the terminal alkyne to yield a copper(I) acetylide which acts as an activated species in the transmetalation step [7]. In the copper-free Sonogashira coupling reaction, the initial oxidative addition is followed by alkyne coordination, and completed by subsequent deprotonation and reductive elimination [37].
A plausible multistep mechanism for the copper-free Sonogashira coupling and heteroannulation reactions is proposed (Scheme 4). First, a copper-free Sonogashira coupling takes place by a Pd(0)-catalyzed reaction, followed by isomerization to the allene intermediate A, enone aldehyde B, and iminium ion C, cyclization to the fused ring systemD, and finally, a base-induced aromatization to afford the product.
Conclusions
In summary, we have developed an efficient and successful copper-free palladium-catalyzed protocol for the synthesis of new 1-amino substituted pyrrolo[1,2-a]quinoline-4-carboxylate esters from alkyl 2-chloroquinoline-3-carboxylates and propargyl alcohol in the presence of secondary amines adopting a one-pot process.
Experimental
Palladium(II) chloride, triphenylphosphine, and propargyl alcohol were purchased from Sigma-Aldrich chemical company, and used without further purification. Acetanilide, phosphorylchlorid, N,N-dimethylformamide, triethylamine, secondry amines, thin-layer chromatography plate, silica gel (particle size, 100–200 mesh), and all the solvents used for the reactions were purchased from Merck. NMR spectra were recorded on a Bruker 300 (300 MHz \(^{1}\hbox {H}\), 75 MHz \(^{13}\hbox {C}\)) spectrometer. \(^{1}\hbox {H}\) NMR signals were reported relative to \(\hbox {Me}_{4}\hbox {Si}\) (\(\delta \) 0.0) or residual \(\hbox {CHCl}_{3}\) (\(\delta \) 7.26). \(^{13}\hbox {C}\) NMR signals were reported relative to \(\hbox {CDCl}_{3}\) (\(\delta \) 77.16). Multiplicities were described using the following abbreviations: s = singlet, d = doublet, t = triplet, and m = multiplet. IR spectra were measured on a Shimadzu IR-435 grating spectrophotometer. Mass spectra were recorded on a 5975C spectrometer (manufactured in Agilent Technologies Company).
Synthesis of 2-chloroquinoline-3-carbaldehyde (1)
To a solution of acetanilide (5 mmol, 0.68 g) in dry DMF (15 mmol, 1.09 g) at 0–5 \(^{\circ }\hbox {C}\), under stirring, phosphoryl chloride (60 mmol, 9.20 g) was added dropwise, and the mixture was stirred at 80–90 \(^{\circ }\hbox {C}\) for 16 h. The mixture was then poured onto crushed ice, stirred well, and the resulting solid was filtered, washed thoroughly with cold water, and dried. The products were purified by recrystallization from \(\hbox {CH}_{3}\hbox {CN}\) [35].
Synthesis of alkyl 2-chloroquinoline-3-carboxylates (2a–c)
A mixture of 2-chloroquinoline-3-carbaldehyde (0.5 mmol, 0.096 g), \(\hbox {K}_{2}\hbox {CO}_{3}\) (3 mmol, 0.25 g), and iodine (2 mmol, 0.51 g) in alcohol (3 mL) was stirred at room temperature until the disappearance of the starting material (monitored by TLC). The reaction mixture was then quenched with saturated aq. \(\hbox {Na}_{2}\hbox {S}_{2}\hbox {O}_{3}\) (5 mL) and water (5 mL). The resulting solid was filtered, washed with water (5 mL), and dried. The crude product was characterized and found to be pure enough to be used as is for further use [36]. The analytic data for 2b and 2c are given below.
