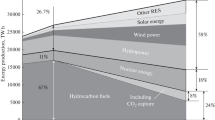
We consider actual problems of production, transportation, and storage of hydrogen and demonstrate the urgency and prospects of subsequent investigations and introduction of hydrogen-based technologies in powder metallurgy. We present predictions of an international organization according to which commercial mass production of hydrogen is expected in the nearest future. This will make hydrogen less costly and more available for the extensive applications in various branches of science and industry. It is indicated that hydrogen is not only a promising alternative energy carrier but also an efficient reducer for numerous metals and alloys and an element applied in various technologies of powder metallurgy aimed at getting broad ranges of high-quality products. It is assumed that hydrogen can find extensive applications in industry in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It is known that metals are rarely encountered in the pure form. As exceptions, we can mention noble metals, copper, and mercury. The other metals are found in the form of compounds with oxygen, sulfur, and halogens. Metals are extracted from these compounds by the process of reduction in which the role of reducers is played by natural gas, methane, hydrogen, aluminum, magnesium, calcium, silicon, etc. One of the most extensively used reducers is carbon.
The advantages of application of carbon in the metallurgical processes of reduction are connected with its sufficiently high efficiency, relatively low cost, and hence, wide availability. An extensive database of the results of investigations formed for a long period of application of this reducer is now used for the development of new technologies of steel-making. Moreover, at present, the research aimed at the use of alternative sources of carbon is continued. Thus, in some cases, it is possible to use, e.g., petroleum coke or charcoal instead of traditional coke. The aim of these investigations is to decrease the cost of reducer because the amount of coal suitable for coking permanently decreases. The carbon footprint of metallurgical production is also a great challenge.
According to the data of the International Energy Agency, in 2018, the total amount of CO2 emissions in the world was equal to 3.31 billion tons and the industrial production was responsible for 40% of these emissions. The fraction of the steel industry constituted about 33.8% of the total amount of industrial emissions [1]. It should be emphasized that the indicated amount of emissions is formed not only by the use of carbon as a reducer but also by the application of carbon-containing components as energy sources and also for some other purposes in this branch of industry, which also lead to the emissions of carbon dioxide.
In order to reduce hazardous emissions of CO2, numerous works and investigations were devoted to the potentially possible and actual optimization of the existing plants by introducing different technologies and methods for the processing and filtration of gases [2,3,4,5,6,7]. Special attention is given to the analysis of the possibilities of application of various types of reducers with an aim of partial or complete replacement of carbon-containing reducers. Moreover, the investigations are carried out not only for the steel industry but also in the field of production and treatment of various other metals and alloys [8,9,10,11,12].
All these facts enable us to conclude that it is necessary to perform additional investigations of the alternative reducers with an aim to reduce CO2 emissions without deterioration of the quality of obtained products and with maximum possible preservation of the production efficiency.
In this connection, hydrogen proves to be one of the most promising reducers. It is already applied in the industry and the possibilities of its extensive commercial applications are of great interest for the researchers and, thus, are studied in numerous works [13,14,15]. In the future, this reducer can replace carbon-containing reducers in the processes of production of various nonferrous metals and steels and their treatment with significant lowering of carbon-dioxide emissions. First of all, this is explained by the high reducing efficiency of this element.
The present work is, in fact, an analytic survey of the contemporary state, problems, and prospects for the subsequent extensive application of hydrogen in the industry and, in particular, in powder metallurgy.
Hydrogen is now used in various fields of science and branches of industry: for the production of ammonia and methanol, in oil refining, and also as a reducer in metallurgy (the traditional methods of getting tungsten and molybdenum include the use of hydrogen, etc.) [16]. Hydrogen-power engineering and hydrogen metallurgy are regarded as the most promising directions [17, 18].
The possibilities of extension of the fields of applicability of hydrogen are restricted by various specific features of its production, storage, transportation, and subsequent application.
At present, hydrogen is mainly produced with the use of natural gas and coal. Only a very small fraction of hydrogen is produced by the electrolysis of water. In this process, water serves as a raw material, which means that the base of raw materials for this process is pure and, in fact, unlimited. This enables us to conclude that, in any case, a potentially ecologically pure raw material has its carbon footprint.
The storage and transportation of hydrogen are complicated by its low density in the gaseous state, low liquefaction temperature, high explosiveness, and negative influence on the properties of structural materials. Actually, these are the main problems restricting the intensification of the use of hydrogen [19].
