A rotating disk method is used to investigate the dissolution rate of metallic iron, nickel, and their alloys in nitric acid media. Discs 2 cm in diameter made of nickel grade N1 and Armco iron, as well as two samples with an Ni:Fe ratio of 15:85 and 50:50 are used. The effect of temperature, initial nitric acid concentration, disk rotation rate, and process duration on specimen dissolution rate are investigated. The rate is determined by the amount of dissolved metal per unit of time from a disk unit surface. Regression analysis is used to describe the effect of nitric acid concentration on specimen dissolution rate. It is found that the dissolution rate of iron metal is up to 20–60 times faster than nickel metal dissolution rate. The effect of HNO3 temperature and initial concentration when dissolving Ni and Fe alloys in nitric acid is investigated. With an increase in temperature from 303 to 353 K ([HNO3] = 0.2 M, Ni:Fe = 50:50) the amount of dissolved Fe and Ni increases by a factor of 8–9. Dissolution of Ni:Fe alloy (15:85) under the same conditions results in an increase in dissolved iron by a factor of four. An increase in nickel concentration in solution occurs within 30 minutes, after which the concentration does not change. In experiments with Ni:Fe alloy = 50:50 the amount of dissolved Fe is 1.8–2.3 times greater than the amount of dissolved Ni depending on the initial acidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Currently according to the estimates of specialists up to 73% of all nickel production is used in manufacturing alloyed materials, in particular alloys based on nickel. The demand for nickel in production of storage batteries remains relatively low, but it will undoubtedly increase.
Within Russia in the last few years nickel production plants have closed (South Ural Nickel Combine, ZAO PO Rezhnikel’, Ufaleinikel’ Combine) in view of enterprise bankruptcy. Nonetheless, the demand for nickel as one of the main elements for stainless steel production proving corrosion resistance remains.
Alloy steel objects have a specific service life after which it requires their utilization and secondary usage [1, 32]. Treatment of this secondary raw material is often accomplished by pyrometallurgical methods. Nickel and iron are the representative phase not only in nickel-containing secondary raw material, but also within metallized nickel mattes, and within ferronickel.
There is increased interest in processing secondary nickel-containing raw material by hydrometallurgical methods [3]. Kinetic features of dissolution of both individual metals and also alloys with a different ratio of components in various solutions is important in order to select the optimum raw material leaching conditions.
Recently in published sources there is mainly information about electrochemical dissolution of iron and nickel alloys [4,5,6]. Dissolution of various materials in nitric acid using rotary disk methods has been the concern of the authors in [7,8,9,10,11]. Research is presented in [12] for dissolution of nickel alloys in red fuming nitric acid.
I. I. Kalinichenko, V. D. Nikitin, et al. have studied dissolution of nickel platelets in nitric acid with t = 60–100°C and concentrations of 0.5–12 M [13].
A. A. Ravdel’ and I. G. Él’kin have used a rotating disk method [14] the aim of their research was determination of fields for occurrence of the process. Ranges for change in parameters: [HNO3] = 3–57%; T = 298–368 K; r = 120–2400 min–1. Dependences have been determined for nickel dissolution rate in nitric on disk rotation frequency at different temperatures.
We have studied chemical dissolution of nickel, iron and their alloys in dilute nitric acid. Use of rotary disk methods makes it possible to provide a more severe hydrodynamic situation, uniform access to a specimen surface, and the possibility of obtaining reliable kinetic dependences for metal dissolution rate on such parameters as temperature, original acidity, mixing intensity, and process duration.
Materials and Methods
The starting alloy specimens were prepared from nickel grade N1 and Armco iron. In order the dissolution of nickel and iron alloys two specimens were used with a ratio Ni:Fe = 15:85 and 50:50. An Armco iron specimen was prepared by rolling, and the rest of the specimens were cast. Discs 2 cm in diameter were prepared from the alloys pressed into a fluoroplastic frame. Before testing a disk soluble surface was polished and degreased with alcohol.
The effect of temperature (303–353 K), disk rotation frequency (350–1500 min–1) and initial HNO3 concentration (0.025–0.2 М) were studied. The dissolved metal concentration was determined by a colorimetric method. The rate was determined for the amount of dissolved metal for a unit of time from a unit of disk surface (v, mole/sec·cm2)).
Results and Discussion
Dissolution of Pure Nickel and Iron. Nickel dissolution is described by reactions (1)–(5) [13]. The change in Gibbs energy in the test temperature range is shown (calculated using HSC 6 program).
Iron dissolution is described by reactions (6)–(10)
Curves are presented in Fig. 1 for the degree of pure nickel and iron dissolution on disk rotation frequency.
With a change in disk rotation frequency from 350 to 1500 min–1 there was an increase in the amount of nickel passing into solution. On kinetic dependences after 10 min horizontal sections appeared in which nickel dissolution ceased and then after 5–10 min it started again.
Iron dissolution rate up to a specimen rotation frequency of 1000 min–1 is proportional to the number rotations in a step of 0.5 that is typical for a dissolution diffusion region (dependences are provided in Fig. 2).