Ethyl 2-chloroquinoline-3-carboxylate (2b)
Dark yellow solid; mp, 106 \(^\circ \hbox {C}\); \(^{1}\hbox {H}\) NMR (300 MHz, DMSO-\(d_{6})\): \(\delta \) 1.35 (t, \(J= 7.0\) Hz, 3H, \(\hbox {OCH}_{2}\hbox {C}\underline{\mathrm{H}}_{3})\), 4.42 (q, J = 7.0 Hz, 2H, \(\hbox {OC}\underline{\mathrm{H}}_{2}\hbox {CH}_{3})\), 7.32–7.70 (m, 4H, 4CH of quinoline), 8.91 (s, 1H, CH of quinoline); IR (KBr): 2931, 1730 \(\hbox {cm}^{-1}\); MS (EI): m/z \([\hbox {M}]^{+}\), 235.
Propyl 2-chloroquinoline-3-carboxylate (2c)
Brown solid; mp, 112 \({^{\circ }}\hbox {C}\); \(^{1}\hbox {H}\) NMR (300 MHz, DMSO-\(d_{6})\): \(\delta \) 1.07 (t, \(J = 7.3\) Hz, 3H, \(\hbox {OCH}_{2}\hbox {CH}_{2}\hbox {C}\underline{\mathrm{H}}_{3})\), 1.68–1.80 (m, 2H, \(\hbox {OCH}_{2}\hbox {C}\underline{\mathrm{H}}_{2}\hbox {CH}_{3})\), 4.32 (t, \(J= 6.8\) Hz, 2H, \(\hbox {OC}\underline{\mathrm{H}}_{2}\hbox {CH}_{2}\hbox {CH}_{3})\), 7.21–7.68 (m, 4H, 4CH of quinoline), 8.90 (s, 1H, CH of quinoline); IR (KBr): 2928, 1730 \(\hbox {cm}^{-1}\); MS (EI): m/z [M]\(^{+}\), 249.
General procedure for synthesis of 1-aminopyrrolo[1,2-a]quinoline-4-carboxylate esters (5a–g)
A mixture of 2-chloroquinoline-3-carboxylate (1 mmol), \(\hbox {Pd}(\hbox {PPh}_{3})_{2}\hbox {Cl}_{2}\) (0.05 mmol, 0.036 g), and \(\hbox {Et}_{3}\hbox {N}\) (3 mmol, 0.30 g) was stirred in CH\(_{3}\)CN (5 mL) at room temperature under an argon atmosphere. Propargyl alcohol (1.25 mmol, 0.07 g) was added, and the mixture was further stirred at 80 \(^{{\circ }}\)C for 3 h. Then, a secondary amine was added, and the mixture was stirred at 80 \(^{{\circ }}\)C for 12 h. The resulting solution was concentrated in vacuo, and the crude residue was subjected to column chromatography (silica gel) using CHCl\(_{3}\) as eluent.
Methyl 1-morpholinopyrrolo[1,2-a]quinoline-4-carboxylate (5a)
Light orange solid; mp, 101–102 \({^{\circ }}\hbox {C}\); \(^{1}\hbox {H}\) NMR (300 MHz, CDCl\(_{3})\): \(\delta \) 2.96–3.05 (m, 2H, \(\hbox {NCH}_{2}\)), 3.16–3.20 (m, 2H, \(\hbox {NCH}_{2}\)), 3.92–4.04 (m, 7H, OCH\(_{3}\), 2 \(\hbox {OCH}_{2}\)), 6.54 (d, \(J= 3.9\) Hz, 1H, CH of pyrrole), 7.23 (d, J = 4.2 Hz, 1H, CH of pyrrole), 7.32–7.37 (m, 1H, CH of quinoline), 7.54–7.60 (m, 1H, CH of quinoline), 7.71–7.72 (m, 1H, CH of quinoline), 7.78 (s, 1H, CH of quinoline), 9.47 (d, \(J= 8.7\) Hz, 1H, CH of quinoline); \(^{13}\hbox {C}\) NMR (75 MHz, CDCl\(_{3})\): \(\delta \) 51.06, 52.01, 65.85, 101.39, 102.34, 115.99, 119.85, 122.52, 122.55, 123.44, 123.75, 128.68, 128.02, 135.32, 140.95, 164.93; IR (KBr): 2928, 1720, 1600 \(\hbox {cm}^{-1}\); MS (EI): m/z [M]\(^{+}\), 310.