Numerous research works are devoted to the solution of these problems. Their results are well known and widely applied. At present, there are various procedures used for the production of hydrogen, ecologically safe and technologically simple (electrolysis of water proves to be the most promising procedure), as well as the ways of its storage and transportation.
As the most promising methods for the production of hydrogen with the minimum carbon footprint, we can mention the alkaline water electrolysis, proton-exchange electrolysis (proton-exchange membrane (PEM) technology) with the use of solid polymer electrolytes, and electrolysis with the use of solid-oxide electrolysis cells (SOEC process). In [20], the authors performed a comparative analysis of these methods and indicated that the choice of technology depends on the external criteria of location of hydrogen-producing plants. In the cited work, the authors also performed the analysis of technologies depending on the predictions of energy requirements, carbon footprint, and production costs in 2030. At present, the procedure of alkaline electrolysis of water proves to be most suitable due to lower costs and energy losses.
In the field of storage and transportation, the accumulation of hydrogen in the solid state seems to be a quite promising method. Due to the high level of safety (unlike its storage in the liquid state when low temperatures are not required) and capacity (its volume density is 100–150 g/liter), this method is capable of guaranteeing compact, stationary, and long-term storage of hydrogen in hydrides.
Moreover, at present, the researchers perform investigations of the possibilities of storage of hydrogen in nanocomposites, microspheres, sorbents, and multicapillary structures. The corresponding methods seem to be quite promising. However, due to the low level of knowledge of the interaction of hydrogen with carbon nanostructures, low charge-discharge rates of the microspheres, and high cost of capillary systems (reasonable only under the conditions of high and ultrahigh pressures), it is still early to speak about the extensive practical application of these methods [19].
It is expected that, by 2030, pure hydrogen will be applied for the production of iron from iron ores on large commercial scales and, by 2035, this technology will become much more widespread. This ways of development certainly promises large economic benefits. Indeed, in view of the fact that the cost of hydrogen fueling stations and hydrogen fuel elements became twice lower for the last decade, experts expect a noticeable decrease in the costs of production of hydrogen in the coming years. Thus, it is predicted that, by 2030, hydrogen will become available for various applications as a result of a decrease in its cost by 60%. As a final result, this will turn hydrogen into a competitive alternative and, in some cases, into the ordinary type of fuel [17, 18]. Hence, the possibility of application of this element as a reducer created by its lower cost and better availability is also expected. In addition, it is worth noting that there is a trend to increase in the consumption of hydrogen throughout the world.
Powder metallurgy is a branch industry that uses hydrogen. It is used not only as a reducing agent for some metals but also in the processes of sintering and treatment of various materials. Hydrogen can be used both in pure form and in the form of a mixture with other gases.
By analyzing the possibilities of application of hydrogen as a reducer, we note that it can be used to reduce iron, cobalt, nickel, copper, molybdenum, tungsten, and some other metals in the solid (mainly powder) state. The reduction of chlorides, fluorides, and sulfides of various metals in the solid state is also possible [13] (Fig. 1).
The possibility of reduction of the indicated compounds was studied by using special software [13]. In the data presented in Fig. 1, the possibility of reduction of radioactive elements is not taken into account. It is worth noting that the indicated elements are mainly reduced just in the powder form.
In the case of reduction of chlorides and fluorides, it is necessary to take into account that these compounds are often hygroscopic and, therefore, in the case where oxides of these elements (Ti, V, Nb, Ta, Cr, and Mn) are irreducible in hydrogen, the absence of oxygen in the working space becomes very important. Moreover, these compounds and their products are characterized by certain corrosion activity, which requires the use of materials with sufficiently high corrosion resistance in the construction of the working space [13].
The reduction of sulfides is strongly restricted because hydrogen sulfide released as a result of reduction is a very toxic inflammable gas.
Thus, the process of reduction of oxides of different metals with the help of hydrogen is the optimal possibility. In the industry, as already indicated, hydrogen is used to reduce molybdenum and tungsten trioxides, which are main sources of these metals. It is also used for the reduction of iron scale and iron ore [21,22,23,24].
In Fig. 2a, we present a schematic diagram of the wasteless production of steel by reduction with hydrogen.
Schematic diagram of wasteless production of steel from the iron ore by reduction with hydrogen [17].
