In this study, phase transformations were examined during low temperature interactions of natural tantalite with powders of aluminum and Ca–Al addition alloy (69.4 wt% Ca) using thermography, X-ray diffraction, and X-ray spectral microanalysis. The phase and elemental compositions of the initial mineral and the products of its metallothermic reduction were determined. Thermodynamic modeling of “mineral–reducing agent” interactions in the systems was performed in the temperature range of 500°C–3000°C, and the temperatures of the resulting products were calculated without taking into account heat losses. Experimental studies on reduction processes were performed under conditions of continuous heating of the mineral with reagents to 1200°C–1550°C in an argon flow. The studied mineral sample was manganotantalite with the composition of Mn0.94(Nb0.495Ta0.505)2.14O6 with a melting point of 1506°C. The aluminothermic reduction proceeded with the formation of intermetallic phases based on the Ta–Nb, Ta–Nb–Mn, and Nb–Mn–Al systems, and upon interaction with the Ca–Al alloy, the metal phase included solid solutions, such as (Nb,Ta)-ss. In both cases, a predominant reduction of niobium and the formation of intermediate suboxides and composite oxides containing niobium and tantalum were noted as a result of the incomplete transformation of manganotantalite during nonisothermal heating in the temperature range under study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The discovery of new application fields of niobium, tantalum, and their compounds in high-tech sectors of the economy has initiated a stable growth in their production and consumption throughout the world since the early 2000s [1, 2]. Despite the richest reserves in Russia of rare metal raw materials, including ores from tantalum deposits, the problem of meeting domestic needs for Nb and Ta remains open because its solution requires the creation of highly profitable production based on new technological approaches. Most recorded reserves relate to rare tin metal ores, including not only complex but also rebellious ores, as up to 60% of the target metals are lost in multi-stage operations for upgrading rough concentrates. Due to the low rates of the mechanical enrichment of tantalum–niobium ores, the development of combined methods is required for their processing, combining the techniques of mechanical and chemical–metallurgical concentrations. In global practice, there are advantageous examples of the pyrometallurgical enrichment of Ta-containing poor raw materials to commercial concentrates, used for the concentrations of Ta and Nb and for the separation of these metals with tin. The pyrometallurgical selection of Ta and Sn via carbothermal reduction has been successfully applied in Germany by H. C. Starck GmbH & Co. KG in the processing of tin slag. A similar technique is used in Australia (Global Advanced Metals) and Brazil (CBMM) [3].
Pavlov et al. [4] was proposed to subject nonwashable polymetallic ores containing niobium and rare earth metals to deep reduction using brown coal. The possibility of using carbon in the reduction of metals from the minerals columbite, tantalite, and wodginite was demonstrated by the authors of [5]. However, this method does not provide a complete recovery of target metals from tantalum-containing raw materials, and some of them transform into molten slags. In addition, the process is accompanied by the formation of several environmentally unsafe gases (NO, NO2, and CO).
A promising field in the development of rare metal technologies is the metal-thermal reduction of tantalum and niobium from complex ores and wastes with further hydrochemical selection of elements [6,7,8,9,10,11]. The metallothermic process is characterized by many advantages as compared with carbothermy due to its exothermicity and simplicity of instrumentation [11,12,13]. However, publicly available data on the aluminothermic process of rare metals mainly concern the reduction of Ta and Nb from pentoxides [7, 14]. With regard to ore minerals of rare metal raw materials, the metallothermic reduction process has not been sufficiently studied, and there is information on the use of aluminothermy in the processing of pyrochlore ores [4, 15]. Moreover, the possibility of metallothermic reduction of tin from cassiterite, a mineral accompanying columbite–tantalite in ores, has been revealed [16, 17].
In the various forms of tantalum and niobium in natural raw materials, the columbite–tantalite group minerals are of the most important industrial importance [18]. These are solid solutions representing a continuous isomorphic series of oxides from niobates to iron and manganese tantalates with the general formula (Fe,Mn)(Ta,Nb)2O6. The justification of the efficiency of the use of metallothermy for the processing of Ta–Nb ore raw materials requires knowledge on phase transformations, macromechanism, and kinetics of the interactions of tantalum niobates, including tantalite, with reducing metals.
This article presents the results of a study on phase formation during the reduction of natural tantalite with aluminum and its alloy with calcium under nonisothermal heating conditions. The reducing agent was chosen due to its high reactivity and availability of aluminum and calcium. Calcium is less convenient to use owing to its high chemical activity, so its combination with aluminum was proposed [7].