Methyl 1-(piperidin-1-yl)pyrrolo[1,2-a]quinoline-4-carboxylate (5b)
Light orange solid; mp, 77–80 \({^{\circ }}\)C; \(^{1}\hbox {H}\) NMR (300 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 1.69–1.95 (m, 6H, 3 \(\hbox {CH}_{2}\)), 2.63–2.71 (m, 2H, \(\hbox {NCH}_{2}\)), 3.31–3.35 (m, 2H, \(\hbox {NCH}_{2}\)), 3.99 (s, 3H, OCH\(_{3})\), 6.48 (d, J = 4.2 Hz, 1H, CH of pyrrole), 7.20 (d, \(J = 3.9\) Hz, 1H, CH of pyrrole), 7.28–7.35 (m, 1H, CH of quinoline), 7.53–7.58 (m, 1H, CH of quinoline), 7.66–7.69 (m, 1H, CH of quinoline), 7.74 (s,1H, CH of quinoline), 9.47 (d, \(J= 8.7\) Hz, 1H, CH of quinoline); \(^{13}\hbox {C}\) NMR (75 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 23.07, 24.81, 51.00, 52.90, 100.82, 102.15, 116.36, 119.86, 122.29, 125.61, 123.05, 123.30, 127.81, 128.39, 135.57, 142.71, 165.11; IR (KBr): 2920, 1721 \(\hbox {cm}^{-1}\); MS (EI): m/z [M]\(^{+}\), 308.
Methyl 1-(pyrrolidin-1-yl)pyrrolo[1,2-a]quinoline-4-carboxylate (5c)
Yellow solid; mp, 85–87 \({^{\circ }}\)C; \(^{1}\hbox {H}\) NMR (300 MHz, CDCl\(_{3})\): \(\delta \) 1.28–1.45 (m, 4H, \(\hbox {2CH}_{2}\)), 2.70–2.86 (m, 2H, \(\hbox {NCH}_{2}\)), 3.20–3.38 (m, 2H, \(\hbox {NCH}_{2}\)), 3.99 (s, 3H, OCH\(_{3})\), 6.53 (d, J = 3.9 Hz, 1H, CH of pyrrole), 7.20 (d, \(J = 3.9\) Hz, 1H, CH of pyrrole), 7.31–7.34 (m, 1H, CH of quinoline), 7.54–7.57 (m, 1H, CH of quinoline), 7.64–7.69 (m, 1H, CH of quinoline), 7.74 (s, 1H, CH of quinoline), 9.22 (d, \(J= 8.7\) Hz, 1H, CH of quinoline); \(^{13}\)C NMR (75 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 24.50, 51.00, 52.08, 100.57, 102.17, 116.34, 119.80, 122.26, 122.69, 123.36, 127.71, 128.32, 129.86, 135.57, 140.23, 166.74; IR (KBr): 2920, 1728 \(\hbox {cm}^{-1}\); MS (EI): m/z [M]\(^{+}\), 294.
Ethyl 1-morpholinopyrrolo[1,2-a]quinoline-4-carboxylate (5d)
Orange solid; mp, 88–90 \({^{\circ }}\)C; \(^{1}\)H NMR (300 MHz, CDCl\(_{3})\): \(\delta \) 1.48 (t,\(\hbox {J}= 7.0\) Hz, 3H, \(\hbox {OCH}_{2}\hbox {C}\underline{\hbox {H}}_{3})\), 2.96–3.05 (m, 2H, \(\hbox {NCH}_{2}\)), 3.16–3.20 (m, 2H, \(\hbox {NCH}_{2}\)), 3.92–4.04 (m, 4H, 2\(\hbox {OCH}_{2}\)), 4.77 (q, J = 7.0 Hz, 2H, \(\hbox {OC}\underline{\hbox {H}}_{2}\hbox {CH}_{3})\), 6.54 (d, \(J= 4.2\) Hz, 1H, CH of pyrrole), 7.23 (d, J = 4.2 Hz, 1H, CH of pyrrole), 7.32–7.38 (m, 1H, CH of quinoline), 7.54–7.60 (m, 1H, CH of quinoline), 7.70–7.75 (m, 1H, CH of quinoline), 7.78 (s, 1H, CH of quinoline), 9.48 (d, \(J= 8.7\) Hz, CH of quinoline); \(^{13}\hbox {C}\) NMR (75 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 14.39, 53.06, 61.09, 68.15, 102.38, 103.38, 117.03, 121.22, 123.55, 124.37, 128.82, 128.99, 129.70, 130.90, 136.33, 1414.97, 167.77; IR (KBr): 2940, 1733 \(\hbox {cm}^{-1}\); MS (EI): m/z [M]\(^{+}\), 324.
