Results of research to assess the effect of increasing the MgO content in the finished sinter from 1.0 to 2.55% on its various metallurgical properties under the conditions of the Egyptian Iron & Steel Company (EISCO) are presented. The increased strength of the sinter in terms of the +5 mm fraction from 84 to 92% due to greater surface melting caused by the lower viscosity and higher fluidity of the primary melt is evidenced by higher bulk density of the sinter samples from 2.78 to 3.11 g/cm3 and a significant decrease in their porosity from 20.54 to 14.87%. This leads to a twofold decrease in the reducibility index of the finished sinter from 85.03 to 39.22%. A decrease in the porosity of the sinter in the secondary cooling zone reduces the secondary oxidation of FeO formed in the combustion zone of solid fuel along with an increased reduction potential of drawn-off gases. Higher MgO content values in the primary melt leads indirectly to an increase in the residual FeO content in the sinter from 13.1 to 16.3% at the same value of solid fuel consumption for sintering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The first HADISOLB-EISCO integrated steel plant began operations in Helwan (Egypt) in 1958–1959. The sinter plant constructed by the German company DEMAG AG consisted of two sintering belts with a sintering area of 50 m2 and two blast furnaces with a volume of 575 m3. Iron ore having a high phosphorus content was delivered from a deposit near the city of Aswan, while the coke for blast-furnace smelting was imported. In addition to the high phosphorus content, the iron ore has an elevated silica content, which increased slag yield and coke consumption, as well as complicating the blast furnace smelting process. Steel from cast iron having a high phosphorus content was smelted in a Thomas converter. Liquid steel was poured into ingots and rolled in German-manufactured rolling mills [1, 2].
Later, in 1967, with the help of the Soviet Union, a project for the plant’s development was devised. The new project was developed by the State Institute for the Design of Metallurgical Plants (Gipromez, Moscow). Modern (for that time) metallurgical equipment was supplied by the South Ural Machine Building Plant (YUMZ) (Orsk, Russia) and other enterprises. The following facilities were built and put into operation: Sinter Plant No. 2 with a powerful modern homogenizing yard, five sintering machines having a sintering area of 75 m2 and equipped with linear sinter coolers; two blast furnaces (BF) comprising a volume of 1033 m3; an oxygen-converter shop with three 90-ton LD oxygen converters (BOF) and six continuous casting machines (CCM); oxygen shop; coke production and other equipment for various auxiliary services of the plant [3, 4].
In the production of fluxed sinter, limestone is used as a flux for smelting cast iron in a blast furnace. The degree of sinter fluxing depends on the composition of the blast furnace charge and the required basicity of the blast furnace slag, including its MgO content. Under a lack of MgO in the main components of the sinter and blast furnace charge, dolomite or other materials containing MgO, for example, dunite and brucite, are usually added to limestone for sinter fluxing. Although dunite is a magnesium-silicate rock with a high MgO content [5, 6], its use is undesirable due to the presence of refractory chromium-containing impurities and the introduction of additional silicate waste rock into the charge. The high refractoriness of dunite up to 1700 °C promotes for its use in furnaces in terms of refractory materials. When dunite is added to sinter charge, a slight decrease in the iron content is observed both with an increase in the specific yield of the blast-furnace slag and the greater coke consumption in the smelting of the cast iron. Brucite (magnesium hydroxide Mg(OH)2) is a high quality magnesium-containing mineral with a small proportion of waste rock, which is imported by Japan from Russia for sinter fluxing instead of dolomite. Replacing dolomite with brucite improves the quality of the sinter, including by slightly increasing the iron content, which reduces coke consumption during cast iron smelting.
The sinter charge at EISCO consists of 55.7% Bahariya iron ore, 6.2% limestone, 3.1% dolomite (33.33% of the total flux), 31.0% return, and 4.1% solid fuel. In terms of fluxes, high-quality limestone from the Bani-Khaled deposits in Minya, consisting of 98–99% calcite, as well as dolomite from the Adabiya quarry in the city of Suez, composed of calcium and magnesium carbonates, are used. The basicity of the sinter is determined by the composition of the blast-furnace charge and is in the range of 0.7–1.2 units. The MgO content in the sinter is maintained in the range of 1.4–1.7%.
