Abstract
Tropical peat swamp forests (PSF) are characterized by high quantities of carbon (C) stored as organic soil deposits due to waterlogged conditions which slows down decomposition. Globally, Peru has one of the largest expanse of tropical peatlands, located primarily within the Pastaza-Marañón river basin in the Northwestern Peru. Peatland forests in Peru are dominated by a palm species—Mauritia flexuosa, and M. flexuosa-dominated forests cover ~ 80% of total peatland area and store ~ 2.3 Pg C. However, hydrologic alterations, land cover change, and anthropogenic disturbances could lead to PSF’s degradation and loss of valuable ecosystem services. Therefore, evaluation of degradation impacts on PSF’s structure, biomass, and overall C stocks could provide an estimate of potential C losses into the atmosphere as greenhouse gases (GHG) emissions. This study was carried out in three regions within Pastaza-Marañón river basin to quantify PSF’s floristic composition and degradation status and total ecosystem C stocks. There was a tremendous range in C stocks (Mg C ha−1) in various ecosystem pools—vegetation (45.6–122.5), down woody debris (2.1–23.1), litter (2.3–7.8), and soil (top 1 m; 109–594). Mean ecosystem C stocks accounting for the top 1 m soil were 400, 570, and 330 Mg C ha−1 in Itaya, Tigre, and Samiria river basins, respectively. Considering the entire soil depth, mean ecosystem C stocks were 670, 1160, and 330 Mg C ha−1 in Itaya, Tigre, and Samiria river basins, respectively. Floristic composition and calcium to Magnesium (Ca/Mg) ratio of soil profile offered evidence of a site undergoing vegetational succession and transitioning from minerotrophic to ombrotrophic system. Degradation ranged from low to high levels of disturbance with no significant difference between regions. Increased degradation tended to decrease vegetation and forest floor C stocks and was significantly correlated to reduced M. flexuosa biomass C stocks. Long-term studies are needed to understand the linkages between M. flexuosa harvest and palm swamp forest C stocks; however, river dynamics are important natural drivers influencing forest succession and transition in this landscape.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tropical forests that experience waterlogged conditions tend to accumulate partially decomposed organic matter, and a sustained accrual of this material on forest floor results in peat formation (Limpens et al. 2008; Rieley et al. 2008; Lähteenoja et al. 2009a). A unique class of tropical forests, referred to as peat swamp forests, are characterized by the presence of high belowground carbon (C) and organic matter at various stages of decomposition. Peatlands occurs wherever rainfall and topography are conducive to poor drainage, causing permanent waterlogging and substrate acidification (Bailey 1951; Bradshaw et al. 2008). Peat swamp forests located at low altitudes occur in river valley basins, watersheds, and subcoastal areas of Southeast Asia, Africa, Caribbean, Central and South America (Hooijer et al. 2010; Posa et al. 2011).
Growing interest in tropical peatlands is mainly due to their increasingly recognized role in the global C cycle (Jauhiainen et al. 2005), greenhouse gas exchange (Kurnianto et al. 2015), paleoecology (Kelly et al. 2014; Lawson et al. 2015), maintenance of biological diversity (Posa et al. 2011), and provision of direct economic gains when peat swamp forests are logged, converted into agricultural farms and oil palm plantations (Roucoux et al. 2013). However, there is a considerable uncertainty in global distribution of tropical peatlands. A best estimate from available global data is 441,025 km2 (Page et al. 2011), combined with discoveries in the Amazon (35,600 km2 (Draper et al. 2014)) and the central Congo Basin (145,500 km2 (Dargie et al. 2017)). Several studies indicate Peru has one of the largest extent of peatlands in the tropics (Gumbricht et al. 2017) and the known extent might increase as more explorations are conducted and new information becomes available. Other than C storage and sequestration, tropical peatlands also play an important role in water regulation, habitat provision for biodiversity, and livelihoods for local communities (Posa et al. 2011; Murdiyarso et al. 2013). However, hydrologic alterations and land cover change by anthropogenic or by natural causes could lead to peat swamp forests degradation, and loss of stored C to the atmosphere as greenhouse gases (GHG) emissions (Hergoualc’h and Verchot 2011, 2014).
South America is estimated to harbor one of the largest area of tropical peatlands worldwide (Gumbricht et al. 2017), of which most are located in the Amazon basin. In Peru, the majority of peatlands are located within Pastaza-Marañón foreland basin (PMFB) in Northwestern region of the Peruvian Amazon, forming an extensive continuous wetland characterized by one of the largest tropical system of fluvial aggradation (Räsänen et al. 1990; Räsänen et al. 1992). The PMFB region has extensive and deep accumulations of peat with depths up to 7.5 m and belowground C stocks ranging from 2 to 20 Pg C (Lähteenoja et al. 2012; Draper et al. 2014). These peat C stocks are significant in the context of national and regional C budgets, because the total aboveground biomass C in the entire Peru are estimated to be 6.9 Pg C (Asner et al. 2014).
Previous studies have suggested that peatlands in this region of Peru support different vegetation assemblages—palm swamp forest, “pole” forests (low stature forest with many thin-stemmed trees), and herbaceous “open” communities (Lähteenoja and Page 2011; Lawson et al. 2015). Palms (family Arecaceae) are the single most abundant arborescent plant family in Amazonian forests, especially in forests with frequent inundation (Kahn and Mejia 1990; Terborgh and Andresen 1998) and poor soil conditions, including shallow rooting depth (Emilio et al. 2014). Some species, such as Mauritia flexuosa, are able to thrive in such conditions and establish nearly mono-dominant stands, which are locally known as aguajales (Kahn et al. 1988). These Mauritia (palm) forests are an important feature of this landscape—both economically and ecologically (Virapongse et al. 2017). A recent study determined actual peatland extent within PMFB to be ~ 35,000 km2 with majority of it covered by palm swamps (Draper et al. 2014). The importance of Mauritia forests among Peruvian peatlands is evident from the fact that they cover ~ 80% of total peatland area and store ~ 2.3 Pg C (Draper et al. 2014).
Over the past few decades, growing local and regional demands for M. flexuosa fruits (Endress et al. 2013) has resulted into increased harvesting pressure on M. flexuosa forests. It is one of the most heavily used plant species for non-timber forest products (NTFPs) with multiple applications in indigenous and rural activities, mostly associated with food, fibers, animal fodder, and construction (Zambrana et al. 2007; Goodman et al. 2013). M. flexuosa fruits are an important source of vitamins and proteins for rural communities (Maria Pacheco 2005) and other products (leaves, oil, wood) derived from M. flexuosa are also economically valuable (Virapongse et al. 2017). The most common practice of harvesting Mauritia fruit involves cutting down fruit-bearing female trees which causes localized reduction in the population of mature trees (Parodi and Freitas 1990; Delgado et al. 2007; Manzi and Coomes 2009). This selective removal of fruiting trees reduces viable seed source in a given forest stand, and unavailability of seeds hinders natural regeneration and recruitment of new Mauritia palms (Horn et al. 2012). Over time, such practices alter the structure and composition of M. flexuosa forests (Hergoualc’h et al. 2017), severely diminishing ecological integrity and loss of ecosystem services derived from these forests. Degradation of Mauritia forests could potentially result in destabilization of peat deposits and reduce its ability to sequester and store C (van Lent et al. 2018). Loss of habitat and food resources also have negative impacts on wildlife including fishes, birds (particularly macaw), large ungulates (such as lowland tapir, peccaries, deer), and primates.