Materials and Methods
The object of this study was a collection of natural tantalites from the Ognevka mine of the Bakenoye deposit (Kalbinsk Range, Republic of Kazakhstan). Based on the chemical analysis results, the sample contained target metals in amount of 23.61 wt.% Nb, 37.82 wt.% Ta, 12.38 wt.% Mn, 0.36 wt.% Sn, and 0.09 wt.% Fe and related components, namely, 0.63% or more Mg, 0.38% Cu, 0.44% Ni, 0.08% Ti, 0.20% Zr, 0.06% Si, and 0.05% W to trace amounts (e.g., S, P, Na, and K). The mineral sample was preliminarily crushed (grinding size = 0.1 mm).
Aluminum powder grade PA 4 containing at least 98% Al was used in the experiments. The particle size was less than 0.14 mm. The combined reducing agent, calcium–aluminum addition alloy (69.4 wt% Ca), was obtained by fusing aluminum and calcium granules at 900 °C–1000 °C in an inert atmosphere. The addition alloy was a two-phase alloy of Ca8Al3 and Ca13Al14, whose heating was accompanied with thermal effects associated with the melting of the eutectic Ca13Al14 + Ca8Al3 ↔ L (540 °C) and the peritectic decomposition of Ca13Al14 ↔ L + CaAl2 (624 °C). The transformation temperature was somewhat lower than in [19] due to the presence of impurities (e.g., Fe, Si, and O) in the addition alloy. In the experiments, an addition alloy ground with a grinding size of 0.1 mm was used.
The phase transformations of the mineral were studied using a Netzsch STA 449 F3 Jupiter thermal analyzer. A portion of tantalite (approximately 15.0 mg) was placed in crucibles made of the Pt–Ir alloy with alundum substrates and lids and heated to 1620 °C at a rate of 20 °C/min in a current (40 cm3/min) of high-purity argon (99.998% Ar). When determining the numerical values of the temperature, the standard functions and settings of the Netzsch Proteus Thermal Analysis software package [20] were used. The temperature measurement error was ± 5 °C.
An experimental study of the metallothermic reduction process was performed through the combined method of thermogravimetric analysis and differential scanning calorimetry (DSC) on a Netzsch STA 449 C Jupiter thermal analyzer in the continuous heating mode to 1200 °C or 1550 °C and cooling to 500 °C. Powders of the mineral and reducing agent, namely, aluminum or aluminum–calcium addition alloy, were thoroughly mixed in a given ratio, compacted, and heated at a rate of 20 °C/min in a current (50 cm3/min) of high-purity argon. The samples were 25.0 to 60.0 mg.
To predict the compositions of the interaction products and regime parameters of the process (temperature and reducing agent consumption), a thermodynamic modeling of the process of reducing metals from tantalites with aluminum and Ca–Al addition alloy was performed using the HSC Chemistry 6.12 (Outotec Oy) software package [21]. Based on the calculation results of the heat balances of the process, the temperature of the interaction products was estimated, which indirectly characterizes the “thermality” of the charge of the reacting components.
The phase composition and crystal structure of the mineral and the products of its reduction were determined from X-ray diffraction (XRD) data obtained on a D8 ADVANCE diffractometer. The quantitative phase composition was estimated by the Rietveld full-profile analysis using the TOPAS program [22].
To study the microstructure and elemental composition of tantalite and the products of its metallothermal reduction, scanning electron microscopy and X-ray spectral microanalysis (XSMA) were used. The studies used a JSM-59000 LV electron microscope with an Oxford INCA Energy 200 energy-dispersive X-ray spectrometer and a TESCAN MIRA 3 LMU field-emission electron microscope equipped with an Oxford Instruments INCA Energy 350 X-max 80 X-ray analyzer.
Results and Discussion
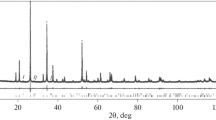
Based on the XRD analysis results, the tantalite sample was represented by a single phase, namely, a solid solution (ss) of manganese tantalate Mn(Nb0.56Ta0.44)2O6 (PDF-2 card No. 01–070–0171 [23]) with an orthorhombic crystal structure of the Pbcn (60) spatial group and parameters of the unit cell (a = 14.4066 (3) Å, b = 5.7563 (1) Å, c = 5.1029 (1) Å, V = 423.166 (13) Å3) (Fig. 1).
