A unified fundamental equation of state has been developed for 2,3,3,3-tetrafluoropropene (R1234yf), a fourth-generation ozone-safe refrigerant, and a method for constructing the equation has been proposed. In the gas region, this equation transforms into an equation of state of the virial form, and in the vicinity of the critical point it satisfies the requirements of the modern scale theory of critical phenomena and transforms into the Widom scale equation. Based on a single fundamental equation of state in accordance with GOST R 8.614-2018, “GSI. State Service of Standard Reference Data. Basic Provisions,” we developed standard reference data GSSSD 380-2020 on density, enthalpy, isobaric heat capacity, isochoric heat capacity, entropy and speed of sound in R1234yf in the temperature ranges 230–420 K and pressures 0.1–20 MPa. We compared the calculated values of equilibrium properties with the most reliable experimental data obtained in well-known global laboratories and tabular data obtained from the known fundamental equations of state R1234yf. Uncertainties of tabulated data for saturated vapor pressure, density, enthalpy, isobaric heat capacity, isochoric heat capacity, entropy and speed of sound of 2,3,3,3-tetrafluoropropene are estimated – standard relative uncertainties for types A, B, total standard relative and expanded uncertainties. The results obtained in this work show that the proposed unified fundamental equation of state adequately describes the equilibrium properties of R1234yf in the above stated range of state parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction. The compound CF3–CF=CH2 (2,3,3,3-tetrafluoro-propene, R1234yf) is a fourth generation ozonefriendly refrigerant. R1234yf has a potential for depleting the Earth’s ozone layer ODP = 0 and a global warming potential GWP = 4. Since 2011, European Union legislation prohibits the use of refrigerants with a GWP > 150 in transport vehicles of new type and in all new vehicles since 2017 [1]. R1234yf replaced the previously used refrigerant R134a (GWP = 1600) in vehicle air conditioning systems. At the same time, the performance characteristics of both refrigerants are similar, which makes it possible to use R1234yf without significant modifications to the air conditioning systems of cars. The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) safety standard assigns R1234yf the classification A2L. Data on the thermodynamic properties of R1234yf [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16] and the equations of state developed on their basis [9, 17] appeared in the open literature relatively recently. Moreover, the fundamental equations of state (FEOS) [9, 17] do not even qualitatively convey the features of the thermodynamic surface in the vicinity of the critical point. Therefore, FEOS [9, 17] cannot be classified as a unified FEOS (UFEOS) [18]. The purpose of this work is to develop a UFEOS for the refrigerant R1234yf and to calculate tables of standard reference data based on it.
Method for constructing UFEOS for R1234yf. To construct a UFEOS, the properties of R1234yf suggest a simple model in the form of the Helmholtz free energy F(ρ, T) [18]:
where F0(ρ, T) is the ideal gas component of free energy [9]; ρ is the density; T is the temperature; R is the gas constant;
Z cr = pcr/(RρcrTcr)·106; Tcr, ρcr, pcr are critical parameters (temperature, density, pressure); Ci, j, D1, D2, D3, a, b are constant coefficients; δ is the critical index of the critical isotherm.
In UFEOS (1), the scale function a(x) of the Helmholtz free energy is determined by the expression [19]:
where x = τ/|Δρ|1/β is the scale variable, which is found from a unique nonanalytic equation of state [20]; (2 – α)a(x = –x0) – – xa′(x = –x0) = 0; τ = T/Tcr – 1; A = –Γkγ1/[2αb2α1(1 – ε)]; B = Γ(2k)–1; α1 = (α – 2)(α – 1); γ1 = γ(γ –1); x1, x2, x3, C are constant parametersr; ε = x1/x2; b2 = (γ – 2β)/[γ(1 – 2β)]; k = [(b2 – 1)/x0]β; Γ is an individual paramete; α, β, γ are critical indices related to the index δ by the Griffiths equalities: 2 – α = βδ + β; γ = βδ – β.
First, the choice of the Helmholtz free energy in form (1) greatly simplifies the algorithm for finding the UFEOS parameters in (1); second, as shown in [18, 20], structure (1) accounts for the experimentally confirmed Benedek hypothesis [21] and the phenomenological theory of the critical point [22]. According to the analysis of the authors of this article, when ρ → 0, UFEOS (1) reduces to a virial equation of the form Z = 1+ ωB(T) + ..., where Z = p/(ρRT) is the compressibility; B(T) is the second virial coefficient, and in the region of strongly developed density fluctuations, UFEOS (1) transitions into a physically based Widom equation [23].