A subsequent increase in disk rotation frequency did not have a marked effect on iron dissolution rate. This is possibly explained by formation of a layer of reaction products on a disk surface that makes iron oxidizing ion diffusion difficult towards the phase interface. At the alloy surface there may be an area of local hydrolysis of trivalent iron accompanied by formation of solid reaction product that is a loose residue of dark brown or black color, readily removed mechanically. The maximum iron dissolution rate (vFe = 1.22∙10–6 mole/(sec∙cm2)) is observed with the following conditions: r = 1000 min–1; Т = 353 K; [HNO3] = 0.1 M; and with r = 1000 min–1; Т = 313 K; [HNO3] = 0.2 M – vFe = 1.2∙10–6 mole/(sec∙cm2).
During nickel dissolution under conditions [HNO3] = 0.1 M and Т = 313 К a change in disk rotation frequency has an insignificant effect on nickel transfer into solution that agrees with data in [14].
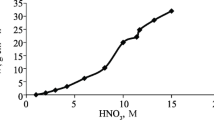
Dependences are provided in Fig. 3 for metal dissolution indices on nitric acid initial concentration and in Fig. 4 there is the dependence of metal dissolution rate on nitric acid concentration.
In the initial time interval (10 min) nickel dissolution rate is faster than in subsequent time intervals. This is explained by the fact that dissolution proceeds more rapidly at a freshly prepared specimen surface.
The dissolution rate for both nickel and iron increases in proportion to an increase in nitric acid initial concentration. Specific dependences are described by the following equations:
An increase in original acid concentration C affects to a greater extent the transfer of iron into solution. In this case iron dissolution rate considerably exceeds that for nickel. With fixed Т = 313 K and r = 1000 min–1, vFe = 2.2∙10–7 mole/(sec∙cm2), vNi = 1.1∙10–8 mole/(sec∙cm2) with 0.025 М HNO3 and vFe = 1.2∙10–6 mole/(sec∙cm2), vNi = 2.1∙10–8 mole/(sec∙cm2) with 0.2 М HNO3, i.e., by a factor of 20–60.
A dependence of ln v on ln C is provided in Fig. 5 that makes it possible to determine the order of metal dissolution reactions for nitric that equal for iron nFe = 0.781, and for nickel nNi = 0.491 respectively.
The calculated value of iron dissolution reaction activation energy was ≈29,000 J/mole that is typical for the diffusion region. Nickel dissolution probably proceeds in a transition (mixed) region (the order of reaction with respect to nitric acid nNi = 0.491).
Dissolution of Ni and Fe Alloys in nitric acid has been studied. Variable parameters: temperature (303 and 353 K); initial concentration HNO3 (0.05 and 0.2 М), alloy composition (Ni:Fe = 50:50 and Ni:Fe = 15:85).
Curves are presented in Figs. 6 and 7 for the dependence of amount of nickel and iron dissolved from alloys with a disk rotation frequency of 1500 min–1.
Calculated values of activation energy during dissolution of nickel from alloy 50:50 from 22,770 J/mole (0.05 M HNO3) tо 39 500 J/mole (0.2 M HNO3); for alloy 15:85 Ea = 29,420 J/mole (0.2 M HNO3) and 41,800 J/mole (0.05 M HNO3). The order of nickel dissolution reaction inn both alloys for nitric acid is T = 353 K is close to one; for 50:50 alloy it equals 0.43, and for alloy 15:85 it equals 1.5.
For iron the activation energy was 11,000–19,000 J/mole, and the order of reactions with respect to nitric acid with T = 353 K (50:50 alloy) equals 1.04 that is typical for the diffusion region for occurrence of the process [15].
A selectivity coefficient has been calculated (nominal value equal to β = А/В, where А = QNi/QFe is the ratio of the amount of nickel and iron in alloy; 50/50 = 1, 15/85 = 0.176; B is the ratio of the amount of nickel and iron transferred into solution. Coefficient β is at a maximum and equals 2.6–3.1 (this signifies that iron is transferred into solution three times more than for nickel) with T = 303 K and [HNO3] = 0.05 M for alloy 50:50; β decreases with an increase in nitric acid concentration to 0.2 М (β = 1.9–2.2) with an increase in temperature to 353 K (β = 1.5–2.1). For alloy 15:85 the selectivity coefficient β = 1.6–2.5 with Т = 303 K and [HNO3] = 0.05 M; it decreases with an increase in nitric acid concentration to 0.2 М (β = 0.95–1.2) and with an increase in temperature to 353 K (β = 0.7–0.95).
Conclusions
Temperature has a significant effect on metal transfer into solution. With a change in temperature from 303 to 353 K the amount of dissolved Fe and Ni increased by a factor of 8–9. During dissolution of Ni:Fe = 15:85 alloy in tests under the same conditions the metal transfer into solution increased by a factor of four.