The XSMA results (Fig. 2, Table 1) showed that tantalite is a monolithic sample consisting of a solid solution of manganese tantalate with an average composition of Mn0.94(Nb0.495Ta0.505)2.14O6, with rare veinlets of quartz and aluminosilicate less than 120 μm wide. The Ta/Nb atomic ratio in tantalite was close to unity. Some small inclusions and veinlets in the mineral were filled with hard coal containing up to 6.6 wt.% Nb and 14.3 wt.% Ta.
A distinctive characteristic of natural and synthetic tantalite–columbites is the presence of ordered and disordered structures [24, 25]. In [24], through the statistical analysis method, Eq. (1) was derived, which determines the degree of order (Q) in the tantalite–columbite structure:
where c and a are the unit cell parameters. For the studied sample of tantalite, the Q value was 81%, which indicates the presence of incompletely ordered structures in it.
According to the thermal analysis data of tantalite (Fig. 3), on the DSC line, effects were revealed with starting and maximum temperatures of 1506 °C/1538 °C upon heating and 1467 °C/1466 °C upon cooling, corresponding to the sample melting and crystallization. There was almost no change in mass during the heating of tantalite.
In the thermodynamic analysis of the aluminothermal reduction process, the studied sample of tantalite was presented as a mixture of its components, i.e., manganese tantalate (MnTa2O6) and manganese niobate (MnNb2O6), based on the chemical and phase composition of the mineral. The thermodynamic values of the compounds taken into account in the simulation (MnNb2O6, MnTa2O6, Mn4Ta2O9, and Mn4Nb2O9), which were absent in the HSC 65.12 program database, were obtained using calculation and experimental methods [26, 27]. The costs of reducing agents were taken in accordance with the stoichiometry of reactions involving a complete reduction of components to metals.
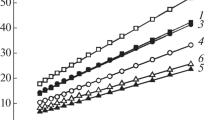
The dependence nature of the equilibrium composition of the interaction products on the consumption of aluminum (Fig. 4) indicates the almost complete extraction of Ta and Nb into the alloy in a temperature range up to 2000 °C at an Al/tantalite molar ratio above four.
Particularly, the niobate part of the mineral reacted with aluminum first. Then, as the consumption of the reducing agent increased, manganese tantalite reacted with it. With the complete reduction of tantalite, the formation of an alloy was expected, whose composition (50 wt.% of Ta; 33 wt.% of Nb, and 17 wt.% of Mn) was practically constant in the temperature range of 500 °C–2000 °C (Fig. 5). At a temperature above 2000 °C, the proportion of tantalum in it increased due to the formation of Ta2Al and the transition of a part of manganese to the oxide phase in the form of MnO·Al2O3 aluminate. Reverse oxidation reactions are also possible for rare metals, which at high temperatures can lead to the generation of corresponding oxide compounds (NbO, Ta2O5, and NbO2). The heat balance calculation of the aluminothermy of tantalite showed that the temperature of the interaction products can reach 2054 °C (excluding heat losses). However, this value is not enough to implement the out-of-furnace process, given the refractoriness of the products and inevitable heat losses in real conditions.
The recovery of metals from tantalite using an aluminum–calcium addition alloy (Fig. 6), at costs calculated for the complete reduction of tantalite, occurs quite completely with the formation of a more fusible slag based on calcium aluminates (Tmelt ≈ 1600 °C–1700 °C). The temperature of the reacting system (Tpr = 2665 °C) may be quite sufficient to conduct the process in the “out-of-furnace” melting mode.
For the experimental verification of the results of the thermodynamic modeling of the interaction of tantalite with aluminum, the consumption of the latter was taken with the calculation of the formation of only metals or metals with their aluminides at an Al/tantalite molar ratio of 4 or 6, respectively, according to the following reactions:
The carefully mixed and compacted reagents in a crucible were heated at a rate of 20 °C/min to 1200 °C–1550 °C. The compositions of the charge and the maximum values of the heating temperature are presented in Table 2.
