A method for enthalpy calibration of differential scanning calorimeters is described which makes it possible to eliminate the existing limits on the temperature range within which this metrological procedure can be carried out. The proposed method does not require use of standard samples of heats of fusion, so it is not limited by the thermal properties of certified materials. This method solves the problem of increasing the accuracy of measurements of specific enthalpy and heats of phase transitions of various substances for all types of differential scanning calorimeters. The effectiveness of the proposed method for solving the accuracy problems is confirmed and illustrated by the authors by comparing the results of measurements of heats of fusion of a number of metals. Results of measurements obtained on differential scanning calorimeters calibrated by the proposed method and on the same instruments, but calibrated in accordance with the generally accepted standard method of the International Union of Pure and Applied Chemistry, are compared. During the experiment, the advantages of the enthalpy calibration method developed here are identified in terms of saving the time and the costs necessary for calibration. The results can be used in work with differential scanning calorimeters, in the development of new materials, the monitoring of technological processes, production, input, and output control, as well as in the development of methods for studying various materials using differential scanning calorimeters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction. Differential scanning calorimeters (DSC) are instruments that make relative measurements. As opposed to adiabatic calorimeters, DSC require transformation of the relative values they obtain into absolute values. The procedure for conversion of the relative into absolute values involves calibration of the DSC. The calibration of a DSC establishes the relationship between the value recorded by the measurement device and the true value of that quantity.
At present, the technique developed by the International Union of Pure and Applied Chemistry (IUPAC) for calibrating DSC based on studies by a working group of the Gesellschaft für Thermische Analyse (GEFTA) [1,2,3] is used in most countries. This technique was accepted in 2000 by the International Confederation for Thermal Analysis and Calorimetry (ICTAC) as a recommendation for its members [4]. This method for enthalpy calibration uses the fusion characteristics of pure substances employed in constructing the International Temperature Scale (ITS-90); the heats of phase transformations of these substances (melting or crystallization) are known with sufficient accuracy. For calibration by the IUPAC technique the following operations are carried out: in the temperature range of interest the heats of fusion Tf of a number of calibration substances are measured and then the calibration coefficient K is calculated for each heat of fusion Tf.
The coefficient K is equal to the ratio of the total true heat of fusion and the heat of fusion recorded by the instrument:
where ΔHΣ is the total true heat of fusion, which is taken to be the certified value of the specific heat of fusion for a given material ΔHsp (for Standards Reference Materials (SRM), this is indicated in the certificate; for Government Standard Samples (GSS), in the test ticket or description of the type) taking into account the mass m of the sample being studied; S is the heat of fusion registered by the instrument determined from the area of the detected signal; P is the recorded power expended in melting of the material; t = te – t0 is the melting time with t0 and te being the coordinates on the time axis corresponding to the initial and final times for melting of the material.
Based on the obtained experimental data Ki(Tf), an interpolated polynomial K = (Ki(Tf)) is constructed for calculating the coefficient K at any temperature within the studied temperature range.
During practical application of the IUPAC technique, it became clear that the speed of the studies, mass of the batch of pure substances, and a number of other factors have a significant effect on the value of the calibration coefficient. Here the error in the calibration coefficient K calculated from the plotted interpolation polynomial for temperatures between the points corresponding to the melting points of the reference materials is always at least 1% [4]. In practice, even at the reference points calculated from the polynomial the values of the heat of the phase transition (fusion) differ from the manufacturer’s data by 0.5–1.0%.
The error in calibration by the IUPAC method [4] can be reduced only by increasing the number of experimental points used to construct the polynomial, i.e., by increasing the number of materials used in the calibration: the Standards Reference Materials (SRM) or Government Standard Samples (GSS). The number of SRM and GSS is extremely limited at present.
Description of the proposed calibration technique. The authors have developed and studied a method for enthalpy calibration of DSC. The method is based on the principle of calibration using certified values of the enthalpy of sapphire (leuco sapphire), a material that is stable in time and with changes in temperature over a wide interval.