In the scheme proposed in Fig. 2, it is assumed that hydrogen is produced by the method of electrolysis of water by using electricity obtained with the help of ecologically friendly technologies [25]. As a final result, we expect the production of steel, which is not only chemically but also ecologically pure.
At the same time, the proposed chain is similar and compatible with the methods of production of metallic powders by reduction and sputtering [26]. Thus, with subsequent introduction of hydrogen-assisted technologies, powder metallurgy may turn into a more ecologically friendly (from the viewpoint of carbon footprint) branch of industry. Under the conditions of permanently increasing requirements to the reduction of harmful emissions and wastes, this is definitely an advantageous feature.
The HDDR (hydrogenation–disproportionation–desorption–recombination) process is also used fairly extensively and includes the application of hydrogen. This process forms a basis of the contemporary method used for the production of magnetic powders and has the following advantages: it is cost-effective and the corresponding procedure of getting anisotropic magnets is quite efficient [27,28,29,30].
Earlier, it was indicated that hydrogen is used as a gas that forms an atmosphere for sintering. In the course of sintering, reducing atmospheres of this kind help to efficiently remove oxide films formed, e.g., on the particles of iron and steel powders. The cermet industry also uses hydrogen as a gas for furnace atmospheres [31, 32].
It is also worth noting that the technologies of powder metallurgy enable us to develop materials suitable for the storage and subsequent transportation of hydrogen [33,34,35,36]. It is reasonable to store hydrogen in carriers produced with the use of different powders, including nanopowders and other nanomaterials (also produced with the use of hydrogen).
Conclusions
The unavoidable necessity of decreasing carbon-dioxide emissions caused by worsening of the ecological situation in the world and the requirements of international organizations and governmental authorities makes topical the search of new ways of solution this problem. The application of hydrogen, as an efficient energy carrier, reducer, and component used in the technologies of powder metallurgy, is a promising alternative of both energy resources and carbon-containing reducers characterized by a significant carbon footprint. The high efficiency of the application of hydrogen for various purposes demonstrated in the present work makes it possible to obtain high-quality products. According to the existing predictions, it is expected that the cost of production of hydrogen will additionally decrease and, hence, the economic effect of its use will become higher. Thus, the subsequent analysis and introduction of hydrogen technologies in the powder metallurgy is an important problem from the viewpoints of both scientific and economical interest, which should lead to a more extensive application of hydrogen in industry.
References
J. Tang, M. Chu, F. Li, C. Feng, Z. Liu, and Y. Zhou, “Development and progress on hydrogen metallurgy,” Int. J. Miner., Met. Mater., 27, No. 6, 713–723 (2020); https://doi.org/10.1007/s12613-020-2021-4.
M. Dreillard, P. Broutin, P. Briot, T. Huard, and A. Lettat, “Application of the DMX TM CO2 capture process in steel industry,” Energy Procedia, 114, 2573–2589 (2020); https://doi.org/10.1016/j.egypro.2017.03.1415.
P. Prathap and D. Senthilkumaran, “Reduction of environmental impact by incorporating performance oriented life cycle assessment,” Environ. Protect. Eng., 42, No. 1, 113–122 (2016).
T. Eglinton, J. Hinkley, A. Beath, and M. Dell’Amico, “Potential applications of concentrated solar thermal technologies in the Australian minerals processing and extractive metallurgical industry,” J. Miner. Met. Mater. Soc., 65, No. 12, 1710–1720 (2013); https://doi.org/10.1007/s11837-013-0707-z.
N. R. Neelameggham, “Soda fuel cycle metallurgy — choices for CO2 reduction,” in: N. R. Neelameggham and R. G. Reddy (editors), Carbon Dioxide Reduction Metallurgy, Metals and Materials Society (Tms) (2008), pp. 135–146.
Q. Bellouard, S. Rodat, M. Grateau, and S. Abanades, “Solar biomass gasification combined with iron oxide reduction for syngas production and green iron metallurgy,” Front. Energy Res., 8, 1–11 (2020); https://doi.org/10.3389/fenrg.2020.00066.
S. Tonomura, N. Kikuchi, N. Ishiwata, S. Tomisaki, and Y. Tomita, “Concept and current state of CO2 ultimate reduction in the steelmaking process (COURSE50) aimed at sustainability in the Japanese steel industry,” J. Sustain. Metall., 2, No. 3, 191–199 (2016); https://doi.org/10.1007/s40831-016-0066-4.