A mixture of aluminum and tantalite powders was taken at a molar ratio of components equal to four 24.5 wt% Al of the tantalite mass. Based on the thermal analysis (Fig. 7) of the mixture, when heated to 1450 °C, three combined exothermic effects at temperatures of 930 °C/1027 °C and 1300 °C/1333 °C/1395 °C were installed on the DSC line, along with the endothermic effect of aluminum melting with the start/maximum temperatures at 651 °C/665 °C. Such temperatures characterized the staged reduction process. A minor endothermic effect at 1414 °C/1432°C (heating) and 1410 °C/1388 °C (cooling) was apparently due to the melting and crystallization of the resulting low-melting phase, such as a solid solution based on the Mn–Nb system [28]. The increase in the sample weight, when heated above 600 °C, was 0.44% at 1450 °C, which was caused by the presence of traces of oxidizing gases in argon. Based on the XRD data, as a result of the interaction of reagents, metal products with the structures of intermetallic compounds Nb0.84Al0.16, Al6Mn, Ta7Mn6, and NbMnAl (Table 2, experiment 2) and intermediate oxides NbO0.7, Mn1.4TaO4.2, and Mn0.55Ta0.45O1.7 were formed, information of which is given in [29, 30]. The formation of a simple niobium suboxide and complex tantalum oxides in the reduction products indicates the sequential nature of the interaction and the higher chemical activity of niobium compared to tantalum. The incomplete conversion of tantalite to metal and the formation of intermediate compounds (NbO0.7, Mn1.4TaO4.2, and Mn0.55Ta0.45O1.7) are apparently associated with the reducing agent deficiency caused by the transition of part of the aluminum into the composition of niobium and manganese intermetallides (Nb0.84Al0.16 and Al6Mn).
An increase in the aluminum content in the mixture to its ratio with tantalite equal to six (36.5% Al) did not significantly affect the course of the DSC line and was expressed in a slight shift of thermal effects toward high temperatures (Fig. 7, b). The results of the phase analysis of the reduction products obtained by heating the reagents to 1200 °C revealed the presence of Al3(Nb0.5Ta0.5) and Ta metal phases, unreacted oxides MnNb2O6 and MnTa2O6, and a small amount of Mn4NbO9 (Table 2). With an increase in the final heating temperature to 1550 °C, manganese was also reduced, which passed into the metal phase in the form of Al6Mn aluminide. At the same time, niobium and tantalum suboxides (NbO, Ta0.83O2) and MnTa2O6 still remained in the interaction products. Despite the similar shape of the DSC lines (Fig. 7), the product compositions of the interaction of tantalite with different amounts of aluminum were somewhat different. When the reducing agent was consumed in accordance with the reaction of Eq. (3) and heated to 1200 °C, the resulting metallic Al3(Nb0.5Ta0.5) and oxide (Al2O3) phases blocked the development of the reaction surface. Moreover, some of the components of the original tantalite remained unreduced, although their structure underwent a certain transformation. The increase in the temperature to 1550 °C also did not lead to a complete reduction of metals, which indicates the need to conduct the process at a higher temperature and/or prolonged (isothermal) exposure.
The XSMA data indicate that the products of heating a mixture of tantalite with aluminum (Al/tantalite = 4) to 1450 °C have a finely dispersed structure consisting of particles of metal and oxide phases. On the section surface (Fig. 8, a), rounded cavities from drops of molten aluminum were clearly seen. The composition of metal phases is heterogeneous, i.e., from two-component (Nb–Ta) to multi-component (Nb–Ta–Mn–Al–W(Sn)) solid solutions (Table 3). In some metal phases, there was almost no aluminum, whereas in others, the aluminum content amounted to 1.5%–11%. Figure 8, b presents a region with discrete oxide phases along its edges, consisting of a solid solution with the composition of 92.3% Al2O3–4.8Ta2O5–1.7MnO. The oxide phases based on the Al2O3 border on the intermediate oxide (Mn,Al)(Ta,Nb)1.2O4 are in contact with the metal inclusions of the average composition Nb0.41Ta0.38Mn0.16W0.05. Some particles (Fig. 8, c) consisted of dispersed inclusions of metallic phases surrounded by aluminum oxide. In addition, some areas (Fig. 8, d) consisted of niobium suboxide (Nb,Ta)O1.06 with an admixture of tantalum and complex oxides MnTa1.7O6 and (Mn,Al)(Ta,Nb)1.2O4. It was depleted in niobium compared with the original manganese tantalum niobate Mn0.94(Nb0.495Ta0.505)2.14O6, which indicates its priority recovery from tantalite. The variety of phases of variable composition, formed when heating a mixture of tantalite with aluminum to 1450 °C, indicate that the process was not completed under nonisothermal conditions. This finding is consistent with the XRD analysis results (Table 2). To complete the process, an increase in the temperature or the consumption of a reducing agent is required.