These anthropogenic pressures, combined with enhanced seasonality in annual precipitation due to climate change pose an unprecedented threat to the peatland forests of Peruvian Amazon. Our study was intended to determine the conditions (status) of Peruvian peat swamp forests that are dominated by M. flexuosa. We estimated total C stocks in these ecosystems by measuring aboveground and belowground vegetation (C in live biomass), downed woody debris and litter (C on forest floor), and C stored in underlying soils. Evaluation of relationships between forest degradation status and C stocks were carried out to understand the role of anthropogenic factors such as palm harvesting on ecosystem C stocks. Role of regional landscape factors such as riverine sediment inputs on peat accumulation processes at a broader scale were also evaluated. In addition, we attempted to provide estimates that will help reduce uncertainties relating to the importance of peat C storage in these forests. An estimate of C stocks in these palm swamp forests will allow better determination of their role as a global sink/source of GHGs and provide additional information for validation of peat distribution models in this region.
2 Material and methods
2.1 Swamp forests in Peru
The Peruvian Amazon basin covers an area of 77,535,384 ha (~ 60% of Peru’s territory) and is the largest physiographical region in the country (Rodríguez 1990). The climate is warm and humid with mean annual temperature ~ 27 °C, with average daily minima around 20–22 °C and maxima around 29–31 °C (Marengo 1998). Rainfall occurs throughout the year with an average annual precipitation of 3087 mm and a weak dry season between June and September (Marengo 1998).
Peru’s wetlands, totaling 65,820 km2 of land cover, can be subdivided into four ecosystem types: (1) peat swamp forests, (2) mangroves, (3) Andean wetlands (bofedales), and (4) coastal marshes and lagoons. Peat swamps with an area of 60,630 km2 account for the greatest proportion of Peru’s wetlands (MINAM 2010; Queiroz et al. 2014). The majority of Mauritia palm swamps (aguajales), pole forests, and open peatlands (~ 27,730 km2) are found within PMFB in the Loreto Department, with smaller patches in the departments of Ucayali and Madre de Dios (MINAM 2010; Draper et al. 2014). The PMFB belongs to the western part of the Amazon basin and is characterized by a broad belt of subsiding basins formed during the Cenozoic uplift of the Andes (Räsänen et al. 1990; Räsänen et al. 1992). Since the Cretaceous period, rivers originating from the Andes have led to the accumulation of several kilometer thick minerogenic deposits in these subsiding forested basins (Lähteenoja et al. 2012). Consequently, the accumulation of organic matter and peat formation in this slowly subsiding basin is a dynamic process, influenced by lateral movements of river channels, e.g., meandering up to > 100 m in a year (Smith et al. 1989) and avulsions (abrupt changes in the location of river stretches) which can cause both burial and erosion of peat deposits (Kalliola et al. 1992; Neller et al. 1992). These hydromorphic processes control the quantity (depth) and location of peat deposits at local and regional scales, leading to spatial variability in belowground C stocks.
2.2 Study area and sampled locations
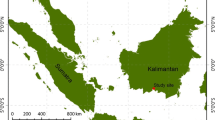
The ecosystem C stocks were measured at 12 peatland swamp forest sites within Amazon basin in the Loreto region of north eastern Peru. At a regional scale, these sites fall into three different river basins/watersheds: Itaya, Tigre and Smiria. Three sites were sampled near Iquitos town close to Itaya River, five sites along the northern reaches of Tigre River, and four sites were sampled inside and in proximity to the Pacaya Samiria National Reserve within the Samiria River basin (Fig. 1 and Table 1). Therefore the sampling sites in these three locations were grouped as Itaya, Tigre, and Samira, respectively. The primary characteristics of all sampled forest sites were the presence of a palm species—Mauritia flexuosa (local name aguaje), and belowground peat. All sampled locations had individuals of M. flexuosa even though this species was not always the most abundant, either because of degradation or natural variation. Sampled sites represented areas with variable degree of degradation—low to high.
2.3 Sample collection
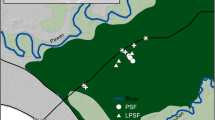
Information necessary to calculate total ecosystem C stocks including standing tree biomass, downed woody debris (dead wood on forest floor), belowground plant biomass, litter biomass on forest floor, and soil C stocks was collected. CIFOR–USFS freshwater peatland forest sampling protocol was used to develop an estimate of total ecosystem C stocks (Kauffman et al. 2016). Aboveground forest structure, composition, and ecosystem C pools were determined at every site by establishing six subplots 50 m apart along a 250-m transect (Fig. 2). Subplot location was determined on the basis of M. flexuosa presence (alive) or past presence (dead), usually 1–2 km inland from the main river channel. Once the first subplot was identified, a 250-m-long straight transect was laid in a random direction. If a deep/unpassable stream/open water area was encountered, then the transect direction was altered so that all six subplots were representative of the forest ecosystem. Vegetation was identified and recorded for species inventory and calculation of aboveground and belowground biomass in live vegetation. Standing dead trees and downed woody debris (DWDs) were measured and counted to determine the quantity of C present in dead and downed woody components of the ecosystem. Vegetation litter on the forest floor was collected and bagged. And lastly, soil samples were obtained (up to 3 m depth) to determine carbon (C) and nitrogen (N) content within the soil profile.
2.3.1 Vegetation
Trees ≥ 5 cm dbh (dbh = stem diameter at 1.3 m above the ground) were measured inside each of the six circular subplots of 10 m radius (area = 314.2 m2), while saplings (vegetation with dbh < 5 cm) were measured only inside a 2-m radius circle (area = 12.6 m2) nested within the larger subplot. All individuals were identified in the field and at least one individual per species was collected. The herbarium vouchers were deposited at the Herbarium Herrerense in Iquitos for future reference. Relative importance value index of species was determined on the basis on their relative abundance, frequency, and dominance in the plots using all trees ≥ 10 cm dbh (Curtis and McIntosh 1951).
The stem height of all palms was measured using a clinometer (Suunto PM-5/360 PC). For palms with slanting trunks, the height of the crown from the ground and slope of the trunk was measured to calculate the length of palm tree trunk. Clinometer was also used to determine the angle of inclination of palm trunk and to determine the angle of elevation of the palm crown with respect to the horizontal ground surface. The length of palm trunk (m) was calculated by using general mathematical principles of trigonometry.
Dead tree measurements were categorized into three classes (I, II, and III) depending on the existing branches and twigs attached to the dead tree at the time of sampling. Class I represented a dead tree with majority of primary and secondary branches attached to the tree, while class III dead tree had no branches attached to the main trunk. Class II category was assigned to a dead tree which possessed primary branches but secondary branches were lost. Aboveground tree biomass (AGB; kg) and belowground tree biomass (BGB; kg) were determined using species-specific allometric equations with stem height (trunk length) as an independent variable in palms (Table 2). Vegetation biomass for dicot trees was determined using generic allometric equations with dbh as an independent variable. Biomass in standing dead trees was calculated based upon their classes. Biomass of class I dead trees was estimated to be 97.5% of a live tree, class II was estimated to be 80% of a live tree, and class III trees were estimated to represent 50% of a live tree (Kauffman and Donato 2012). Vegetation biomass (kg) was converted into megagram per hectare before converting into carbon mass per hectare. Biomass of trees (both above and below ground) was converted to C mass using specific conversion ratios. A conversion factor of 0.47 was used for aboveground C and 0.39 for belowground C (Kauffman and Donato 2012).
2.3.2 Downed woody debris and litter
Downed woody debris were measured using planar intercept technique (Brown 1974; Harmon and Sexton 1996). A survey tape was run 12 m from the plot center in four mutually perpendicular directions, oriented at 45° angle from the azimuth of main transect (Fig. 2). Woody debris intersecting the survey tape were recorded at each of the six subplots. Downed wood ≥ 7.5 cm in diameter at the point of intersection with tape was classified as “large” and measured along the entire length of 12 m long tape. Large wood (pieces ≥ 7.5 cm in diameter) were separated in two decay categories: sound and rotten. Woody debris that were ≥ 2.5 cm but < 7.5 cm in diameter at the point of intersection were called “medium” and were measured along the last 5 m of each of the four DWD transects. To determine wood densities of DWDs, a one-time collection of ~ 20–25 pieces of each woody debris class was made. Each piece was measured for volume using the water mass displacement method and dried in oven to obtain dry mass. These values were used to compute wood density and obtain quadratic mean diameter (QMD) for medium woody debris size class (= 3.98 cm). Wood density (g cm−3) was 0.25 and 0.59 for large rotten and large solid woody debris, respectively, and 0.44 g cm−3 for medium debris size class. A conversion factor of 0.47 was used to convert DWD mass into C mass.