Ethyl 1-(piperidin-1-yl)pyrrolo[1,2-a]quinoline-4-carboxylate (5e)
Orange solid; mp, 79–81 \({^{\circ }}\)C; \(^{1}\hbox {H}\) NMR (300 MHz, \(\hbox {CDCl}_{3})\): 1.37 (t, \(J = 7.2\) Hz, 3H, \(\hbox {OCH}_{2}\hbox {C}\underline{\hbox {H}}_{3})\), 1.65–1.80 (m, 6H, 3\(\hbox {CH}_{2}\)), 2.51–2.60 (m, 2H, \(\hbox {NCH}_{2}\)), 3.20–3.23 (m, 2H, \(\hbox {NCH}_{2}\)), 4.35 (q, \(J= 7.2\) Hz, 2H, \(\hbox {OC}\underline{\hbox {H}}_{2}\hbox {CH}_{3})\), 6.37 (d, \(J= 4.2\) Hz, 1H, CH of pyrrole), 7.10 (d, \(J= 4.5\) Hz, 1H, CH of pyrrole), 7.18–7.23 (m, 1H, CH of quinoline), 7.41–7.47 (m, 1H, CH of quinoline), 7.55–7.61 (m, 1H, CH of quinoline), 7.63 (s, 1H, CH of quinoline), 9.36 (d, \(J= 8.7\) Hz, 1H, CH of quinoline); \(^{13}\hbox {C}\) NMR (75 MHz, \(\hbox {CDCl}_{3})\): \(\delta \) 14.15, 25.85, 29.68, 53.94, 61.02, 101.80, 103.17, 117.39, 121.22, 123.31, 167.79, 124.43, 128.77, 129.41, 130.90, 136.58, 143.73, 167.79; IR (KBr): 2931, 1730 \(\hbox {cm}^{-1}\); MS (EI): m/z [M]\(^{+}\), 322.
Propyl 1-morpholinopyrrolo[1,2-a]quinoline-4-carboxylate (5f)
Orange solid; mp, 92–94 \({^{\circ }}\)C; \(^{1}\hbox {H}\) NMR (300 MHz, \(\hbox {CDCl}_{3}\)): 1.11 (t, J = 7.5 Hz, 3H, \(\hbox {OCH}_{2}\hbox {CH}_{2}\hbox {C}\underline{\hbox {H}}_{3})\), 1.85–1.92 (m, 2H, \(\hbox {OCH}_{2}\hbox {C}\underline{\hbox {H}}_{2}\hbox {CH}_{3})\), 2.96–3.05 (m, 2H, \(\hbox {NCH}_{2}\)), 3.16–3.20 (m, 2H, \(\hbox {NCH}_{2}\)), 3.92–4.03 (m, 4H, \(\hbox {2OCH}_{2}\)), 4.38 (t, \(J = 6.6\) Hz, 2H, \(\hbox {OC}\underline{\hbox {H}}_{2}\hbox {CH}_{2}\hbox {CH}_{3})\), 6.54 (d, \(J = 4.2\) Hz, 1H, CH of pyrrole), 7.23 (d, J = 4.2 Hz, 1H, CH of pyrrole), 7.32-7.39 (m, 1H, CH of quinoline), 7.54–7.60 (m, 1H, CH of quinoline), 7.70–7.75 (m, 1H, CH of quinoline), 7.78 (s, 1H, CH of quinoline), 9.48 (d, J = 8.7 Hz, 1H, CH of quinoline); \(^{13}\hbox {C}\) NMR (75 MHz, \(\hbox {CDCl}_{3}\)): \(\delta \) 9.64, 21.12, 52.02, 65.70, 65.86, 101.35, 102.33, 115.99, 120.23, 122.50, 122.62, 123.33, 123.88, 128.67, 129.85, 135.30, 140.93, 164.63; IR (KBr): 2920, 1720 \(\hbox {cm}^{-1}\); MS (EI): m/z [M]\(^{+}\), 338.