With this method, the difference in the enthalpies ΔH of the sapphire is taken over a specific temperature interval. During development of the method, the most representative temperature interval was chosen to be ±20 K from the temperature of the phase transition that was studied:
where Tph is the temperature of the phase transition being studied.
We calculate the calibration coefficient using the formula
where Ss is an area equal to the sum of the areas under the curves recorded by the instrument during scanning of the sapphire sample and the empty crucibles over the temperature range Ti–1–Ti.
The desired value of the heat of the phase transition of the test material ΔHf is given by the formula
where Sx is the area under the curve recorded by the DSC during scanning of the test material.
An important operation in reducing the error in integrating when determining the area recorded during scanning of the sapphire, empty crucibles, or a sample is the operation of equalization (matching the curves) for the values of the recorded power in the isothermal regime before and at the end of scanning (the procedure “Slope” in the program Pyris for Perkin- Elmer instruments). Equalization is carried out before taking the integral.
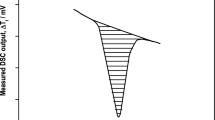
Figure 1 shows curves recorded by the instrument during measurement of the enthalpy of sapphire and empty crucibles within the range of the melting temperatures of tin, as well as the recorded melting curve for tin, and the areas used in calculations of the calibration coefficient and the true value of the heat of fusion of the material.
Calculating the calibration coefficient by the proposed method: 1, 3, 5) the heat flux Φ obtained in measurements of tin, sapphire, and empty crucibles, respectively; 2) an area equal to the heat of fusion of tin; 4) an area equal to the enthalpy of fusion of sapphire over the temperature range corresponding to the temperature range for the melting of tin ±20 K.
The advantages of the proposed approach are:
-
the user has access to the numerical values of the comparison standard at any temperature over the widest temperature range of 10–2300 K. In Russia, data on the enthalpy of sapphire are provided in the description of the standard sample type of thermodynamic properties (SOTS-1) GSO 149-86P No. 3039 and in international practice, in Ref. 5;
-
the values of the comparison standard are characterized by an error of 0.1%, i.e., less than the errors in the heats of fusion of SRM or GSS, recommended by the IUPAC for calibration of DSC [4]; and
-
the experimental value of the calibration coefficient corresponds directly to a narrow temperature interval within which the instrument records the transition process being studied.
Results of an experimental study of the enthalpy calibration technique. For experimental testing of the prospects for applying the proposed method, we have measured the heats of fusion of indium In, tin Sn, bismuth Bi, and zinc Zn on a Perkin-Elmer DSC8000. For each material three experiments were conducted with samples of different mass. All the measurements were made on an instrument with the enthalpy calibration (in accordance with IUPAC) turned off and at a scan rate of 10 K/min.
The results are shown in Table 1, where mi is the mass of the test sample, Sxi is the area under the melt curve recorded by the instrument during scanning of the test sample, Ki are the values of the calibration coefficient obtained for each of the materials that was studied relative to sapphire, ΔHfi is the heat of the phase transition for each sample obtained by the enthalpy calibration method, ΔHsp.fi is the specific heat of fusion for each sample, ΔHav is the specific heat of fusion of a material, equal numerically to the average value of the specific heat of the samples of each material, ΔHcert is the certified value of the specific heat of a material, and δ is the relative deviation of the obtained value of the specific heat of a material from the certified value. In the Russian Federation, the certified values are taken to be data from the description of the type in the Set of standard samples of temperatures and heats of phase transitions (the SOTSF complex) GSO 2312-82/2316-82 (UNIIM, certification confirming the type of standard samples No. 6133, valid until September 6, 2024) and in international practice (values given in parentheses), from Refs. 6,7,8. The discrepancies between the observed and certified values of the specific heats of these materials did not exceed 0.56%.
Table 2 lists the results of measurements of the energy characteristics of the certified standard samples (SS) obtained by the IUPAC method and the enthalpy calibration technique (ΔHsp.f is the specific heat of fusion of the standard samples of indium, tin, bismuth, and zinc). The values of the specific heat of fusion of the standard samples obtained on calibrating the DSC8000 by the proposed method are characterized by an error comparable to, and for some temperature ranges, less than when the IUPAC method is used to calibrate the DSC8000.