The results of the phase formation during the aluminothermic reduction of natural tantalite showed that during continuous heating to 1450 °C–1550 °C, tantalum and solid solutions based on the intermetallic compounds of the Ta–Nb, Mn–Al, Ta–Nb–Mn, and Nb–Mn–Al systems passed into the metal phase. The recovery process was accompanied by the formation of oxides with a composition of Nb0.7, Ta0.83O2, and Mn1.4TaO4.2, Mn0.55Ta0.45O1.7, along with aluminum oxide. Complete winning of metals requires high temperatures, long interaction, or component transfer to a molten state, which provides conditions for the separation of metal and oxide phases.
The interaction of tantalite with the Ca–Al alloy with consumption of 45.2% of the tantalite weight, assuming the reduction of metals according to the equation
was considered when heated to 1250 °C. On the DSC line (Fig. 9), during heating, endothermic effects were noted with start/maximum temperatures of 540 °C/545 °C and 625 °C/633 °C, associated with the phase transformations of the addition alloy (eutectic melting and peritectic reaction). Exothermic effects with maximum temperatures of 575 °C, 669 °C, 844 °C, and 975 °C indicate the interaction of the reagents. The alternation of these effects was apparently due to the wavelike course of the process, which starts with the melting of the reducing agent, its interaction with the oxide, then the melt diffusion through the layer of solid products, and a new development of reactions with the repetition of the cycle described. When the sample was cooled on the DSC line, there was a minor effect (1174 °C/1165 °C) on the fusible phase crystallization.
According to the XRD data, when tantalite with the Ca–Al alloy was heated to 1250 °C, the interaction products contained more than 37% of the metal phase, predominantly niobium aluminides (Table 4). With longer heating up to 1470 °C, additional tantalum passed into the alloy in the form of solid solutions (13%) and pure metal (7%). Essentially, in both cases, the composite oxide compounds of tantalum and niobium with calcium were formed in the oxide phase, and in case 1, this was tantalum niobate Ca4(Ta,Nb)2O9. When heated to 1470 °C, calcium aluminate Ca2(Ta,Nb)AlO6 containing Ta and Nb was added to it. At a low temperature, their content (26%) was two times higher than that when heated to 1470 °C (12% in total). Hence, calcium tantalum niobates were considered as intermediate products formed at the beginning of the metallothermic process through the replacement of manganese with calcium in tantalite [31]. The main part of all oxides, which was approximately 70%, when heated to a high temperature, is represented by calcium aluminates Ca3Al2O6 and Ca5.65Al7O16.15.
The calculation of the rational phase composition of the product of the interaction of a mixture of tantalite with the Ca–Al alloy heated to 1470 °C, according to the XSMA (Fig. 10, Table 5), generally agrees with the XRD results. The main oxide phase is represented by calcium aluminates Ca3Al2O6 (Fig. 10, point 1), containing neither niobium nor tantalum. The intermediate product, identified via the XRD as calcium tantalum niobate Ca4(Ta,Nb)2O9, is Ca4Ta2O9 tantalate with an admixture of niobium (Fig. 10, points 2, 3). A similar characteristic of the composition was also noted for the Ca2(Ta,Nb)AlO6 phase where the rare metal component is represented almost exclusively by tantalum (Fig. 10, point 6). In the metallic phase, on the contrary, in the composition of the (Nb,Ta)-ss solid solution, niobium predominates in the atomic ratio, which is 1.7 times more than that of tantalum (Fig. 10, point 4). However, metal particles with an equal ratio of Nb and Ta were also found in the reduction product (Fig. 10, point 5). The revealed characteristics of the niobium and tantalum distribution between the oxide and metal components of the products of the interaction of tantalite with the Ca–Al alloy are quite understandable, given the difference in the reducibility of the mineral components, that is, the higher reactivity of niobium compared to that of tantalum. In particular, the Nb0.5Ta0.5 metal phase did not contain calcium and aluminum, whereas in the solid solutions (Nb,Ta)-ss and (Nb,Mn,Ta)-ss, up to 2.0 wt.% Ca and 0.7 wt.% Al were revealed.