Propyl 1-(piperidin-1-yl)pyrrolo[1,2-a]quinoline-4-carboxylate (5g)
Orange solid; mp, 89–91 \({^{\circ }}\)C; \(^{1}\)H NMR (300 MHz, \(\hbox {CDCl}_{3})\): 1.10 (t, \(J = 7.5\) Hz, 3H, \(\hbox {OCH}_{2}\hbox {CH}_{2}\hbox {C}\underline{\hbox {H}}_{3})\), 1.47–1.94 (m, 8H, \(\hbox {4CH}_{2}\)), 2.62–2.71 (m, 2H, \(\hbox {NCH}_{2}\)), 3.31–3.46 (m, 2H, \(\hbox {NCH}_{2}\)), 3.36 (t, \(J = 6.6\) Hz, 2H, \(\hbox {OC}\underline{\hbox {H}}_{2}\hbox {CH}_{2}\hbox {CH}_{3})\), 6.47 (d, \(J= 3.9\) Hz, 1H, CH), 7.20 (d, J = 4.2 Hz, 1H, CH), 7.29–7.34 (m, 1H, CH of quinoline), 7.55–7.58 (m, 1H, CH of quinoline), 7.67–7.71 (m, 1H, CH of quinoline), 7.78 (s, 1H, CH of quinoline), 9.47 (d, \(J = 8.7\) Hz, 1H, CH of quinoline); \(^{13}\hbox {C}\) NMR (75 MHz, \(\hbox {CDCl}_{3})\): 10.69, 22.17, 25.68, 29.72, 53.49, 66.67, 68.17, 101.82, 103.19, 117.39, 121.27, 123.31, 123.61, 123.99, 124.45, 128.61, 129.41, 136.58, 143.72, 165.85;; IR (KBr): 2925, 1720 \(\hbox {cm}^{-1}\); MS (EI): m/z [M]\(^{+}\), 336.
References
Sonogashira K, Tohda Y, Hagihara N (1975) Convenient synthesis of acetylenes-catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett 16:4467–4470. doi:10.1016/S0040-4039(00)91094-3
Cassar L (1975) Synthesis of aryl- and vinyl-substituted acetylene derivatives by the use of nickel and palladium complexes. J Organomet Chem 93:253–257. doi:10.1016/S0022-328X(00)94048-8
Li J, Gribble GW (2000) Palladium in heterocyclic chemistry; tetrahedron organic chemistry series, vol 20. Pergamon, Amsterdam
Willy B, Muller T (2010) Three-component synthesis of benzo[b][1,5]thiazepines via coupling-addition-cyclocondensation sequence. Mol Divers 14:443–453. doi:10.1007/s11030-009-9223-z
Diederich F, Stang P, Tykwinsk R (2005) Acetylene chemistry: chemistry, biology, and material science. Wiley-VCH, Weinheim. doi:10.1002/3527605487
Francke V, Mangel T, Müllen K (1998) Synthesis of \(\alpha \),\(\omega \)-difunctionalized oligo- and poly(p-phenyleneethynylene)s. Macromolecules 31:2447–2453. doi:10.1021/ma971429m
Sonogashira K (2002) Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp\(^2\)-carbon halides. J Organomet Chem 653:46–49. doi:10.1016/S0022-328X(02)01158-0
Vasconcelos SN, Shamim A, Ali B, Oliveira IM, Stefani HA (2016) Functionalization of protected tyrosine via Sonogashira reaction. Mol Divers 20:469–481. doi:10.1007/s11030-015-9642-y
Arumugasamy E, Yu-Hsiang W, Tong-Ing H (2003) Sonogashira coupling reaction with diminished homocoupling. Org Lett 5:1841–1844. doi:10.1021/ol034320+
Siemsen P, Livingston RC, Diederich F (2000) Acetylenic coupling: a powerful tool in molecular construction. Angew Chem Int Ed 39:2632–2657. doi:10.1002/1521-3773(20000804)39:15<2632::AID-ANIE2632>3.0.CO;2-F
Cheng J, Sun Y, Wang F, Guo M, Xu J, Pan Y, Zhang Z (2004) A Copper- and amine-free sonogashira reaction employing aminophosphines as ligands. J Org Chem 69:5428–5432. doi:10.1021/jo049379o