Carbon mass in the litter component of sampled areas was determined by quantifying accumulated vegetation litter on the forest floor. A quadrat with fixed dimensions (0.38 m × 0.38 m) was used to collect litter at the far end of two DWD transects at each of the six subplots. This resulted in 12 samples from sampled site and provided a good representation of litter deposition at a site. Vegetation litter was brought to the lab, weighed, and ~ 100 g subsample was dried in an oven at 40 °C. The dried subsample was weighed to determine moisture content (as loss in the weight), and was subsequently homogenized by using a combination of mechanical grinder and a mortar-pestle. Dried and finely ground litter samples from both quadrats at each subplot were mixed together and analyzed for N and C content. Carbon content in homogenized litter sample was used to determine overall C mass in forest litter on areal basis (C ha−1). Total vegetation C (C ha−1) at each location was derived by adding C in above and belowground vegetation, DWDs, and litter.
2.3.3 Soils
Intact soil cores were obtained at each subplot up to a depth of 3 m and soil samples at specified depth intervals within soil profile were collected. For majority of sites, Russian peat auger (Eijkelkamp Peat Auger) was used; however, an open face auger was used at few subplots at Itaya sites. The auger consists of a semi-cylindrical chamber (radius = 2.87 cm for open face auger and 2.07 cm for Russian peat auger) and a detachable handle to extend its length and enable soil cores extraction up to 3 m depth. The soil core was divided into depth intervals of 0–15, 15–30, 30–50, 50–100, 100–150, 150–200, 200–250, and 250–300 cm. A 5-cm section of soil from these depth intervals was obtained for laboratory determination of C. If there were interbedded mineral and organic soil layers within these zones, 5 cm section was collected from an area including both mineral and organic layer proportionally such that it was representative of that depth interval. Soil depth to point of refusal (minerogenic sediment) was measured at three locations around each plot center by inserting a graduated aluminum pole. Two probes were used with inference capacity of either 2.65 or 5.3 m (longer probe was available only during some part of the sampling). The pH of stagnant undisturbed water at each location was measured by a portable handheld pH measuring device (Oakton EcoTestr pH 2 waterproof tester) (Table 1).
Forest soil, litter, and DWD samples were transported to the laboratory at Instituto de investigaciones de la Amazonía Peruana (IIAP), Iquitos, for weighing, drying, and grinding. Carbon and N concentrations in dry homogenized samples were determined by the induction furnace method in a Costech EA C-N analyzer at the analytical laboratory of University of Hawaii-Hilo, Hawaii, United States of America. A smaller subset of total soil samples (count = 87) was tested for calcium (Ca) and magnesium (Mg) content by using ammonium acetate to extract the cations (Simard 1993), followed by analysis of the solution on a Varian Vista CCD ICP-OES. Total Ca and Mg content is helpful in identifying the influence of riverine transport and external inputs of minerals and nutrients at a given location and is helpful in determination of nutrient status of a site. If Ca/Mg ratio of a peat soil is above 6 (avg. ratio in the rainwater), it indicates influence or inputs of river water, and would render a site minerotrophic, whereas lower Ca/Mg ratios are indicative of low-nutrient status or ombrotrophic systems (Lähteenoja et al. 2009a). The selected samples for Ca and Mg analysis included soils from different depth in soil profile to allow determination of changes in the nutrient status of a site as preserved in its stratigraphy.
Soil C pools were obtained as the product of soil C concentration, bulk density, and plot specific soil depth measurements. Carbon masses from each soil section representing different depth horizons were added to derive total soil C stocks at a site. Soil C stocks are reported for two depth boundaries—(1) up to 1 m soil depth and (2) for the entire soil profile. Top 1 m soil profile C allows for an easy comparison among sites but C stocks in the entire soil depth reflect the variability in the soil profile C stocks at different sites. Finally, total ecosystem C stocks were obtained by adding C stocks in aboveground and belowground vegetation, DWDs, litter, and soil profile at each site.
2.3.4 Degradation criteria
Each site was assigned a degradation class to assess the effect of forest degradation on ecosystem C pools. These classes were based on the sum of three criteria: quantity of DWD, M. flexuosa height distribution, and presence of M. flexuosa seedlings at a site. Each site was scored with 1 if a degradation criterion was present or 0 if the criterion was absent, respectively.
The criteria for DWD were based on the count of DWD (diameter > 20 cm) at a site relative to the total DWD (diameter > 20 cm) counted at all sites. The presence of large quantity of DWD on the forest floor could be an indicator of disturbances (natural or anthropogenic) (Palace et al. 2012). A score of 1 was assigned if the count at a site was above the overall median, or 0 if this condition was not met. The relationship between M. flexuosa height distribution and degradation was based upon the study by Hergoualc’h et al. (2017). The height distribution of standing palms was split in five ranges (1–7, 7–13, 13–19, 19–25, and 25–31 m). A site was categorized as degraded when the height distribution was skewed towards small or tall individuals, as found by Hergoualc’h et al. (2017). A score of 1 was assigned when the height ranges < 13 m represented < 25% of the standing M. flexuosa palms, or when the height ranges > 19 m represented less than 28% of the standing M. flexuosa palms. An absence of seedling recruitment could also indicate recurrent seed removal following fruit collection. Sites where no young palms (class 1–7 m) were present were scored 1. The degradation class for each site was calculated by summation of the scores assigned for these above-mentioned three criteria. The maximum score or class of 3 suggests high degradation, the minimum score or class of 0 suggests low degradation.
2.3.5 Data analysis
All carbon parameters were tested for normality using skewness and kurtosis, with limits at ± 1. If parameters did not conform to the assumptions of parametric statistics, non-parametric methods were employed and Kruskal-Wallis tests were used to compare mean C estimates between locations and sites. In other cases, ANOVA’s and Bonferroni post-hoc tests were used. All statistical operations were performed with the software IBM SPSS Statistics for Windows 21.0 (IBM Corp. 2012).
3 Results
3.1 Structure and composition of palm swamp forests
In total, 2338 individual living trees were counted and measured across the 12 sampled sites (Appendix Table 1), corresponding to 58 families and 214 species. Approximately 16–28 families of trees with dbh ≥ 5 cm were encountered at each sampled location, with a slightly lower number in Samiria region. Number of species varied among different locations (27–52 species), with highest number of species at Las Brisas (46), Llanque (52), and Monteverde (52) sites (Appendix Table 1). Mauritia flexuosa was present in all sites and it was the most abundant, frequent, and dominant species in most sites considering individuals with dbh ≥ 10 cm (Table 3). Other important trees and palms (dbh ≥ 10 cm) from these sites represent diversity of vegetation encountered at the sampled locations (Table 3). Species found to be abundant in peatland pole forest such as Pachira brevipes (Malvaceae), Oxandra mediocris (Annonaceae) and Platycarpum loretensis (Rubiaceae) (Draper et al. 2017) were present at all sites except Monteverde in Tigre region.
Two attributes of vegetation characteristics: basal area (m2 ha−1) and tree density (trees ha−1) — provide an indication of the forest structure at a location. Basal area ranged from 7 to 24 m2 ha−1 for dicots, 0.2 to 5.5 m2 ha−1 for other palms, and 1.3 to 11.7 m2 ha−1 for M. flexuosa among all sampled sites (Table 4). Highest basal area for dicot trees was observed at Avispa while lowest was observed at San Martin. Among the 11 sites where dbh of palms was measured, highest basal area for M. flexuosa was observed at Monteverde while lowest was observed at San Julian. Only four sites (San Julian, Avispa, Chanchari, and Shiringal) had basal area represented by all palms amounting to less than 40% of total dicot tree basal area, which indicated that Arecaceae family was less prevalent in these forests.