A. Babich, D. Senk, and S. Born, “Interaction between co-injected substances with pulverized coal into the blast furnace,” ISIJ Int., 54, No. 12, 2704–2712 (2014); https://doi.org/10.2355/isijinternational.54.2704.
C. Pichler and J. Antrekowitsch, “Pyrolysis gas as a renewable reducing agent for the recycling of zinc- and lead-bearing residues: A status report,” J. Miner. Met. Mater. Soc., 69, No. 6, 999–1006 (2017); https://doi.org/10.1007/s11837-017-2341-7.
T. Griessacher and J. Antrekowitsch, “Utilization of biomass at the recycling of heavy metal containing wastes,” Waste Biomass Valor., 3(3) (2012), pp. 369–374; https://doi.org/10.1007/s12649-012-9126-6.
T. Griessacher, J. Antrekowitsch, and S. Steinlechner, “Charcoal from agricultural residues as alternative reducing agent in metal recycling,” Biomass Bioenergy, 39, 139–146 (2012); https://doi.org/10.1016/j.biombioe.2011.12.043.
C. Baumgart, C. Weigelt, A. Lißner, S. Martin, C. G. Aneziris, and L. Krüger, “Processing of 17Cr7Mn6Ni TRIP steel powder by extrusion at room temperature and pressureless sintering,” Adv. Eng. Mater., 22, No. 6 (2020).
S. Luidold and H. Antrekowitsch, “Hydrogen as a reducing agent: Thermodynamic possibilities,” J. Miner. Met. Mater. Soc., 59, No. 10, 58–62 (2007); https://doi.org/10.1007/s11837-007-0133-1.
M. Kundak, L. Lazic, and J. Crnko, “CO2 emissions in the steel industry,” Metalurgija, 48, No. 3, 193–197 (2009).
Z. Chen, J. Dang, X. Hu, and H. Yan, “Reduction kinetics of hematite powder in hydrogen atmosphere at moderate temperatures,” Metals, 8, No. 10, 751 (2018); https://doi.org/10.3390/met8100751.
N. L. Solodova, R. R. Minigulov, and E. A. Emel’yanycheva, “Hydrogen as a promising energy carrier. Contemporary methods for the production of hydrogen,” Vest. Kazan. Tekhnol. Univ., No. 3 (2015); URL: https://cyberleninka.ru/article/n/vodorod-kakperspektivnyy-energonositel-sovremennye-metody-polucheniyavodoroda (Date of access: 22.07.2020).
Hydrogen Council. Hydrogen Scaling up, a Sustainable Pathway for the Global Energy Transition [Electronic Resource]; URL: https://hydrogencouncil.com/wp-content/uploads/2017/11/Hydrogen-scaling-up-Hydrogen-Council.pdf (Date of access: 07.07.2020).
Hydrogen Council. Path to Hydrogen Competitiveness, a Cost Perspective [Electronic Resource]; URL: https:// hydrogencouncil.com/wp-content/uploads/2020/01/Path-to-Hydrogen-Competitiveness_Full-Study-1.pdf (Date of access: 09.07.2020).
V. N. Fateev, O. K. Alekseeva, S. V Korobtsev, and E. A. Seregina, “Problems of accumulation and storage of hydrogen,” Kimya Problemleri, No. 4 (16), 453–483 (2018); URL: https://cyberleninka.ru/article/n/problemy-akkumulirovaniya-ihraneniyavodoroda (Date of access: 22.07.2020).
N. Tenhumberg and K. Buker, “Ecological and economic evaluation of hydrogen production by different water electrolysis technologies,” Chem. Ing. Tech., 92, No. 2, 1–11 (2020).
X. Liu, G. Cao, Y. He, M. Yang, and Z. Liu, “Reduction of oxide scale with hydrogen,” J. Iron Steel Res. Int., 21, No. 1, 24–29 (2014); https://doi.org/10.1016/s1006-706x(14)60005-4.
Y. Mohassab, M. Elzohiery, and H. Y. Sohn, “Flash reduction of magnetite and hematite concentrates with hydrogen in a lab-scale reactor for a novel ironmaking process,” in: Proc. of the 7th Internat. Symp. on High-Temperature Metallurgical Processing (February 14–18, 2016, Downtown Nashville, Tennessee), Wiley (2016), pp. 3–10.
Z.-F. Li, Y. Gao, G.-M. Cao, and Z.-Y. Liu, “High-efficiency reduction behavior for the oxide scale formed on hot-rolled steel in a mixed atmosphere of hydrogen and argon,” J. Mater. Sci., 55, No. 4, 1826–1839 (2020).