Zhong H, Wang J, Lia L, Wang R (2014) The copper-free Sonogashira cross-coupling reaction promoted by palladium complexes of nitrogen-containing chelating ligands in neat water at room temperature. Dalton Trans 43:2098–2103. doi:10.1039/C3DT52970C
Leadbeater NE, Tominack BJ (2003) Rapid, easy copper-free Sonogashira couplings. Tetrahedron Lett 44:8653–8656. doi:10.1016/j.tetlet.2003.09.159
Böhm V, Herrmann WA (2000) A copper-free procedure for the palladium-catalyzed Sonogashira reaction. Eur J Org Chem 2000:3679–3681. doi:10.1002/1099-0690(200011)2000:22<3679::AID-EJOC3679>3.0.CO;2-X
Anderson KW, Buchwald SL (2005) General catalysts for the Suzuki–Miyaura and Sonogashira coupling reactions of aryl chlorides and for the coupling of challenging substrate combinations in water. Angew Chem Int Ed 44:6173–6177. doi:10.1002/anie.200502017
Yi Ch, Hua R (2006) Efficient copper-free \(\text{ PdCl }_{2}\)(PCy\(_{3}\))\(_{2}\)-catalyzed Sonogashira coupling of aryl chlorides with terminal alkynes. J Org Chem 71:2535–2537. doi:10.1021/jo0525175
Zhang Z, Lu W, Huang W, Li Y, Gao H, Luo Y (2006) Copper-free Sonogashira reaction using 7-chloro camptothecins. Tetrahedron 62:2465–2470. doi:10.1016/j.tet.2006.01.001
Fleckenstein CA, Plenio H (2008) Aqueous/organic cross coupling: sustainable protocol for Sonogashira reactions of heterocycles. Green Chem 10:563–570. doi:10.1039/B800154E
Chandra A, Singh B, Upadhyay S, Singh RM (2008) Copper-free Sonogashira coupling of 2-chloroquinolines with phenyl acetylene and quick annulation to benzo[b][1,6]naphthyridine derivatives in aqueous ammonia. Tetrahedron 64:11680–11685. doi:10.1016/j.tet.2008.10.010
Eicher T, Hauptmann S (2003) The chemistry of heterocycles, 2nd edn. Wiley-VCH, Weinheim 316
Michael JP (2007) Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep 24:223–246. doi:10.1039/B509528J
Pearson W, Fang W (2000) Synthesis of benzo-fused 1-azabicyclo[m.n.0]alkanes via the Schmidt reaction: a formal synthesis of gephyrotoxin. J Org Chem 65:7158–7174. doi:10.1021/jo0011383
Wei L, Hsung RP, Sklenicka HM, Gerasyuto AI (2001) A novel and highly stereoselective intramolecular formal [3+3] cycloaddition reaction of vinylogous amides tethered with \(\alpha \),\(\beta \)-unsaturated aldehydes: a formal total synthesis of (+)-gephyrotoxin. Angew Chem Int Ed 40:1516–1518. doi:10.1002/1521-3773(20010417)40:8<1516::AID-ANIE1516>3.0.CO;2-V
Dillard RD, Pavey DE, Benslay DN (1973) Synthesis and antiinflammatory activity of some 2,2-dimethyl-1,2-dihydroquinolines. J Med Chem 16:251–253. doi:10.1021/jm00261a019
Joshi AA, Viswanathan CL (2006) Recent developments in antimalarial drug discovery. Anti Infect Agents Med Chem 5:105–122. doi:10.2174/187152106774755626
Kidwai M, Negi N (1997) Synthesis of some novel substituted quinolines. Monatsh Chem 128:85–89. doi:10.1007/BF00807642
Alqasoumi I, Al-Taweel AM, Alafeefy AM, Noaman E, Ghorab MM (2010) Novel quinolines and pyrimido[4,5-b]quinolines bearing biologically active. Eur J Med Chem 45:738–744. doi:10.1016/j.ejmech.2009.11.021