The flux materials affect the microstructure of the resulting sinter along with its physical and chemical properties. The metallurgical properties of the sinter for blast-furnace smelting mainly depend on its mineralogical composition and the sintering technology. The metallurgical properties of the sinter to a large extent determine the stability and cost-effectiveness of blast-furnace smelting. The average chemical composition of the raw material used for sintering is presented in Table 1.
The Present Study is Focused on the effect of a gradual increase in the MgO content in the sinter on the change in sintering parameters and sinter metallurgical properties, including strength, porosity, reducibility, and temperature range of softening. The MgO content was varied by gradually replacing the flux limestone with dolomite containing CaO and MgO.
Research Methodology
Sintering experiments involving the gradual replacement of limestone with dolomite were carried out in a “sinter pot” laboratory sintering unit (Fig. 1). The design of this unit was described in a previously published article [7].
The size of the sintered Bahariya iron ore and fluxes with solid fuels was 0–10 and 0–3 mm, respectively. The moisture content of the pelletized sinter charge comprised 12%. Before sintering, the sinter charge was mixed for 4 min to obtain a granular charge. After each sintering procedure, the specific production rate (t/(m2·h)) was calculated according to the vertical sintering speed (mm/min) as follows [7,8,9]:
Bulk density, apparent porosity, and reducibility of the sinter were determined according to the accepted standard methods [7, 10,11,12]:
where Wa — mass of the dry sample, kg; WC — mass of the test sample immersed in liquid, kg; Wd — mass of the test sample suspended in air, kg; D — density of the immersion liquid at the temperature prevailing during the test;
where W0 — initial mass of the sinter sample after moisture removal, kg; Wt — mass of the sample during the reaction, kg; Oxygen, % — mass of the sinter oxygen (%) in the composition of FeO and Fe2O3.
The softening factor of the sinter was determined according to the accepted standard methods. In this study, the softening range was evaluated taking into account the 40% shrinkage [7, 13, 14].
Chemical analysis of the raw materials used in the experiments was performed by the Shimadzu-MXF 2400 XRF spectrometer. The structure of the resulting sinter samples was studied using a scanning electron microscope (SEM) equipped with the FEI unit (S-Netherlands).
Results and Discussion
The composition of the sinter samples with varying MgO content, obtained by successive replacement of limestone with dolomite, is provided in Table 2. Changes in the sintering parameters with various indicators of the sinter quality are given in Table 3.
The MgO content has a significant effect on the slag regime in the production of cast iron in a blast furnace. The MgO content in the blast furnace slag ranging within 6–12% has a positive effect on the fluidity of the slags, lowering their viscosity and increasing the desulfurizing ability of the slag. The fluidity, desulfurizing ability, and ability of the slag to remove alkalis can be improved by using the sinter with a high MgO content [15,16,17]. In addition, with an increase in the MgO content, the proportion of the glass phase in the finished sinter may decrease. This improves the reducibility of the sinter at high temperatures and the index of the “softening-melting” range [18, 19]. For the low-basicity sinter, the improvement of sintering, composition and structure of the sinter, as well as the increase in its strength are closely related to an increase in the MgO content [20,21,22,23]. According to the study of [24], the “softening-melting” indices were improved under an increase in the MgO content from 2.66 to 3.86%, with the strength of the sinter reaching its maximum value when the MgO content was 3.56%.
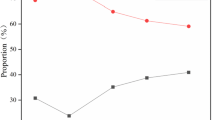
The successive replacement of the limestone with the dolomite led to an increase in the MgO content in the sinter from 1.0 to 2.55% along with a corresponding decrease in the CaO content from 9.72 to 7.35%. At the same time, the FeO content in the sinter gradually increased from 13.1 to 16.2% (Fig. 2).
Due to a decrease in the porosity of the sinter, the bulk density increased from 2.78 to 3.11 g/cm3, which naturally increased the strength of the sinter in terms of the +5 mm fraction from 84 to 92% (Fig. 3).