Tree density ranged from 292 to 1517 ha−1 for dicots, 11–435 ha−1 for other palms, and 16–170 ha−1 for M. flexuosa among sampled sites. Highest tree density for dicot trees was observed at Avispa, while lowest was observed at Shiringal. Highest M. flexuosa density was observed at Quistococha, while lowest was at San Julian. Two sites in Tigre (Chanchari and Monteverde) and three sites in Samiria (Pobre Cocha, San Martin, and Shiringal) had total palm density amounting to more than 40% of dicot trees density.
3.2 Above and belowground biomass and soil C stocks
The highest and lowest aboveground vegetation C stock were found in Itaya region (Quistococha—97.7 ± 15 Mg C ha−1 and San Julian—36.8 ± 10.5 Mg C ha−1; Appendix Table 3). There was no difference in the mean aboveground vegetation C stocks among three regions, nor did individual sites differ significantly (Fig. 3a). Mean belowground vegetation C stocks displayed similar variation across the sites, with highest and lowest belowground vegetation C stocks also in Itaya region (Quistococha—24.9 ± 4.1 Mg C ha−1 and San Julian—8.8 ± 2.5 Mg C ha−1; Fig. 3b). In addition, there was no difference in the total vegetation C stocks among different sites across regions (Fig. 3c).
Aboveground (a) and belowground (b) C stocks for M. flexuosa (MF), dicot trees (trees), and other palms; (c) total vegetation, downed woody debris (DWD), and litter C stocks; (d) soil C stocks for soil layer < 1 and > 1 m. Error bars represent one standard error for the entire stacked bar. Note the scales of y-axes differ
Within the sites sampled in Itaya region, Quistococha and Las Brisas had vegetation C stocks of 122.5 ± 19 and 86.7 ± 15.2 Mg C ha−1, respectively; however, the third site: San Julian—had only 45.6 ± 12.9 Mg C ha−1. In Tigre region, highest vegetation C stocks were found at Avispa (116.1 ± 14.2 Mg C ha−1) and lowest at Nueva York (57.6 ± 13.3 Mg C ha−1). Within sites in Samiria region, largest vegetation C stocks were present in San Miguel (117.8 ± 27.6 Mg C ha−1) whereas lowest vegetation C stocks were found at Pobre Cocha (63.9 ± 15.2 Mg C ha−1). Fraction of total aboveground vegetation C stocks contributed by palms ranged from 26 to 70% within three regions, with no one region having high or low C stocks in palms (Table 4 and Appendix Table 3).
Downed woody debris C ranged from 2.1 ± 0.7 to 23.1 ± 14.9 Mg C ha−1 among different sites (Fig. 3c, Table 5). Carbon in DWD was relatively small except for three sites (San Julian, Nueva York, and Pobre Cocha) where it amounted to approximately 22–36% of the total vegetation C stocks (Table 5). Litter C was generally uniform among the sites representing ~ 6.9% of total vegetation C. Lowest litter C was observed at San Miguel (2.3 ± 0.2 Mg C ha−1) and highest at Quistococha (7.8 ± 1.3 Mg C ha−1) and Pobre Cocha (7.8 ± 0.6 Mg C ha−1).
The major repository of C in these forested ecosystems are the soils. Variability in soil C storages along soil depth profile and among six subplots at sampled site reveals similarity within sampled regions, but wide variation exists between different regions (Fig. 3d; Appendix Tables 4, 5, 6, and 7). Mean soil C stocks in top 1 m of soil profile for Itaya, Tigre, and Samiria regions were 298 ± 96, 471 ± 39, and 225 ± 42 Mg C ha−1, respectively. When the entire soil depth was included, the mean soil C stocks were 577 ± 238, 1067 ± 116, and 234 ± 44 Mg C ha−1 for these three regions, respectively.
3.3 Soil physico-chemical properties
There was a considerable variation in soil depths across the three regions (p < 0.01), and among the sampled sites (p < 0.01) (Table 1). The majority of sites had a surface layer of organic peat, followed by peaty clay, and then a dense gleyed clay horizon. Bulk density (g cm−3) was typically low within the surface soils (1 m soil profile) suggesting a prevalence of organic matter, except in sections where underlying clay deposits were encountered (Appendix Tables 4, 5, and 6). For instance, within the Itaya region, Las Brisas and Quistococha had deep organic soils; however, San Julian soils were shallow and had higher bulk density (Table 1). In Tigre region, bulk density was low (< 0.3 g cm−3) in the surface soils (top 1 m soil) at all sampled sites. Soils in Samiria region were shallower and thick-textured with higher bulk density than the other two regions (p < 0.01). Carbon concentration in surface 1-m soil profile in Itaya region ranged from 50 to 53% in Quistococha and Las Brisas and considerably lower at San Julian (17%; Appendix Table 4). Low C content and high bulk density in San Julian soils reflect mineral nature of sampled soils instead of peat observed at other sites, which was specifically visible in subplots 4, 5, and 6 at this site (Appendix Table 4). As a consequence, peat C density at San Julian was low in comparison to peat density in the other two sites in Itaya region (p < 0.01) (Table 1). In Tigre region, C content in top 1 m soil ranged from 26 to 53% with Chanchari having highest (> 52%) and Monteverde lowest (< 30%) C concentration. In Samiria region, C content in top 1 m soil ranged from 20 to 34% among sites (Table 1). Carbon density was on average higher in Tigre region (4.6 g C cm−3) in comparison to Itaya (3.2 g C cm−3) and Samiria (3.1 g C cm−3) regions (p < 0.01).
The Ca/Mg ratio profiles from the sampling locations yielded information about the prevailing conditions during soil/peat formation at these palm swamp forests. All sampled sites in Samiria showed Ca/Mg > 6 (Fig. 4a, b), suggesting presence of riverine inputs in this region. In Itaya region, Ca/Mg ratio at San Julian was < 6 indicating minimal influence of riverine inputs over short time horizons. Only one site in Tigre region (Chanchari) had a Ca/Mg ratio > 6 while other four sites had low Ca/Mg ratio where periodic river inputs may have been minimal. The depth profile of Ca/Mg ratio at Chanchari showed an interesting pattern, with a low Ca/Mg ratio (< 6) at the surface and high Ca/Mg ratio (~ 13) at about 125 cm, and then again low ratio at 175 cm depth followed by high Ca/Mg ratio again at deeper depths. This may be indicative of flooding events that took place at various times in the history, and its signature (in the form of high Ca/Mg ratio) got embedded within the peat layer.
a Variation in calcium/magnesium ratios within soil profile at different sampling locations. b Distribution of sites on the basis of calcium/magnesium ratios within 1 m deep soil profile. Dashed line indicates maximum Ca/Mg ratio observed in the rain water. Low C content at sites occur below 30 cm depth (shown in green rectangle)
Among the sampled sites, strong relationships between soil nutrients and bulk density were observed within the top 1 m of soils (Fig. 5a, b). Soil N content increased linearly with increasing C content while bulk density decreased logarithmically with increasing C content. Within the top 1 m of soil profile, > 70% of samples had a bulk density < 0.2 g cm−3 and a C content > 20%. Surface soil (0–30 cm) contained majority of C stored in these soils and soil C content declined dramatically with increasing soil depth (Fig. 5b).