Baumann M, Baxendale IR (2015) Batch and flow synthesis of pyrrolo[1,2-a]-quinolines. J Org Chem 80:10806–10816. doi:10.1021/acs.joc.5b01982
Sarkar S, Bera K, Jalal S, Jana U (2013) Synthesis of structurally diverse polyfunctional pyrrolo[1,2-\(a\)]quinolines by sequential Iron-catalyzed. Eur J Org Chem 27:6055–6061. doi:10.1002/ejoc.201300659
Glukhareva TV, D’yachenko EV, Morzherin YY (2002) Synthesis of spiro derivatives. Chem Heterocycl Compd 38:1426–1427. doi:10.1023/A:1022107332320
Keivanloo A, Bakherad M, Rahmani M, Rahimi A (2013) A Novel one-pot access to 2-formyl/acetyl-1-substituted pyrrolo[2,3-b]quinoxalines under Sonogashira reaction conditions. Monatsh Chem 144:859–863. doi:10.1007/s00706-012-0887-1
Bakherad M, Keivanloo A, Samangooei S (2012) Synthesis of 1-aryl-substituted-4-chloroimidazo[1,2-a]quinoxalines catalyzed by \(\text{ PdCl }_{2}\) in water. Tetrahedron Lett 23:1447–1449. doi:10.1016/j.tetlet.2012.01.028
Bakherad M, Keivanloo A, Jajarmi S (2012) Synthesis of pyrrolo[2,3-b]quinoxalines by the Pd/C-catalyzed multicomponent reaction of 1,2-dichloroquinoxaline with hydrazine hydrate, phenylacetylene, and a variety of aldehydes in water. Tetrahedron 68:2107–2112. doi:10.1016/j.tet.2012.01.045
Keivanloo A, Bakherad M, Rahimi A, Taheri SAN (2010) One-pot synthesis of 1,2-disubstituted pyrrolo[2,3-b]quinoxalines via palladium-catalyzed heteroannulation in water. Tetrahedron Lett 51:2409–2412. doi:10.1016/j.tetlet.2010.02.123
Meth-Cohn O, Narine B, Tamowski B (1981) A versatile new synthesis of quinolines and related fused pyridines. J Chem Soc Perkin Trans 1:1520–1530. doi:10.1039/P19810001520
Sharma N, Asthana M, Nandini D, Singh RP, Singh RM (2013) An economical nucleophilic route toward facile synthesis of pyrano[4,3-b]quinolin-1-ones via 6-endo-dig cyclization of o-alkynylquinoline esters. Tetrahedron 69:1822–1829. doi:10.1016/j.tet.2012.12.068
Ljungdahl T, Bennur T, Dallas A, Emtenäs H, Mårtensson J (2008) Two competing mechanisms for the copper-free Sonogashira cross-coupling reaction. Organometallics 27:2490–2498. doi:10.1021/om800251s
Acknowledgments
We gratefully acknowledge the financial support of the Research Council of the Shahrood University of Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11030_2016_9694_MOESM1_ESM.docx
The supporting information for this work is available, as follows: copies of 1H and 13C spectra of all the 1-amino substituted pyrrolo[1,2-a]quinoline-4-carboxylate esters.(doc 1.83MB)
Rights and permissions
About this article
Cite this article
Keivanloo, A., Kazemi, S.S., Nasr-Isfahani, H. et al. Efficient one-pot synthesis of new 1-amino substituted pyrrolo[1,2-a]quinoline-4-carboxylate esters via copper-free Sonogashira coupling reactions. Mol Divers 21, 29–36 (2017). https://doi.org/10.1007/s11030-016-9694-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9694-7