N. M. Gaballah, A. F. Zikry, M. G. Khalifa, A. B. Farag, N. A. El-Hussiny, and M. E. H. Shalabi, “Kinetic reduction of mill scale via hydrogen,” Sci. Sinter., 46, No. 1, 107–116 (2014).
Y. Z. Lang, R. R. Arnepalli, and A. Tiwari, “A review on hydrogen production: methods, materials and nanotechnology,” J. Nanosci. Nanotechnol., 11, No. 5, 3719–3739 (2011).
E. M. Fainshmidt, V. F. Pegashkin, and L. A. Babysheva, “Raw materials for the production of sintered components in mechanical engineering,” Izv. Vyssh. Uchebn. Zaved. Mashinostr., No. 1 (2008); URL: https://cyberleninka.ru/article/n/syrie-dlya-polucheniya-spechennyhdetaley-mashinostroeniya (Date of access: 17.07.2020).
F. Akagi and Y. Ishii, “Effects of anisotropy field dispersion and grain boundary on coercivity and squareness ratio for HDDR-processed NdFeB powders,” AIP Adv., 8, No. 5, 056201 (2018).
L.-W. Cai, S. Guo, G.-F. Ding, R.-J. Chen, J. Liu, D. Lee, and A.-R. Yan, “Influence of RE-rich phase distribution in initial alloy on anisotropy of HDDR powders,” Chin. Phys. B, 24, No. 9, 097505 (2015); https://doi.org/10.1088/1674-1056/24/9/097505.
H. R. Cha, J. G. Lee, Y. K. Baek, J. H. Yu, H. W. Kwon, and Y. D. Kim, “Synthesis of ultra-fine grained Nd–Fe–B magnetic powder by the control of DR speed during HDDR process,” Korean J. Met. Mater., 51, No. 5, 371–376 (2013).
S. K. Pal, K. Güth, T. G. Woodcock, L. Schultz, and O. Gutfleisch, “Properties of isolated single crystalline and textured polycrystalline nano/sub-micrometre Nd2Fe14B particles obtained from milling of HDDR powder,” J. Phys. D: Appl. Phys., 46, No. 37, 375004–375012 (2013).
V. I. Kostikov, V. Yu. Dorofeev, and Zh. V. Eremeeva, “Protective atmospheres in powder metallurgy,” Tekhnol. Met., No. 12, 30–33 (2007).
V. I. Kostikov, V. Yu. Dorofeev, D. A. Chumak-Zhun’, A. P. Ul’yanovskii, Zh. V. Eremeeva, and D. L. Yaitskii, “Reduction of oxide films during consolidation of the charge used to make powder semifinished products,” Metallurg, No. 7, 55–57 (2008); English translation: Metallurgist, 52, 415–419 (2008).
L. Kral and J. Cermak, “Improvement of hydrogen storage properties of Mg by catalytic effect of Al-containing phases in Mg–Al–Ti–Zr–C powders,” Int. J. Hydrogen Energy, 44, No. 26, 13,561–13,568 (2019).
Y.-C. Pan, J.-X. Zou, X.-Q. Zeng, and W.-J. Ding, “Hydrogen storage properties of Mg–TiO2 composite powder prepared by arc plasma method,” Trans. Nonferr. Met. Soc. China, 24, No. 12, 3834–3839 (2014).
M. Meyer and L. Mendoza-Zelis, “Mechanically alloyed Mg–Ni–Ti and Mg–Fe–Ti powders as hydrogen storage materials,” Int. J. Hydrogen Energy, 37, No. 19, 14,864–14,869 (2012).
A. A. Kovalevskii, A. S. Strogova, V. A. Labunov, and A. A. Shevchenok, “Nano- and microstructural silicon powders in the synthesis and storage of hydrogen,” in: Z. Bartul and J. Trenor (editors), Advances in Nanotechnology, Vol. 18, Chapter 5, pp. 173–189, Nova Science (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 65, No. 3, pp. 63–67, March, 2021.
Rights and permissions
About this article
Cite this article
Akhmetov, A.S., Eremeeva, J.V. Prospects for the Extensive Application of Hydrogen in Powder Metallurgy. Metallurgist 65, 314–319 (2021). https://doi.org/10.1007/s11015-021-01159-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-021-01159-0