3.4 Total ecosystem carbon stocks and effect of palm swamp forests degradation
Total ecosystem C stocks in palm swamp forest (PSF) of Peruvian Amazon ranged from 200 to 1000 Mg C ha−1 in Itaya, 900–1600 Mg C ha−1 in Tigre, and from 300 to 400 Mg C ha−1 in Samiria region (See Appendix Table 7 for complete dataset). Mean ecosystem C stocks in Samiria (337.9 ± 38.1 Mg C ha−1) were lowest in comparison to mean ecosystem C in Itaya (677 ± 254.1 Mg C ha−1) and Tigre (1164.7 ± 114.1 Mg C ha−1; p < 0.01). Carbon stocks in Itaya region were significantly lower than carbon stocks in Tigre region (p < 0.01). The largest contribution to total ecosystem C stocks was by forest soils for all sites; however, C stocks in vegetation were important fractions in these systems (Table 6). Sampled sites in Samiria region had relatively higher fraction of C stock in vegetation biomass ~ 25% of total ecosystem C stocks (when both above and belowground fractions were combined). Soils C typically represented 70–90% of total ecosystem C stocks. Carbon stocks in downed woody debris and litter were sizeable (~ 10–15 Mg C ha−1); however, in comparison to vegetation and soil C compartments, these together constituted ~5% of the total ecosystem C stocks (Table 6).
The average degradation scores for Itaya, Tigre, and Samiria regions were 1.3, 1.4, and 2.3, respectively. There were no differences between regions (p = 0.42); degradation classes ranged from low disturbance to higher levels of disturbance within each region (Table 1). Degradation did not correlate with soil C up to 1 m depth, nor the entire soil profile. However, increased levels of degradation showed a trend for lower total vegetation C and forest floor C (DWD + litter) (Fig. 6a). Moreover, the C stock in above and belowground M. flexuosa vegetation showed a clear and significant reduction with increased degradation (r2 = 0.60, p < 0.01) (Fig. 6b). The C stock in trees, other palms, and the forest floor was not significantly correlated to degradation class.
4 Discussion
Palm swamp forests account for most of Peru’s wetlands and the majority of this wetland type is found within the Pastaza-Marañón foreland basin in the western Amazon. Our study was aimed at quantitative assessment of C stocks in these swamp forests, evaluation of C stocks variability across the Pastaza-Marañón region, and appraisal of potential of overharvesting of M. flexuosa on disruption of forest structure and loss of ecosystem functions leading to soil C losses.
4.1 Carbon dynamics on a regional scale
Total ecosystem C stocks determined in this study (200–1600 Mg C ha−1) were within the range reported by Lähteenoja et al. (2009b), Lähteenoja et al. (2012), and Draper et al. (2014) from north eastern region of Peru. Soil C typically represented 70–90% of total ecosystem C, followed by vegetation C as second largest component, followed by DWD and litter. The C stocks in primary (384 Mg C ha−1) and secondary (230 Mg C ha−1) forests of Colombia (Sierra et al. 2007) were much smaller in comparison to the Peruvian palm swamp forests C stocks because of the absence of C rich peat layer. Although litter C represent only a small fraction, a significant amount of soil C originates from residual refractory C that accumulates on the forest floor as litter and does not decompose due to prevailing anoxic conditions of swamp forests.
All sampled locations in Itaya and Tigre regions had high C stocks when the complete soil profile was accounted (except for San Julian and sites in Samiria region) where peat depths were shallow (< 1 m). This represents an interesting finding since the standing vegetation (both aboveground and belowground) C in the three sampled regions were similar; although soil C and peat depth varied between site-to-site. This suggests that landscape level factors (sediment inputs and hydrodynamic processes controlled by annual river flooding) could play an important role in organic matter accumulation, peat formation, and stabilization or erosion in this region. Since the Cretaceous period, rivers originating from the Andes have led to the accumulation of several kilometer thick minerogenic deposits in these subsiding basins (Lähteenoja et al. 2012). Consequently, the accumulation of peat is a dynamic process and is influenced by meandering of river channels (Smith et al. 1989) and avulsions which can cause both burial and erosion of peat deposits (Kalliola et al. 1992; Neller et al. 1992). Moving rivers may be responsible both for burial and erosion of peat located in their path (Morozova and Smith 2003).
The complex stratigraphy observed in many of the sites provide evidence of peat burial and mixing with minerogenic sediments in areas influenced by river flooding and sediment inputs. The peat layer within Tigre region was relatively thick and the upper soil layers did not contain minerogenic deposits (Fig. 4); however, sites from Samiria region had shallow peat layer with overlying clay deposits. Carbon content in Tigre region was highest but N % was relatively low (Fig. 5a). This could be indicative of humification process where recalcitrant C accumulates while N is reduced along the soil profile. In Itaya and Samiria regions, high soil bulk density was observed underneath 30 cm depth horizon (Fig. 5b), whereas bulk densities of surficial layers were relatively low. This may indicate a past incident of flooding or river migration leaving clayey deposits. Subsequent accumulation of vegetation litter and organic matter over clayey sediment resulted in forming a high C content surficial layer, which overtime formed the peaty layer we encountered.
At Tigre sites however, bulk density remained generally low throughout the deeper soil horizons. Lack of clayey riverine sediment deposits in Tigre region is also indicative of lower hydrodynamic energy of the system, which enhances accumulation and accrual of organic matter and is conducive of peat formation (Bailey 1951). Sites in Tigre basin seem to be stable over a historic period of time and do not seem to be affected by landscape shaping characteristics of the main river (Draper et al. 2014). Interestingly, soil bulk density and C content at Chanchari did not show differences with other sampled locations in Tigre region but the Ca/Mg ratio at this site changed dramatically with soil depth, and was dissimilar to other sites (Fig. 4). While it is reasonable to assume that river mediated inputs of sediments have been relatively low or absent for sites situated ~ 12–15 km downstream of Chanchari, it is possible that the river dynamics affecting and shaping the palm forests of Chanchari were different than other sites in the Tigre basin. On the other hand in Samiria region, relatively shallow depth of organic layer, fine clay deposits, and high Ca/Mg ratio suggests that sampled sites were directly affected by the Samiria river dynamics (Figs. 4 and 5).
Using low Ca/Mg ratios as an indicator of ombrotrophic conditions and limited riverine influences, it can be surmised that majority of C stored in the top 1 m soil surface in Itaya and Tigre regions is autochthonous. Where as, high Ca/Mg ratio in Samiria region suggested riverine influence and so soil C at these sites may be associated with the mineral and fine clay particles brought by the river/flood waters. This observation is also supported by elevated soil bulk density in Samiria sites (Fig. 5) which provide another evidence of river flooding in depositing mineral sediments and shaping soil characteristics. A steady decline in Ca/Mg ratio from high (~ 13) at 130 cm to low (~ 5.8) at the surface at Chanchari (Fig. 4a) shows an interesting dynamic process in action. It represents late successional stage of minerotrophic mire and its transition into an ombrotrophic system (Lähteenoja et al. 2009a), possibly facilitated by lower hydrodynamic energy of the system (reduced flooding) and vegetation change.