In practice, the value of the FeO content serves as an indirect estimate of the surface melting degree of the finished sinter. The change in the degree of sinter surface melting can be estimated by the change in the porosity index. A decrease in the sinter porosity from 20.54 to 14.67% leads to an increase in the residual FeO content from 13.1 to 16.2%, which, accordingly, reduces the reducibility index from 85.03 to 39.22%, i.e., by more than two times (Fig. 4). A reduction in the sinter porosity from 20.54 to 14.67% doubled the softening temperature range from 60 to 120 °C (see Fig. 4). During the melting of such a sinter in a blast furnace, the specific consumption of coke for iron smelting will increase.
The analysis of the obtained results demonstrated that the optimization of the properties and quality indicators of the sinter by changing the content of MgO or FeO should be carried out taking into account the assessment of the blast furnace smelting indicators.
Conclusions
As a result of the studies on the effect of increasing the MgO content in the sinter from 1.0 to 2.55% on various metallurgical properties, the following conclusions can be drawn:
– the strength of the sinter in terms of the +5 mm fraction consistently increases from 84 to 92% due to a higher degree of surface melting, as evidenced by a significant decrease in porosity from 20.54 to 14.87% and an increase in the bulk density from 2.78 to 3.11 g/cm3;
– a decrease in porosity from 20.54 to 14.87% leads to a twofold decrease in the sinter reducibility index from 85.03 to 39.22%, which should increase the specific coke consumption for smelting cast iron;
– an increase in the MgO content of the primary melt can cause a decrease in its viscosity and a higher degree of surface melting, which, in turn, reduces the porosity of the finished sinter and, accordingly, the degree of secondary oxidation of FeO formed in the combustion zone of solid fuel with an increase in its residual content from 13.1 to 16.3% under constant consumption of solid fuel for sintering;
– an increase in the FeO content in the sinter by 3.2% contributed to a reduction in the softening start point by 15 °C, doubling the softening range from 60 to 120 °C, which, in turn, should lead to an increase in the specific coke consumption during smelting cast iron.
The obtained results indicate that an increase in the MgO content in the sinter from 1.0 to 2.55% led to significant changes in the quality indicators of the sinter and its metallurgical properties for the conditions of blast furnace smelting. Therefore, for each specific case, depending on the composition of the sinter charge and the requirements of blast furnace smelting for the minimum coke specific consumption, it is necessary to carry out end-to-end industrial tests in order to determine the rational content of MgO and FeO in the sinter and to optimize the strength and reducibility of the latter, taking into account the indicators of blast furnace smelting.
References
M. Y. Ashour, Study of some Physicochemical Parameters Affecting the Sintering Process of Baharia Iron Ores in Helwan Iron & Steel Works, M. Sci. Thesis, 1–3 (1986).
K. Taha, An Attempt for Decreasing the Consumption of Ferromanganese by Increasing the Residual Manganese at the End of the Blow During the Process of Top-Blowing, M. Sci. Thesis, Tabbin Institute for Metallurgical Studies, 1–2 (1999).
M. W. Salem, Physic-Chemical and Mechanical Properties of Aswan Ores and Sinters, Ph. D. Thesis, 9–12 (1970).
M. Meraikib, “Behavior of sodium chloride on smelting of Egyptian iron ores,” Stahl U. Eisen, Bd. 102, 1269 (1982).
T. Umadevi, A. K. Roy, P. C. Mahapatra, M. Prabhu, and M. Ranjan, “Influence of magnesia on iron ore sinter properties and productivity — use of dolomite and dunite, JSW Steel Limited, Toranagallu — 583275, Bellary, Karnataka, India,” Steel Research Int., 80, No. 11, 800–807 (2009).
A. N. Shapovalov, E. V. Ovchinnikova, V. B. Gorbunov, R. R. Dema, and O. B. Kalugina, “The effect of the composition of magnesia flux on the sinter structure and properties,” IOP Conf. Series: Materials Science and Engineering, 625, 012009 (2019).
A. I. Hassan, G. K. Mohamed, A. M. Mohamed, and S. A. Belal, “Sintering of Bahariya iron ore using anthracite at the Egyptian Iron & Steel Company,” Metallurgist, 65, No. 1-2, 3–12 (2021).