The latter means that within the framework of the approach proposed by the authors of this article, power-law dependences are fulfilled at all scales, including the dependences for isochoric CV and isobaric Cp heat capacities, respectively:
Consequently, UFEOS (1), in accordance with the requirements of the scaled theory [24], describes a thermodynamic surface in the asymptotic vicinity of the critical point.
The UFEOS parameters in (1) were found using the data set [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16] in accordance with GSSSD ME 247-2016, “Method for calculating the thermodynamic properties of 2,3,3,3-tetrafluoropropane in the temperature range from 230 K to 370 K and pressures from 0.1 MPa to 10 MPa”:
the coefficients Ci, j are presented in Tables 1, 2. The parameter Γ = 5.568426059213551 was calculated according to the method considered in [25] and based on the Lysenkov–Rykov similarity relation [26].
The calculated values of the properties of R1234yf from UFEOS (1) for checking the computer code are presented in Table 3, where H is the enthalpy; S is entropy.
Analysis and discussion of research results. Using UFEOS (1), we computed tables of standard reference data (GSSSD 380-2020) for the properties of R1234yf, G = (ρ, H, S, CV, CP, w, L), where L is the specific heat of vaporization, for the temperature range 230–420 K and pressures 0.1–20 MPa.
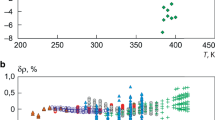
The p, ρ, T, CV, CP, w values calculated from UFEOS (1) are compared with the corresponding experimental data [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. In Figs. 1–4 we show the relative deviations δr = (rexp – req)/rexp·100 of the values req calculated from UFEOS (1) from the experimental data rexp of the corresponding property r. It was found that UFEOS (1) describes with low uncertainty (within the error of experimental data) both a thermal surface (Figs. 1, 2) and experimental data on isochoric CV (Fig. 3) and isobaric CP heat capacities (Figs. 4, 5). Unlike FEOS [9, 17], the UFEOS developed within the proposed method (1) describes the thermodynamic surface of R1234yf in the vicinity of the critical point in accordance with scaled theory of critical phenomena [24] (cf. Fig. 5). Therefore, we also calculated detailed tables of standard reference data G = (ρ, H, S, CV, CP, w, r) for the properties of R1234yf in a broad neighborhood of the critical point.
Relative divergence values of density δρ calculated as per the equations presented in this study UFEOS (1) as compared with the experimental data [9].
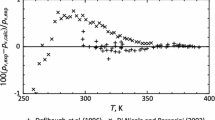
Relative divergence values of pressure δp calculated as per the equations presented in this study UFEOS (1) as compared with the experimental data [12].
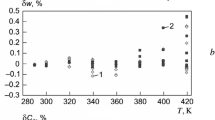
Relative divergence values of isobaric heat capacity δCP calculated as per the equations presented in this study UFEOS (1) as compared with the experimental data [8].
Behavior of isobaric heat capacity in the critical region on isothermal lines: 1, 4) 373.15 K; 2, 5) 393.15 K; 3, 6) 413.15 K; 1–3) experimental data [13]; 4–6) calculated as per UFEOS (1).
For a more complete assessment of the uncertainty of information on the equilibrium properties of R1234yf, obtained from UFEOS (1), two approaches were used. The first approach is based on GOST 34100.3-2017/ISO/IEC Guide 98-3:2008, “Measurement Uncertainty. Part 3. Guide to the Expression of Uncertainty in Measurement.” The standard uncertainty of type A is calculated by comparing the experimental values of ri with the corresponding properties found by the recommended equation (1). The uncertainty uA for the property r is estimated by the expression
where Δri = ri – rav is the absolute deviation from the experimental values ri of the property rav = r(x, y) calculated using UFEOS (1) and arguments (x, y) at the ith point; n is the number of experimental values ri selected in the array X = (r, x, y) to estimate the uncertainty uA(r).
Based on Eq. (5) and GOST 34100.3-2017, the standard deviation S(r) is introduced in the form of the standard relative uncertainty
where δri = Δri·100.