In Peru, peatlands are found at both low (Lähteenoja et al. 2009b; Householder et al. 2012; Lähteenoja et al. 2013; Draper et al. 2014) and high altitudes (Hribljan et al. 2016; Hribljan et al. 2017) and can be ombrotrophic or minerotrophic (Lähteenoja et al. 2009a) which support the growth of different vegetation types (Roucoux et al. 2013; Draper et al. 2017). No difference in total ecosystem C stocks was observed between ombrotrophic or minerotrophic sites, even though areas that are less influenced by river systems may continue to accumulate organic matter and transform into elevated ombrotrophic bogs (Lähteenoja et al. 2009a; Lawson et al. 2014). It is also likely that vegetation composition (species diversity) may vary between ombrotrophic and minerotrophic sites (Roucoux et al. 2013; Kelly et al. 2017). At Chanchari, where we observed declining Ca/Mg ratios (site transitioning from minerotrophic to ombrotrophic status), most important vegetation species was Virola duckei (Table 2) instead of M. flexuosa. M. flexuosa density can range from 130 to 250 individuals per ha in extensive stands of palm swamp forests in Peru (Kahn 1991), however at Chanchari we found lowest M. flexuosa density (48 palms ha−1) among the five sampled sites in Tigre basin. The reduction in M. flexuosa density could be due to anthropogenic impacts (M. flexuosa fruit harvesting) or due to natural forest succession. Nothwithstanding this transition, Chanchari has maintained itself as a peatland and has the highest overall C stocks in the region (Fig. 3, Appendix Table 7). Based on the floristic composition, some of the sites sampled in Tigre region contained species that are found in ombrotrophic pole forests. For example, species such as Pachira brevipes, Oxandra mediocris, and Platycarpum loretensis found in Avispa and Chanchari, respectively, may be indicative of succession of palm swamp forests to the pole forests (Draper et al. 2017). Therefore, the potential stability of peatlands in this region may also relate to natural succession of the vegetation, and some sites may be undergoing a transition to a pole forest as suggested by the floristic composition of a site (e.g., evidence of pollen record at San Jorge in Rio Amazonas (Kelly et al. 2017)). While dominance of a pioneer species, Cecropia membranacea, at San Julian in Itaya region indicates heavy loss of canopy cover which can be attributed to the degradation caused by anthropogenic impacts. As a result, the degradation score was 3 and vegetation C stocks at San Julian were only 45.6 ± 12.9 Mg C ha−1, lowest of all 12 sampled sites.
4.2 Vegetation characteristics, degradation, and ecosystem carbon stocks
The aboveground vegetation C stock in our sites averaged at 69.9 Mg C ha−1, which was similar to values presented in a review from Southeast Asian peatlands (Hergoualc’h and Verchot 2011) and from palm swamp peatlands of Peru at different levels of degradation (Hergoualc’h et al. 2017). Comparing to Southeast Asian peatlands, our values were closer to the average aboveground C stocks of logged peat swamp forests (85.1 Mg C ha−1), than to virgin peat swamp forests (181.9 Mg C ha−1) (Hergoualc’h and Verchot 2011). Utilizing established metric of classifying forest degradation (Thompson et al. 2013) based on tree density and magnitude of DWDs, sampled sites were categorized into different degradation classes. The impact of anthropogenic forest degradation in this sense is relatively recent in some sites (e.g., Las Brisas, Shiringal) and is not reflected in the observed variation of soil C. We observed that our score of degradation is reflected in the vegetation C; recurrent harvesting of M. flexuosa likely resulted in lower vegetation C. Since swamp forests soil C typically represented 70–90% of total ecosystem C (Table 6), we did not observe any relationship between soil C stocks and our assigned degradation classes. Although the effect of degradation in our sites was not clearly evident on the total ecosystem C stocks, contribution to belowground C by forest vegetation in the form of litter (Melillo et al. 1989; Yule and Gomez 2009) and root biomass might be lower at a degraded site, and it could negatively impact overall C accumulation rates at that site in long term.
Vegetation C fraction was the second largest component and showed large variation both within and between regions, but only showed a trend of reduced C with increased level of degradation (Fig. 6a). However, the observed C stocks in M. flexuosa palms did uniformly decrease in all sites and regions with increased level of degradation (Fig. 6b). The overall variation observed in M. flexuosa C stocks within one degradation class was substantial, e.g., for category 1 this ranged from 12 to 58 Mg C ha−1. This indicates an additional source of variation related to, for example, natural disturbances or succession and less so to anthropogenic disturbances. It is possible that natural succession (transition of PSF into pole forests) may contribute to a “higher” score on degradation scale in our protocol, however separating natural disturbances and succession from anthropogenic disturbances is quite challenging in absence of a long-term study. The degradation scores based on DWD captures impacts within a short time scale (0–5 years), after which the palm trunks gradually disintegrate and disappear from forest floor. The palm height distribution and seedling absence are typically visible for longer time periods after selective harvesting and were used to capture disturbance, but these effects could also be due to natural disturbances or vegetation succession. The only way to truly distinguish between natural and anthropogenic disturbances is by monitoring sites for long time periods and by conducting interviews with local villagers about local practices and land use history, or by monitoring origin of fruits at local markets. Other researchers have recognized this lack of understanding between M. flexuosa harvest and palm swamp forest dynamics (Endress et al. 2013; Virapongse et al. 2017). Evidence of natural succession is evident in Pobre Cocha and Shiringal in Samiria region where Mauritia flexuosa density was low and species representative of seasonally flooded forest were dominant (e.g., Euterpe precatoria and Hura crepitans) suggesting the sites corresponded to more recently developed palm swamps. Palaeo-environmental records from two peatland sites from the studied region (San Jorge and Quistococha) showed that the vegetation changes during early phases of peat initiation were a result of autogenic succession and fluvial influence (Roucoux et al. 2013; Kelly et al. 2017). Pollen data and sedimentary evidence from these two sites confirmed the dominant role of flooding in vegetation transition and succession. Stratigraphic record also indicated multiple abrupt transitions, repetitions, and existing complexity in the processes that shaped the current landscape. Existing palm swamp forests and its peat deposits are an outcome of several dynamic and unpredictable processes that has been active for past 1000s of years, and anthropogenic disturbances need to be minimized to maintain coninuity in that process.
4.3 Conclusions
The results of this study on quantification of C stocks in PSFs in Peruvian Amazonia are meant to highlight the importance of these ecosystems as C repositories. Our investigation was an attempt to quantify existing C stocks in these areas and emphasize the potential role in C storage and global climate change that these ecosystems could play. We utilized a quantitative metric to differentiate peat swamp forests on the basis of existing disturbances and assigned different degradation scores to the studied sites. There was a large amount of variation caused by many factors in our dataset, and the degradation score was only able to explain a small portion of variation in the ecosystem C stocks. Clayey sediments embedded in the peat deposits, Ca/Mg ratios, and discontinuous elevated bulk densities in the soil profiles indicated the influence of river flooding in shaping this landscape. Forest transition and loss of M. flexuosa can be caused by natural succession or by anthropogenic extraction of M. flexuosa fruits; however, the only way to truly distinguish between natural and anthropogenic disturbances is by monitoring sites for long time periods. Since our study protocol does not lend that ability, perhaps a diagnostic criterion can be added in future studies to distinguish natural variation from degradation, and to capture characteristic of pole forests, or successional environments naturally succeeding away from palm swamps.
We found that the Tigre region has been less influenced by rivers during the formation of its peat and consist of large ombrotrophic peatlands with greater depths that have likely formed over longer time-scales. The stability and lower frequency of river avulsions in this region probably were caused due to isolation of Rio Tigre from the Rio Pastaza some 8000 years BP (Bernal et al. 2011). Northern parts of Rio Tigre basin therefore experienced build-up of peat over time and transitioned from high- to low-nutrient status (minerotrophic to ombrotrophic), and may experience vegetation succession to pole forests (Draper et al. 2014). The area in Samiria basin is more influenced by river dynamics and likely underwent sediment deposition, erosion, and burial of peat in not so distant past. Moreover, we see that largest C stocks are in the Tigre river outside of the currently protected Pacaya Samiria reserve. This might point towards a need for new conservation areas in this region for the conservation of palm swamp and pole forests where the largest carbon stocks are stored (Draper et al. 2014). Long-term site-specific studies aimed towards investigating linkages of forest degradation with soil C stocks may provide useful information in this context. In the degraded palm swamps, management plans or interventions to conserve fruit production and genetic resources are needed to have greater potential to protect existing C stocks, while simultaneously preserving all the ecosystem services associated with it.