Conclusions
The studied mineral is tantalite (Ognevka deposit, Kalbinsk Range), which is practically a single phase manganotantalite with the chemical formula Mn0.94(Nb0.495Ta0.505)2.14O6 and a melting point of 1506 °C.
The interaction of aluminum with tantalite during continuous heating started in the temperature range of 930 °C–950 °C. At 1450 °C–1550 °C, it led to the formation of solid solutions in the metal phase based on the intermetallic compounds of the Ta–Nb, Mn–Al, Ta–Nb–Mn, and Nb–Mn–Al systems with a predominant reduction of niobium. The reduction process at a stoichiometric consumption of aluminum led to the formation, along with aluminum oxide, of intermediate oxides Mn1.4TaO4.2, NbO0.7, and Mn0.55Ta0.45O1.7. The increase in the temperature to 1550 °C did not induce the complete reduction of metals, which indicates the need to increase it and/or increase the holding time of the process.
The use of a combined Ca–Al alloy containing 69.4% calcium as a metal reducing agent from natural tantalite when the reagents were heated to 1250 °C–1470 °C resulted in the formation of solid solutions based on tantalum and niobium and contributed to the formation of calcium aluminates, which practically do not dissolve rare metals.
The obtained results can be used to develop the theoretical foundations for the technology of metallothermic reduction of natural tantalum niobates.
This work was supported by the Russian Foundation for Basic Research (project No. 18-29-2405_mk) using the equipment of the Ural-M Center for Collective Use.
References
E. E. Nikishina, D. V. Drobot, and E. N. Lebedeva, “Niobium and tantalum: the state of the global market, areas of application, raw materials. Part 1,” Izv. Vyssh. Ucheb. Zav. Tsvetn. Metallurg., No. 5, 28–34 (2013).
E. E. Nikishina, D. V. Drobot, and E. N. Lebedeva, “Niobium and tantalum: the state of the global market, areas of application, raw materials. Part 2,” Izv. Vyssh. Ucheb. Zav. Tsvetn. Metallurg., No. 1, 29–41 (2014).
C. A. Faria Sousa, The Evolution of FeNb Manufacturing, (2012); URL: http://cbmm.com.br/portug/sources/techlib/science_techno/table_content/sub_1/images/pdfs/006.pdf.
M. V. Pavlov, I. V. Pavlov, V. F. Pavlov, O. V. Shabanova, and A. V. Shabanov, “Aspects of the processes of pyrometallurgical processing of polymetallic ores of the Chuktukonskoye deposit (Krasnoyarsk Territory),” Khim. Interes. Ustoych. Razvit., No. 23, 263–266 (2015).
V. M. Chumarev, V. P. Maryevich, A. N. Mansurova, and S. M. Kazhakhmetov, “Phase formation and reduction kinetics of metals in the interaction of columbite, tantalite, and wodginite with carbon,” Metally, No. 2, 10–15 (2008).
V. M. Orlov, E. N. Kiselev, and M. V. Kryzhanov, “Powders of niobium and tantalum from waste products of lithium niobate and tantalate production,” Khimich. Tekhnolog., 18, No. 4, 146–150 (2017).
R. Munter, A. Parshin, L. Yamshchikov, V. Plotnikov, V. Gorkunov, and V. Kober, “Reduction of tantalum pentoxide with aluminum and calcium: thermodynamic modeling and scale skilled tests,” Proc. Estonian Acad. Sci., 59, No. 3, 243–252 (2010).
J.-C. Stoephasius, B. Friedrich, and J. Hammerschmidt, A New Processing Route for Titanium Alloys by Aluminothermic Reduction of Titanium Dioxide and Refining by ESR: The 10th World Conf. on Titanium (Hamburg, Germany, July 2003) (2003).
W. George, Pat. US 4169722. Fletcher Aluminothermic Process, submitted 08/18/1977; published 10/02/1979.
M. V. Kryzhanov, V. M. Orlov, and V. V. Sukhorukov, “Thermodynamic modeling of magnesiothermic reduction of niobium and tantalum from pentoxides,” Russ. J. Appl. Chem., 83, No. 3, 379–383 (2010).
D. K. Bose and C. K. Gupta, “Extractive metallurgy of tantalum,” Miner. Process. Extr. Metall. Review, 22(2), 389–412 (2002); https://doi.org/10.1080/08827500208547422.