The estimate of non-excluded systematic relative errors δri has the form of the standard relative uncertainty of type B:
where bi– = ri – ai–; bi+ = ri – ai+; ai–, ai+ are the lower and upper boundaries of the range of ri changes (see GOST 34100.3-2017, Sec. 4.3.8).
The total standard relative uncertainty for the property r is determined by the formula
The expanded uncertainty at k = 2 and the confidence level P = 0.95 for the property r is calculated from the formula
From Eqs. (5)–(9) we compute the uncertainties for saturated vapor pressure ps (from the data [3, 7, 9, 10, 15]), density ρ (from the data [4, 7, 9, 16]), isochoric CV and isobaric heat capacity CP (using [11] and [5, 8], respectively), as well as the speed of sound w (using [2, 13]), %:
Within the framework of the second approach, to assess the accuracy of the GSSSD 380-2020 tables, the following statistical characteristics (criteria) with relative units of measurement (in percent) were selected [27]:
– absolute average deviation of the property p (absolute average deviation, AAD)
where δpi = 100Δpi/pi exp; Δpi = pi exp – pi eq; pi exp, pi eq are experimental and calculated pressure values, respectively;
systematic deviation (BIAS)
– standard deviation (SDV)
– root-mean square deviation (RMS)
Table 4 shows the values of the criteria calculated from Eqs. (10)–(13) and characterizing the deviations of the experimental values of the equilibrium properties r of the refrigerant R1234yf [2, 6,7,8,9, 11, 13, 14] from those calculated from UFEOS (1), for N experimental points.
On the line of elasticity, the experimental data [12] are described in the framework of UFEOS (1) within the experimental uncertainty of these data. The maximum relative deviation
of the calculated value of the saturated steam pressure from the experimental data of J. Yin et al. (2019) [12] is δps max = 0.1%, while for FEOS M. Richter et al. (2011) [9] give δps max = 0.35%.
Conclusion. The method proposed by the authors of this article for constructing the UFEOS of the refrigerant R1234yf enables increasing the accuracy of calculating the equilibrium properties as a result of a physically based choice for the structure of the equation of state both in the regular part of the thermodynamic surface and in the vicinity of the critical point. The use of statistical characteristics (AAD, BIAS, SDV, RMS) and GOST 34100.3-2017 in estimating the calculated characteristics of the UFEOS, adopted in international thermophysical laboratories of leading metrological institutes (for example, NIST USA), makes it possible to objectively estimate the accuracy of known experimental information of equilibrium properties of R1234yf and of tables of standard reference data developed on the basis of UFEOS.
References
Montreal Protocol on Substances that Deplete the Ozone Layer. 2010 Report of the Refrigeration, Air Conditioning and Heat Pumps Technical Options Committee, 2010 Assessment, pp. 4–14.
Y. Kano,Y. Kayukawa, K. Fujii, and H. Sato, Int. J. Thermophys., 31, 2051–2058 (2010), https://doi.org/https://doi.org/10.1007/s10765-010-0885-7.
G. Di Nicola, F. Polonara, and G. Santori, J. Chem. Eng. Data, 55, 201–204 (2010), https://doi.org/https://doi.org/10.1021/je900306v.
C. Di Nicola, G. Di Nicola, M. Pacetti, et al., J. Chem. Eng. Data, 55, 3302–3306 (2010), https://doi.org/https://doi.org/10.1021/je100102q.
K. Tanaka, Y. Higashi, and R. Akasaka, J. Chem. Eng. Data, 55, 901–903 (2010), https://doi.org/https://doi.org/10.1021/je900515a.
G. Qiu, X. Meng, and J. Wu, J. Chem. Thermodyn., 60, 150–158 (2013), https://doi.org/https://doi.org/10.1016/j.jct.2013.01.006.
L. Fedele, S. Bobbo, F. Groppo, et al., J. Chem. Eng. Data, 56, 2608–2612 (2011), https://doi.org/https://doi.org/10.1021/je2000952.
N. Gao, Y. Jiang, J. Wu, and Y. He, Fluid Phase Equil., 376, 64–68 (2014), https://doi.org/https://doi.org/10.1016/j.fluid.2014.05.029.