References
Asner GP, Knapp DE, Martin RE, Tupayachi R, Anderson CB, Mascaro J, Sinca F, Chadwick KD, Sousan S, Higgins M (2014) The high-resolution carbon geography of Peru. Carnegie Airborne Observatory and the ministry of environment of Perú
Bailey HH (1951) Peat formation in the tropics and subtropics. Soil Sci Soc Am J 15(C):283–284
Bernal C, Christophoul F, Darrozes J, Soula J-C, Baby P, Burgos J (2011) Late Glacial and Holocene avulsions of the Rio Pastaza Megafan (Ecuador–Peru): frequency and controlling factors. Int J Earth Sci 100:1759–1782
Bradshaw CJ, Sodhi NS, Brook BW (2008) Tropical turmoil: a biodiversity tragedy in progress. Front Ecol Environ 7:79–87
Brown JK (1974) Handbook for inventorying downed woody material. General Technical Report GTR-INT-16. in Service UF (ed) Missoula
Curtis JT, McIntosh RP (1951) An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 32:476–496
Dargie GC, Lewis SL, Lawson IT, Mitchard ET, Page SE, Bocko YE, Ifo SA (2017) Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 542:86–90
Delgado C, Couturier G, Mejia K (2007) Mauritia flexuosa (Arecaceae: Calamoideae), an Amazonian palm with cultivation purposes in Peru. Fruits 62:157–169
Draper FC, Roucoux KH, Lawson IT, Mitchard ET, Coronado ENH, Outi L, Montenegro LT, Sandoval EV, Baker TR (2014) The distribution and amount of carbon in the largest peatland complex in Amazonia. Environ Res Lett 9:124017
Draper FC, Coronado ENH, Roucoux KH, Lawson IT, Pitman NC, Fine PV, Phillips OL, Montenegro LAT, Sandoval EV, Mesones I, García-Villacorta R (2017) Peatland forests are the least diverse tree communities documented in Amazonia, but contribute to high regional beta-diversity. Ecography. https://doi.org/10.1111/ecog.03126
Emilio T, Quesada CA, Costa FR, Magnusson WE, Schietti J, Feldpausch TR, Brienen RJ, Baker TR, Chave J, Álvarez E (2014) Soil physical conditions limit palm and tree basal area in Amazonian forests. Plant Ecol Divers 7:215–229
Endress BA, Horn CM, Gilmore MP (2013) Mauritia flexuosa palm swamps: composition, structure and implications for conservation and management. Forest Ecol Manag 302:346–353
Goodman RC, Phillips OL, del Castillo Torres D, Freitas L, Cortese ST, Monteagudo A, Baker TR (2013) Amazon palm biomass and allometry. Forest Ecol Manag 310:994–1004
Gumbricht T, Roman-Cuesta RM, Verchot L, Herold M, Wittmann F, Householder E, Herold N, Murdiyarso D (2017) An expert system model for mapping tropical wetlands and peatlands reveals South America as the largest contributor. Glob Chang Biol 23:3581–3599
Harmon ME, Sexton J (1996) Guidelines for measurements of woody detritus in forest ecosystems. US LTER Network Office, Seattle
Hergoualc’h K, Verchot LV (2011) Stocks and fluxes of carbon associated with land use change in southeast Asian tropical peatlands: a review. Glob Biogeochem Cycles 25(2). https://doi.org/10.1029/2009GB003718
Hergoualc’h K, Verchot L (2014) Greenhouse gas emission factors for land use and land-use change in southeast Asian peatlands. Mitig Adapt Strateg Glob Chang 19:789–807
Hergoualc’h K, Gutiérrez-Vélez VH, Menton M, Verchot LV (2017) Characterizing degradation of palm swamp peatlands from space and on the ground: an exploratory study in the Peruvian Amazon. Forest Ecol Manag 393:63–73
Hooijer A, Page S, Canadell JG, Silvius M, Kwadijk J, Wösten H, Jauhiainen J (2010) Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences 7(5):1505–1514
Horn CM, Gilmore MP, Endress BA (2012) Ecological and socio-economic factors influencing aguaje (Mauritia flexuosa) resource management in two indigenous communities in the Peruvian Amazon. Forest Ecol Manag 267:93–103
Householder JE, John PJ, Mathias WT, Susan P, Outi L (2012) Peatlands of the Madre de Dios River of Peru: distribution, geomorphology, and habitat diversity. Wetlands 32:359–368
Hribljan JA, Suárez E, Heckman KA, Lilleskov EA, Chimner RA (2016) Peatland carbon stocks and accumulation rates in the Ecuadorian páramo. Wetl Ecol Manag 24:113–127
Hribljan JA, Suarez E, Bourgeau-Chavez L, Endres S, Lilleskov EA, Chimbolema S, Wayson C, Serocki E, Chimner RA (2017) Multidate, multisensor remote sensing reveals high density of carbon-rich mountain peatlands in the páramo of Ecuador. Glob Chang Biol 23:5412–5425
Jauhiainen J, Takahashi H, Heikkinen JE, Martikainen PJ, Vasander H (2005) Carbon fluxes from a tropical peat swamp forest floor. Glob Chang Biol 11:1788–1797
Kahn F (1991) Palms as key swamp forest resources in Amazonia. Forest Ecol Manag 38:133–142
Kahn F, Mejia K (1990) Palm communities in wetland forest ecosystems of Peruvian Amazonia. For Ecol Manag 33:169–179
Kahn F, Mejia K, de Castro A (1988) Species richness and density of palms in terra firme forests of Amazonia. Biotropica 20:266–269
Kalliola R, Salo J, Puhakka M, Rajasilta M, Häme T, Neller RJ, Räsänen ME, Danjoy Arias WA (1992) Upper amazon channel migration. Naturwissenschaften 79:75–79
Kauffman JB, Donato D (2012) Protocols for the measurement, monitoring and reporting of structure, biomass and carbon stocks in mangrove forests. Center for International Forestry Research Center (CIFOR) Working paper 86
Kauffman JB, Arifanti VB, Basuki I, Kurnianto S, Novita N, Murdiyarso D, Donato DC, Warren MW (2016) Protocols for the measurement, monitoring, and reporting of structure, biomass, carbon stocks and greenhouse gas emissions in tropical peat swamp forests. Center for International Forestry Research (CIFOR), Bogor
Kelly TJ, Baird AJ, Roucoux KH, Baker TR, Honorio Coronado EN, Ríos M, Lawson IT (2014) The high hydraulic conductivity of three wooded tropical peat swamps in northeast Peru: measurements and implications for hydrological function. Hydrol Process 28:3373–3387
Kelly TJ, Lawson IT, Roucoux KH, Baker TR, Jones TD, Sanderson NK (2017) The vegetation history of an Amazonian domed peatland. Palaeogeogr Palaeoclimatol Palaeoecol 468:129–141
Kurnianto S, Warren M, Talbot J, Kauffman B, Murdiyarso D, Frolking S (2015) Carbon accumulation of tropical peatlands over millennia: a modeling approach. Glob Chang Biol 21:431–444
Lähteenoja O, Page S (2011) High diversity of tropical peatland ecosystem types in the Pastaza-Marañón basin, Peruvian Amazonia. J Geophys Res Biogeosci 116(G2). https://doi.org/10.1029/2010JG001508
Lähteenoja O, Ruokolainen K, Schulman L, Alvarez J (2009a) Amazonian floodplains harbour minerotrophic and ombrotrophic peatlands. Catena 79:140–145
Lähteenoja O, Ruokolainen K, Schulman L, Oinonen M (2009b) Amazonian peatlands: an ignored C sink and potential source. Glob Chang Biol 15:2311–2320
Lähteenoja O, Reátegui YR, Räsänen M, Torres DDC, Oinonen M, Page S (2012) The large Amazonian peatland carbon sink in the subsiding Pastaza-Marañón foreland basin, Peru. Glob Chang Biol 18:164–178
Lähteenoja O, Flores B, Nelson B (2013) Tropical peat accumulation in Central Amazonia. Wetlands 33:495–503
Lawson IT, Jones TD, Kelly TJ, Coronado ENH, Roucoux KH (2014) The geochemistry of Amazonian peats. Wetlands 34:905–915