Yu. L. Pliner and G. F. Ignatenko, Recovery of Metal Oxides with Aluminum [in Russian], Metallurgiya, Moscow (1967).
N. P. Lyakishev, Yu. L. Pliner, G. F. Ignatenko, and S. I. Lappo, Aluminothermy [in Russian], Metallurgiya, Moscow (1978).
M. N. Gasik, N. P. Lyakishev, and B. I. Emlin, Theory and Technology of Ferroalloy Production [in Russian], Metallurgiya, Moscow (1988).
K. Gupta, “Extractive metallurgy of niobium, tantalum, and vanadium,” Int. Met. Rev., 29, No. 6, 405–444 (1984).
S. N. Tyushnyakov, R. I. Gulyaeva, L. Yu. Udoeva, S. A. Sergeeva, and S. A. Petrova, “Metallothermic reduction of natural cassiterite,” Metallurg, No. 7, 52–61 (2021).
V. M. Chumarev, S. M. Kozhakhmetov, A. D. Vershinin, T. A. Kokoveshnikova, and A. N. Mansurova, “Study of the kinetics and macromechanism of the recovery of tin from cassiterite,” Kompleks. Ispolz. Mineral. Syr'ya, No. 4, 56–62 (2011).
A. N. Zelikman, B. G. Korshunov, A. V. Elyutin, and A. M. Zakharov, Niobium and Tantalum [in Russian], Metallurgiya, Moscow (1990).
K. Ozturk, L.-Q. Chen, and Z.-K. Liu, “Thermodynamic assessment of the Al–Ca binary system using random solution and associate model,” J. Alloys Compound., 340, No. 1–2, 199–206 (2002).
Netzsch Proteus Software. Thermal Analysis. Version 4.8.3.
A. Roine, HSC Chemistry 6.0 User’s Guide. Chemical Reaction and Equilibrium Software with Extensive Thermochemical Database and Flowsheet Simulation, OutotecResearchOy, Pori (2006).
DIFFRAC Plus : TOPAS Bruker AXS GmbH, Ostliche, Rheinbruckenstraße 50, D-76187, Karlsruhe, Germany (2008).
Powder Diffraction File PDF4 + ICDD Release 2016.
T. S. Ercit, M. A. Wise, and P. Cerny, “Compositional and structural systematics of the columbite group,” Am. Mineral., 80, 613–619 (1995).
S. C. Tarantino and M. Zema, “Mixing and ordering behavior in manganocolumbite-ferrocolumbite solid solution: A singlecrystal X-ray diffraction study,” Am. Mineral., 90, 1291–1299 (2005).
A. N. Mansurova, R. I. Gulyaeva, V. M. Chumarev, and V. P. Mar’evich, “Thermochemical properties of MnNb2O6,” J. Therm. Anal. Calorim., 101, 45–47 (2010).
R. I. Gulyaeva, S. A. Petrova, V. M. Chumarev, and E. N. Selivanov, “High-temperature heat capacity and thermal expansion of the MnTa2O6,” J. Alloys Compound, 834, 155153 (2020).
Sh. Liu, B. Hallstedt, D. Music, and Y. Du, “Ab initio calculations and thermodynamic modeling for the Fe–Mn–Nb system,” CALPHAD: Comput. Coupl. Phase Diagrams Thermochem., 38, 43–58 (2012).
A. C. Turnock, “Mn–Ta oxides: phase relations at 1200°C,” J. Am. Ceram. Soc., 49, 382–384 (1966).
S. Esmaeilzadeh, S. Lidin, M. Nygren, and J. Grins, “Single crystal refinement of the incommensurately modulated Mn0.55Ta0.45O1.7, an oxygen deficient fluorite type compound,” Z. Org. Allg. Chem., 626, 148–159 (2000).
K. T. Jacob and A. Rajput, “Phase relations in the system Ca–Ta–O and thermodynamics of calcium tantalates in relation to calciothermic reduction of Ta2O5,” J. Alloys Compound, 620, 256–262 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 66, No. 2, pp. 75–85, February, 2022. Russian DOI: https://doi.org/10.52351/00260827_2022_02_75.
Rights and permissions
About this article
Cite this article
Gulyaeva, R.I., Udoeva, L.Y., Petrova, S.A. et al. Phase Transformation during Metallothermic Reduction of Tantalite. Metallurgist 66, 200–214 (2022). https://doi.org/10.1007/s11015-022-01316-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-022-01316-z