The original acidity with a temperature of 353 K has a considerable effect on metal transfer into solution. With [HNO3] = 0.2 М and Ni:Fe = 50:50 the amount of Fe and Ni dissolved is greater than with [HNO3] = 0.05 М by factors of 8 and 5.7 respectively.
In tests with Ni:Fe 50:50 alloy the amount of iron dissolved is greater by a factor of 1.8–2.3 than for dissolved nickel in relation to the original acidity.
Specific kinetic dependences have been determined for dissolution of nickel, iron and their binary alloys in nitric acid on the parameters indicated.
These studies have demonstrated a significant role of hydrodynamics. In view of this hydrometallurgical equipment should provide good mixing and the original materials should have a developed surface (turnings, powder, etc.).
References
V. F. Mysik, “Scrap processing — glance into the future,” Ghern. Met. Byull. NTiÉI, No. 10 (1426), 61–67 (2018); ISSN: 0135–5910eISSN: 2619–0753.
Manisha Sahoo, Sidhartha Sarkar, Ajoy C. R. Das, Gour Gopal Roy, and Prodip Kumar Sen, “Role of scrap recycling for CO2 emission reduction in steel plant: A model based approach,” Steel Research Inter. First published: 21 June 2019; https://doi.org/10.1002/srin.201900034.
A. T. Dadakhodzhaev and N. N. Mamtaliev, “Methods for extracting nickel from waste product and its application,” Universum: Tekhnicheskie Nauki: Élektron. Nauch. Zh., No. 4(61) (2019); URL: http://7universum.com/ru/tech/archive/item/7217 (referral date: 30.01.2020).
F. Balbaud, G. Sanchez, G. Santarini, and G. Picard, “Cathodic reactions involved in corrosion processes occurring in concentrated nitric acid at 100°C,” European J. of Inorganic Chemistry, No. 4, 665–674 (2000).
R. D. Armstrong, G. E. Cleland, and G. O. H. Whillock, “Effect of dissolved chromium species on the corrosion of stainless steel in nitric acid,” J. of Applied Electrochemistry, 28, No. 11, 1205–1211 (1998).
D. G. Kolman, D. K. Ford, D. P. Butt, and T. O. Nelson, “Corrosion of 304 stainless steel exposed to nitric acid-chloride environments,” Corrosion Science, 39, No. 12, 2067–2093 (1997).
G. Peev, A. Nikolova, and D. Peshev, “Solid dissolution in a thin liquid film on a horizontal rotating disk,” Heat and Mass Transfer/Waerme — und Stoffuebertragung, 43, No. 4, 397–403 (2007).
S. Thomas, A. Faghri, and W. Hankey, “Experimental analysis and flow visualization of a thin liquid film on a stationary and rotating disk,” J. of Fluids Engineering, Trans. of the ASME, 113, No. 1, 73–80 (1991).
D. S. Reutov, B. D. Khalezov, L. A. Ovchinnikova, and A. S. Gavrilov, “Study of copper and zin ferrite dissolution kinetics by a rotary disk method,” Scientific Bases and Treatment Technology for Ores and Technogenic Raw Material: Proc. Internat. Sci.-Tech. Conf. (Yekaterinburg, 10–13 Apr, 2018).
A. I. Pichugina, S. A. Gortsevich, and V. I. Lutsik, “Dissolution kinetics for millerite and heazlewoodite in nitric acid solutions,” Byull. Nauki Praktiki, No. 11, 106–111 (2016).
R. C. Woodhouse, C. Boxall, and R. J. Wilbraham, “Nitric acid reduction on 316L stainless steel under conditions representative of reprocessing,” Symp. on Corrosion in Nuclear Energy Systems: From Cradle to Grave — 223rd Meeting of the Electrochemical Society (Toronto, ON; Canada. 12–16 May 2013), 53, No. 21, 33–44 (2013).
L. Ya. Gurevich and A. D. Zhirnov, “Corrosion resistance of nickel alloys with chromium and silicon in red fuming nitric acid,” Account of VIAM Research 1994–201636, FGUP VIAM (1994).
I. I. Kalinichenko, V. D. Nikitin, M. R. Stronberg, G. M. Kir’yanova, and K. A. Kotyaeva, “Dissolution of nickel in nitric acid,” Zh. Prikl. Khim., 4, 24–43 (1959).
A. Ravdel’, “Determination of the fields of nickel dissolution in nitric acid by a rotary disk method,” Zh. Prikl. Khim., No. 5, 966 (1967).
A. N. Zelikman, G. M. Vol’dman, and L. V. Belyaevskaya, Hydrometallurgical Process Theory [in Russian], Metallurgiya, Moscow (1975).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 65, No. 10, pp. 36–41, October, 2021. Russian DOI: 10.52351/00260827_2021_10_36.
Rights and permissions
About this article
Cite this article
Elfimova, L.G., Naboichenko, S.S. Kinetics of Nickel, Iron, and their Alloys Dissolution in Nitric Acid. Metallurgist 65, 1100–1107 (2022). https://doi.org/10.1007/s11015-022-01252-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-022-01252-y