M. Richter, M. O. McLinden, and E.W. Lemmon, J. Chem. Eng. Data, 56, 3254–3264 (2011), https://doi.org/https://doi.org/10.1021/je200369m.
Z. Yang, L. Kou, W. Mao, et al., J. Chem. Eng. Data, 59, 157–160 (2014), https://doi.org/https://doi.org/10.1021/je400970y.
Q. Zhong, X. Dong, Y. Zhao, et al., J. Chem. Thermodyn., 125, 86–92 (2018), https://doi.org/https://doi.org/10.1016/j.jct.2018.05.022.
J. Yin, G. Zhao, and S. Ma, Int. J. Refrig., 107, 183–190 (2019), https://doi.org/https://doi.org/10.1016/j.ijrefrig.2019.08.008.
M. Z. Lukawski, M. P. E. Ishmael, and J. W. Tester, J. Chem. Eng. Data, 63, 463–469 (2018), https://doi.org/https://doi.org/10.1021/acs.jced.7b00946.
S. Lago, P. A. Giuliano Albo, and S. Brignolo, J. Chem. Eng. Data, 56, 161–163 (2011), https://doi.org/https://doi.org/10.1021/je100896n.
K. Tanaka and Y. Higashi, Int. J. Refrig., 33, 474–479 (2010), https://doi.org/https://doi.org/10.1016/j.ijrefrig.2009.10.003.
L. Fedele, J. S. Brown, L. Colla, et al., J. Chem. Eng. Data, 57, 482–489 (2012), https://doi.org/https://doi.org/10.1021/je201030g.
R. Akasaka, K. Tanaka, and Y. Higashi, Int. J. Refrig., 33, 52–60 (2010), https://doi.org/https://doi.org/10.1016/j.ijrefrig.2009.09.004.
I. V. Kudryavtseva, V. A. Rykov, and S. V. Rykov, E. E. Ustyuzhanin, J. Phys. Conf. Ser., 946, 012118 (2018), https://doi.org/https://doi.org/10.1088/1742-6596/946/1/012118.
S. V. Rykov, V. A. Rykov, I. V. Kudryavtseva, et al., Math. Montisnigri, 47, 124–136 (2020), https://doi.org/10.20948/mathmontis-2020-47-11.
A. D. Kozlov, V. F. Lysenkov, P. V. Popov, and V. A. Rykov, J. Eng. Phys. Thermophys., 62, No. 6, 611–617 (1992), https://doi.org/https://doi.org/10.1007/BF00851887.
G. B. Benedek, Polarization Matie et Payonnement, Livre de Jubile en l’Honneur du Proffesor A. Kastler, Press. Univ. de Paris, Paris (1968), p. 71.
V. A. Rykov, S. V. Rykov, I. V. Kudryavtseva, and A. V. Sverdlov, J. Phys. Conf. Ser., 891, 012334 (2017), https://doi.org/https://doi.org/10.1088/1742-6596/891/1/012334.
B. Widom, J. Chem. Phys., 43, 255–262 (1965), https://doi.org/https://doi.org/10.1063/1.1696618.
Sh. Ma, Modern Theory of Critical Phenomena [Russian translation], Mir, Moscow (1980).
S. V. Rykov, I. V. Kudryavtseva, V. A. Rykov, and A. V. Sverdlov, Vestn. Mezhdunar. Akad. Kholoda, No. 2, 79–85 (2020), https://doi.org/10.17586/1606-4313-2020-19-2-79-85.
S. V. Rykov, I. V. Kudryavtseva, and V. A. Rykov. J. Phys. Conf. Ser., 1385, 012014 (2019), https://doi.org/https://doi.org/10.1088/1742-6596/1385/1/012014.
R. Mares, O. Profous, and O. Sifner, Int. J. Thermophys. 20, 933–942 (1999), https://doi.org/https://doi.org/10.1023/A:1022647605881.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izmeritel’naya Tekhnika, No. 2, pp. 9–15, February, 2021
Rights and permissions
About this article
Cite this article
Kolobaev, V.A., Rykov, S.V., Kudryavtseva, I.V. et al. Methodology for Constructing the Equation of State and Thermodynamic Tables for a New Generation Refrigerant. Meas Tech 64, 86–93 (2021). https://doi.org/10.1007/s11018-021-01901-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11018-021-01901-9