The proposed method does not yield a clear gain in accuracy for DSC of the compensation type, but it does permit the use of leuco sapphire for an almost unlimited time. But the use of standard samples for calibration of the heats of fusion provided by the IUPAC is limited by the number of melting thermal cycles after which it becomes necessary to obtain new SRM and GSS.
In Tables 3 and 4 the results of measurements of the heat of fusion of materials with calibration of the DSC of an uncompensated type (DSC1, Mettler-Toledo) by the proposed method with the heats of fusion determined with this same instrument but calibrated by the IUPAC method. The following notation is introduced in Table 3: Ss is the area under the curve recorded by the instrument when measuring sapphire in the range of temperatures which corresponds to the temperature interval for melting of the specific material (type of standard sample) ±20 K; ΔH is the obtained value of the specific heat of fusion of the material without using the calibration coefficient. The results (see Table 3) indicate that the enthalpy calibration technique is suitable for uncompensated type DSC. while according to the results of Table 4 this method offers promise and has an advantage (higher accuracy) for calibration of uncompensated-type DSC.
Conclusion. Use of the enthalpy calibration technique developed here to reduce the instrument error is appropriate for DSC of an uncompensated type, since for calorimeters of the compensated type the discrepancy from the certified values of the specific heats of fusion are comparable to those for calibration by the IUPAC method. However, by using the proposed technique for all forms of DSC it is possible to reduce the component error in measurements of the enthalpies or heats of phase transitions owing to drift of the calibration coefficient over time because of a reduction in the calibration time and the possibility of making the calibration and measurements of the test material within a single day.
The enthalpy calibration technique can be used for all types of DSC and over any working temperature range within the interval from 10–2300 K, since it is not limited by the temperature characteristics of the existing standard samples and makes it possible to measure the calibration coefficient for a narrow temperature interval within which the the phase transition process is recorded. The enthalpy calibration process developed here can be recommended for precision studies of the energy characteristics of phase transitions.
References
St. M. Sarge, E. Gmelin, G. W. H. Höhne, et al., Thermochim. Acta, 247, No. 2, 129–168 (1994), https://doi.org/10.1016/0040-6031(94)80118-5.
St. M. Sarge, G. W. H. Höhne, H. K. Cammenga, et al., Thermochim. Acta, 361, No. 1–2, 1–20 (2000), https://doi.org/10.1016/s0040-6031(00)00543-8.
E. Gmelin and St. M. Sarge, Pure & Appl. Chem., 67, No. 11, 1789–1800 (1995), https://doi.org/10.1351/pac199567111789.
G. D. Gatta, M. J. Richardson, St. M. Sarge, and S. Stølen, Pure & Appl. Chem., 78, No. 7, 1455–1476 (2006), https://doi.org/10.1351/pac200678071455.
D. G. Archer, J. Phys. Chem. Ref. Data, 22, 1441–1453 (1993), https://doi.org/10.1063/1.555931.
F. Grønvold, J. Chem. Thermodyn., 25, No. 9, 1133–1144 (1993), https://doi.org/10.1006/jcht.1993.1110.
D. G. Archer, J. Chem. & Eng. Data, 49, No. 5, 1364–1367 (2004), https://doi.org/10.1021/je049913p.
F. Grønvold and S. Stølen, Thermochim. Acta, 395, No. 1–2, 127–131 (2002), https://doi.org/10.1016/s0040-6031(02)00217-4.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Metrologiya, No. 4, pp. 40–52, October–December, 2021.
Rights and permissions
About this article
Cite this article
Kompan, T.A., Kulagin, V.I. & Vlasova, V.V. Enthalpy Calibration Method for Differential Scanning Calorimeters. Meas Tech 64, 999–1003 (2022). https://doi.org/10.1007/s11018-022-02035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11018-022-02035-2