Lawson IT, Kelly T, Aplin P, Boom A, Dargie G, Draper F, Hassan P, Hoyos-Santillan J, Kaduk J, Large D (2015) Improving estimates of tropical peatland area, carbon storage, and greenhouse gas fluxes. Wetl Ecol Manag 23:327–346
Limpens J, Berendse F, Blodau C, Canadell J, Freeman C, Holden J, Roulet N, Rydin H, Schaepman-Strub G (2008) Peatlands and the carbon cycle: from local processes to global implications—a synthesis. Biogeosciences 5:1475–1491
Manzi M, Coomes OT (2009) Managing Amazonian palms for community use: a case of aguaje palm (Mauritia flexuosa) in Peru. Forest Ecol Manag 257:510–517
Marengo J (1998) Climatología de la zona de Iquitos, Perú Geoecologia y desarrollo Amazonico: estudio integrado en la zona de Iquitos. Peru University of Turku Press, Turku, pp 35–57
Maria Pacheco SL (2005) Nutritional and ecological aspects of buriti or aguaje (Mauritia flexuosa Linnaeus filius): a carotene-rich palm fruit from Latin America. Ecol Food Nutr 44:345–358
Melillo JM, Aber JD, Linkins AE, Ricca A, Fry B, Nadelhoffer KJ (1989) Carbon and nitrogen dynamics along the decay continuum: plant litter to soil organic matter. Plant Soil 115:189–198
MINAM (2010) Mapa del Patrimonio Forestal Nacional. In: Dirección General de Evaluación VyFdPN (ed) Ministerio del Ambiente-MINAM, Lima, Peru
Morozova GS, Smith ND (2003) Organic matter deposition in the Saskatchewan River floodplain (Cumberland Marshes, Canada): effects of progradational avulsions. Sediment Geol 157:15–29
Murdiyarso D, Kauffman JB, Verchot LV (2013) Climate change mitigation strategies should include tropical wetlands. Carbon Manag 4:491–499
Neller R, Salo J, Rasanen M (1992) On the formation of blocked valley lakes by channel avulsion in Upper Amazon foreland basins. Z Geomorphol 36:401–411
Page SE, Rieley JO, Banks CJ (2011) Global and regional importance of the tropical peatland carbon pool. Glob Chang Biol 17:798–818
Palace M, Keller M, Hurtt G, Frolking S (2012) A review of above ground necromass in tropical forests. In: Sudarshana P, Nageswara-Rao M, Soneji JR (eds) Tropical forests. Intech (online publisher), pp 215–252. Published online at: http://www.intechopen.com/books/tropical-forests
Parodi JL, Freitas D (1990) Geographical aspects of forested wetlands in the lower Ucayali, Peruvian Amazonia. Forest Ecol Manag 33:157–168
Posa MRC, Wijedasa LS, Corlett RT (2011) Biodiversity and conservation of tropical peat swamp forests. Bioscience 61:49–57
Queiroz JSD, Silva F, Ipenza C, Hernick C, Batallanos L, Griswold D, Rogers AE (2014) Peru tropical forest and biodiversity assessment. USAID. Published online at http://www.usaidgems.org/Documents/FAA&Regs/FAA118119LAC/Peru%20FAA%20118%20119%20FB%20Assessment%20August%202014.pdf
Räsänen ME, Salo J, Jungnert H, Pittman LR (1990) Evolution of the western Amazon lowland relief: impact of Andean foreland dynamics. Terra Nova 2:320–332
Räsänen M, Neller R, Salo J, Jungner H (1992) Recent and ancient fluvial deposition systems in the Amazonian foreland basin, Peru. Geol Mag 129:293–306
Rieley J, Wüst R, Jauhiainen J, Page S, Wösten H, Hooijer A, Siegert F, Limin S, Vasander H, Stahlhut M (2008) Tropical peatlands: carbon stores, carbon gas emissions and contribution to climate change processes. In: Peatlands and climate change, vol 12. International Peat Society, Vapaudenkatu, pp 148–182
Rodríguez F (1990) Los suelos de áreas inundables de la Amazonía Peruana: potencial, limitaciones y estrategias para su investigación. Folia Amazónica IIAP 2:7–25
Roucoux KH, Lawson IT, Jones TD, Baker TR, Coronado EH, Gosling WD, Lähteenoja O (2013) Vegetation development in an Amazonian peatland. Palaeogeogr Palaeoclimatol Palaeoecol 374:242–255
Sierra CA, del Valle JI, Orrego SA, Moreno FH, Harmon ME, Zapata M, Colorado GJ, Herrera MA, Lara W, Restrepo DE, Berrouet LM, Loaiza LM, Benjumea JF (2007) Total carbon stocks in a tropical forest landscape of the Porce region, Colombia. Forest Ecol Manag 243:299–309
Simard RR (1993) Ammonium acetate-extractable elements. In: Carter MR (ed) Soil sampling and methods of analysis. Canadian Society of Soil Science, Lewis Publishers, Boca Raton, pp 39–49
Smith ND, Cross TA, Dufficy JP, Clough SR (1989) Anatomy of an avulsion. Sedimentology 36:1–23
Terborgh J, Andresen E (1998) The composition of Amazonian forests: patterns at local and regional scales. J Trop Ecol 14:645–664
Thompson ID, Guariguata MR, Okabe K, Bahamondez C, Nasi R, Heymell V, Sabogal C (2013) An operational framework for defining and monitoring forest degradation. Ecol Soc 18:20
van Lent J, Hergoualc’h K, Verchot L, Oenema O, van Groenigen JW (2018) Greenhouse gas emissions along a peat swamp forest degradation gradient in the Peruvian Amazon: soil moisture and palm roots effects. Mitig Adapt Strateg Glob Chang 1–19. https://doi.org/10.1007/s11027-018-9796-x
Virapongse A, Endress BA, Gilmore MP, Horn C, Romulo C (2017) Ecology, livelihoods, and management of the Mauritia flexuosa palm in South America. Glob Ecol Conserv 10:70–92
Yule CM, Gomez LN (2009) Leaf litter decomposition in a tropical peat swamp forest in Peninsular Malaysia. Wetl Ecol Manag 17:231–241
Zambrana NYP, Byg A, Svenning J-C, Moraes M, Grandez C, Balslev H (2007) Diversity of palm uses in the western Amazon. Biodivers Conserv 16:2771–2787
Acknowledgements
The research was a collaborative effort with partners from Instituto de investigaciones de la Amazonía Peruana (IIAP), US Department of Agriculture and CIFOR. Authors would like to thank Prof. Martin Herold, Dennis Del Castillo, Monica Aleman and Gloria Arellano and a group of enthusiastic people who enabled data collection in the field. We acknowledge the effort of field crew members—Nicole M Riviera, Julio Irarica, Diego Martin, Jack Pacaya, Victor Ruiz, Cecilia B Falcón, Ricardo Z Young, Elvis J Paredes, Rique B Estrada, Jose Manuel R Huaymacari, Maria E R Pena, Jhon del A Pasquel, Carlos G Hidalzo Pizano, and Luisa N Huaratapairo. The Analytical Lab, University of Hawaii, Honolulu, US is also acknowledged for performing soil elemental analysis. We also thank the SERNANP for providing research permit (No 009-2015-SERNAP-RNPS-JEF) that enabled sampling in the Pacaya Samiria National Reserve. Finally, we thank two anonymous reviewers and associate editor for their insightful comments that greatly improved this manuscript.
Funding
This study was made possible by a grant from the US Department of Agriculture, Forest Service (FS), Washington Office, and FS International Programs, implemented by Department of Environmental Sciences, Wageningen University, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 90 kb)
Rights and permissions
About this article
Cite this article
Bhomia, R.K., van Lent, J., Rios, J.M.G. et al. Impacts of Mauritia flexuosa degradation on the carbon stocks of freshwater peatlands in the Pastaza-Marañón river basin of the Peruvian Amazon. Mitig Adapt Strateg Glob Change 24, 645–668 (2019). https://doi.org/10.1007/s11027-018-9809-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11027-018-9809-9