S. Saveliev and M. Kondratenko, “Analysis and synthesis of factors determining the sintering speed of sinter charge,” E3S Web of Conf., 166, 06010 (2020).
M. Matsumura, Ya. Yamaguchi, M. Hara, Ch. Kamijo, T. Kawaguchi, and Y. Nakagawa, “Improvement of sinter productivity by adding return fine on raw materials after granulation stage,” ISIJ Int., 53, No. 1, 34–40 (2013).
J. H. Chesters, “Refractories: production and properties,” Iron and Steel Inst., 53 (1973).
C. E. Loo and W. Leung, “Factors influencing the bonding phase structure of iron ore sinters,” ISIJ Int., 43, 1393–1402 (2003).
N. A. El-Hussiny and M. E. H. Shalabi, “A self-reduced intermediate product from iron and steel plant waste material using a briquetting process,” Powder Technology, 205, No. 1-3, 217–223 (2011).
O. A. Mohamed, M. E. H. Shalabi, S. N. Boulis, and M. H. Youssef, “The effect of amide ollic acid on the sintering of an iron ore,” 3rd Mining, Petroleum and Metallurgy Conf. Cairo University. Faculty of Engineering, 2–4 February (1992).
H. Li-Heng, “Effect of raw material composition on the sintering properties,” ISIJ Int., 45, No. 4, 551–559 (2005).
Z. G. Liu, M. S. Chu, H. T. Wanf, W. Zhao, and X. X. Xue, “Effect of MgO content in sinter on the softening-melting behavior of mixed burden made from chromium-bearing vanadium-titanium magnetite,” Int. J. Miner. Met. Mater., 23, 25–32 (2016).
M. Zhou, S. T. Yang, T. Jiang, and X. X. Xue, “Influence of MgO in form of magnesite on properties and mineralogy of high chromium, vanadium, titanium magnetite sinters,” Ironmak. Steelmak, 42, 217–224 (2015).
D. Papanastassiou, P. Nicolaou, and A. Send, “Effect of Al2O3 and MgO contents on the properties of the blast furnace slag,” Stahl Und Eisen., 120, 59–64 (2000).
Q. Li, Z. Z. Huang, T. Jiang, Y. B. Yang, and G. H. Li, “Effect of dolomite and serpentine on sinter quality and microstructure,” Iron Steel, 41, 10–14 (2006).
S. R. Yu, S. J. Yu, Y. H. Liu, and Y. M. Li, “Influence of MgO content on the sinter index and the metallurgical property,” Angang Technol., 353, 23–26 (2008).
M. S. Zhou and Y. R. Li, “Laboratory study on reasonable MgO content in the sinter of Anshan Iron and Steel Group Co.,” Sinter and Pelletizing, 6, 1–4 (2005).
Z. Zhao, “Laboratory sintering study with adding dolomite fines,” Sinter and Pelletizing, 28, 25–27 (2003).
U. S. Yadav, B. D. Pandey, B. K. Das, and D. Jene, “Influence of magnesia on sintering characteristics of iron ore,” Ironmak. Steelmak, 29, 91–95 (2002).
L. X. Yang and L. Davis, “Assimilation and mineral formation during Sintering for blends containing magnetite concentrate and hematite/pisolite sintering fines,” ISIJ Int., 39, 239–245 (2007).
S. Yang, W. Tang, M. Zhou, T. Jiang, X. Xue, and W. Zhang, “Effects of dolomite on mineral compositions and metallurgical properties of chromium-bearing vanadium-titanium magnetite sinter,” Minerals, 7, 210 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 65, No. 10, pp. 19–24, October, 2021. Russian DOI: 10.52351/00260827_2021_10_19.
Rights and permissions
About this article
Cite this article
Hassan, A.I., Khalifa, M.G., Meraikib, M.A. et al. Effect of MgO Content on Properties of Bahariya Iron Ore Sinter under the Conditions of Egyptian Iron & Steel Company (EISCO). Metallurgist 65, 1077–1084 (2022). https://doi.org/10.1007/s11015-022-01249-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-022-01249-7